L-Arginine Modulates Neonatal Leukocyte Recruitment in a Gestational Age-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Compliance with Ethical Standards
2.3. Isolation of Polymorphonuclear Leukocytes (PMNs)
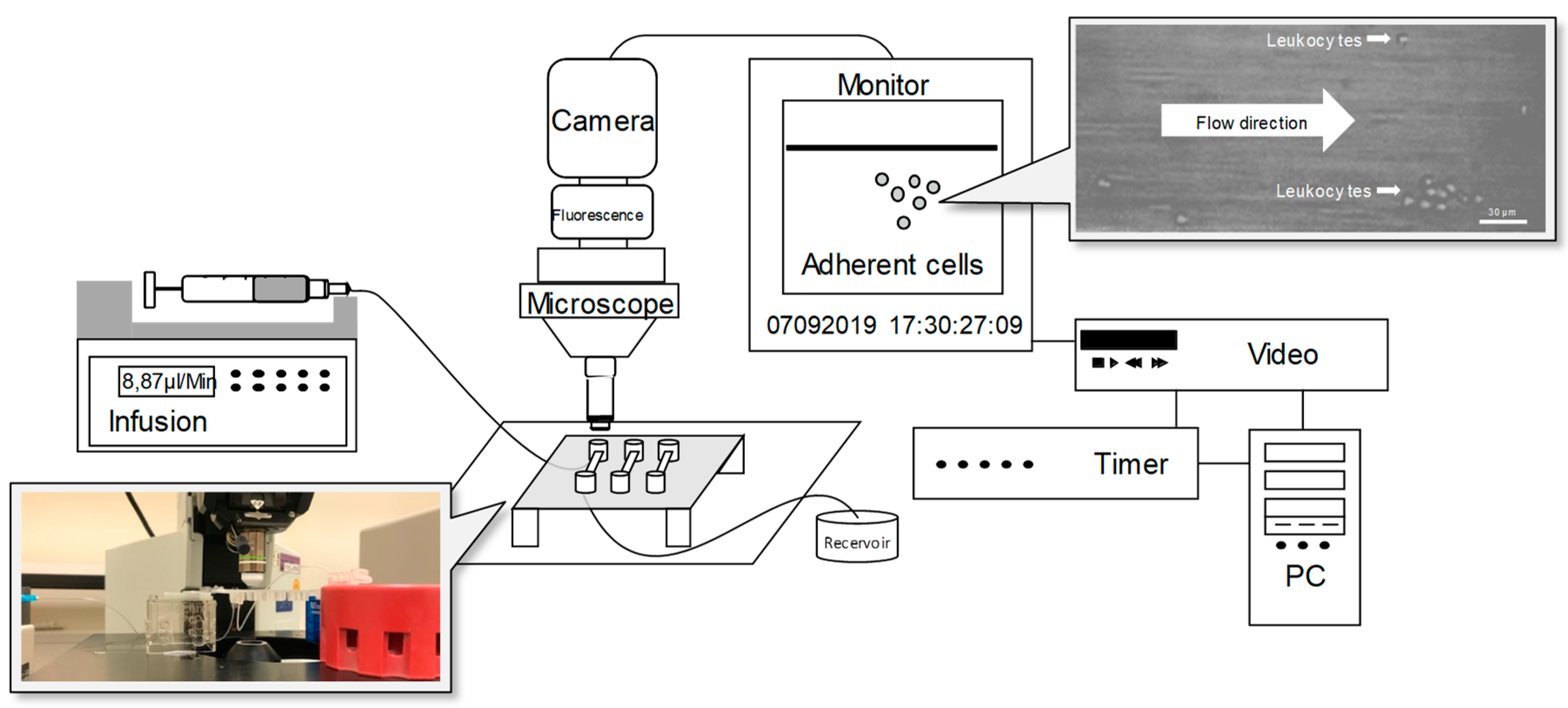
2.4. Flow Chamber Experiments
2.5. Transmigration of Polymorphonuclear Leukocytes (PMNs)
2.6. Flow Cytometry
2.7. Statistics
3. Results
3.1. Study Population
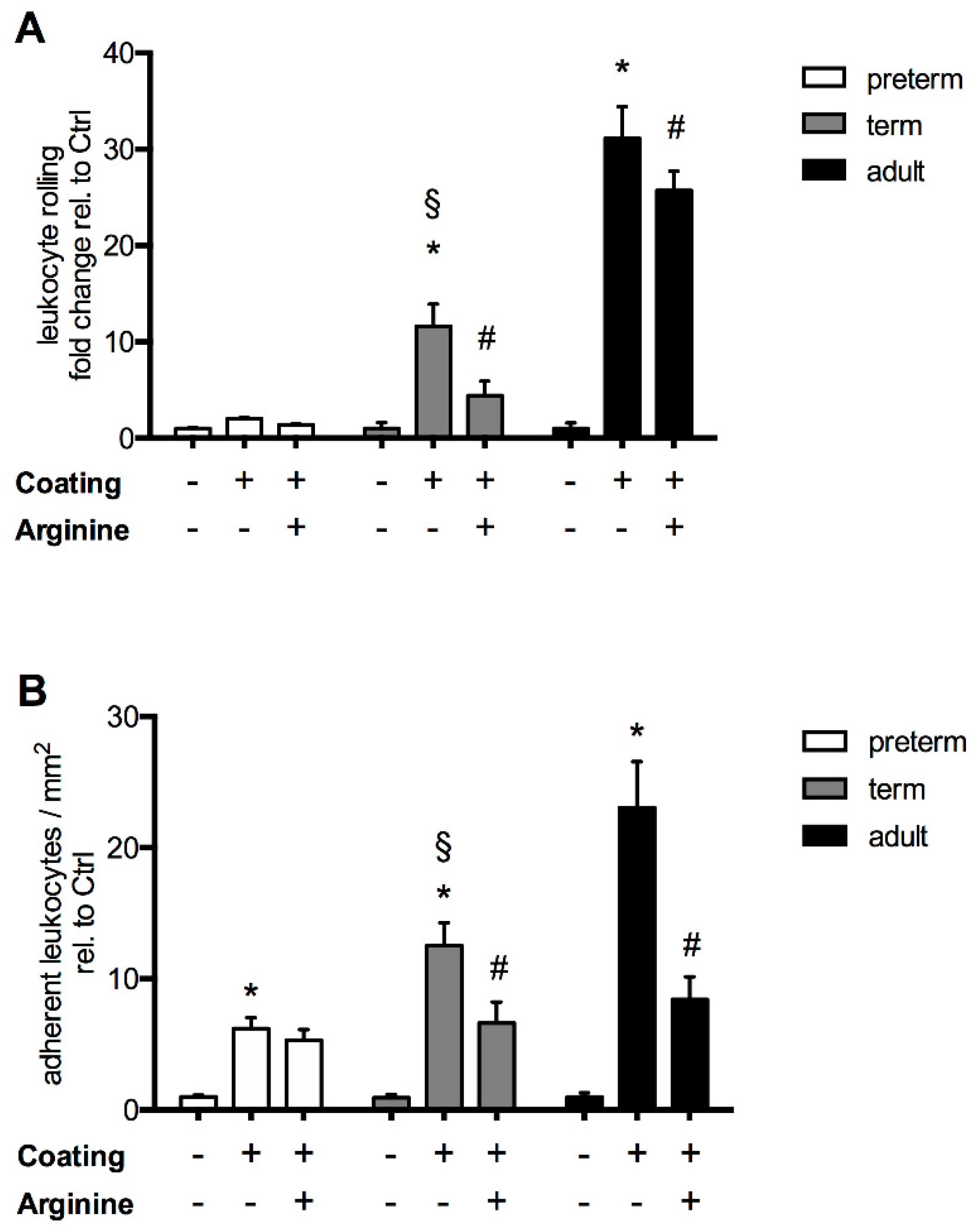
3.2. The Response of L-Arginine Does Not Depend on Clinical Characteristics at Birth and Throughout Neonatal Development
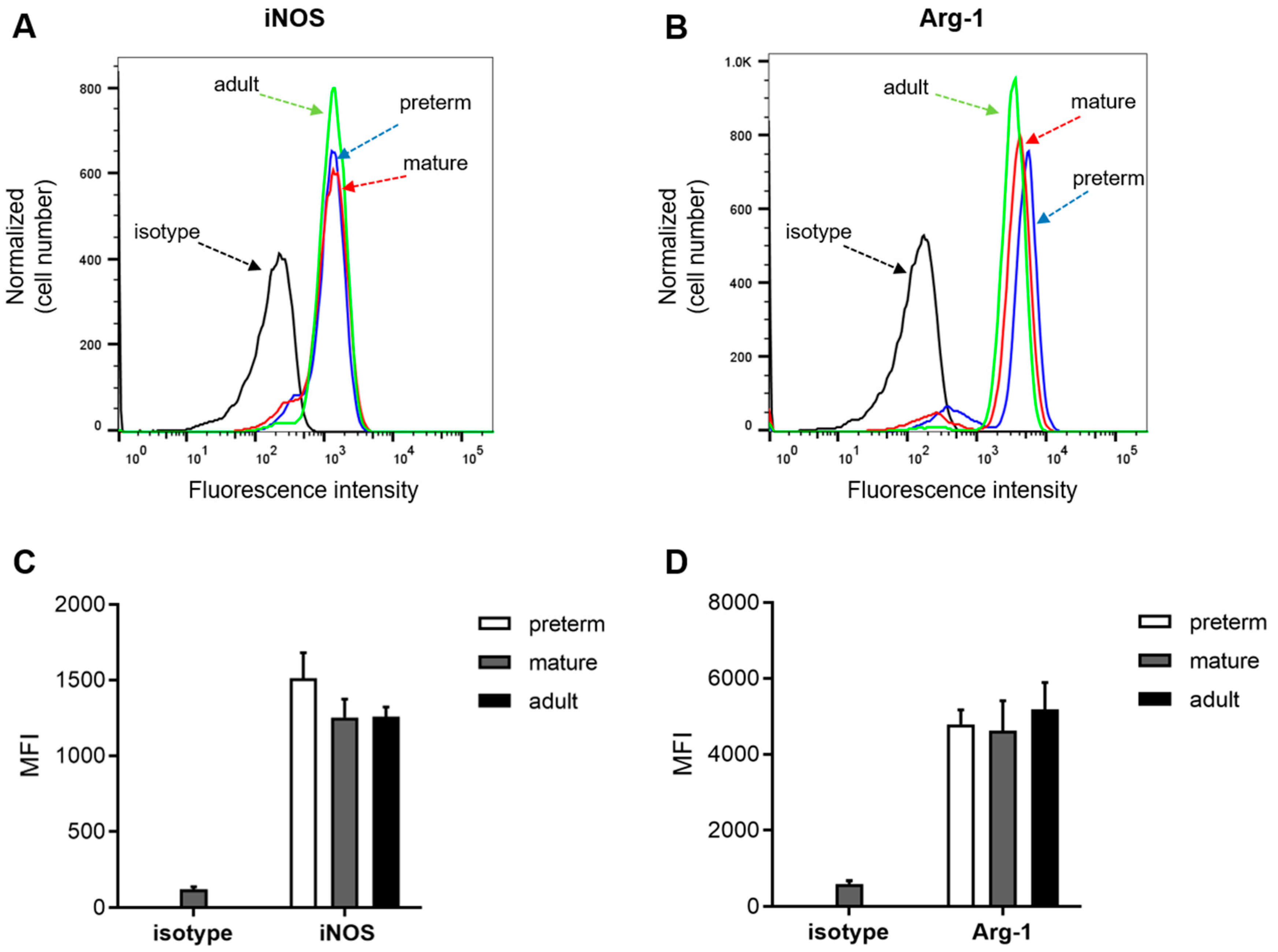
3.3. Expression of Arginase 1 and iNOS Is not Ontogenetically Regulated
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genzel-Boroviczény, M.O.; MacWilliams, S.; Von Poblotzki, M.; Zoppelli, L. Mortality and major morbidity in premature infants less than 31 weeks gestational age in the decade after introduction of surfactant. Acta Obstet. Gynecol. Scand. 2006, 85, 68–73. [Google Scholar] [CrossRef]
- Fanaroff, A.A.; Stoll, B.J.; Wright, L.; Carlo, W.A.; Ehrenkranz, R.A.; Stark, A.R.; Bauer, C.R.; Donovan, E.F.; Korones, S.B.; Laptook, A.R.; et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007, 196, 147.e1–147.e8. [Google Scholar] [CrossRef]
- Camacho-Gonzalez, A.; Spearman, P.; Stoll, B.J. Neonatal infectious diseases: Evaluation of neonatal sepsis. Pediatr. Clin. N. Am. 2013, 60, 367–389. [Google Scholar] [CrossRef] [Green Version]
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Veter Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996, 32, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Karenberg, K.; Hudalla, H.; Frommhold, D. Leukocyte recruitment in preterm and term infants. Mol. Cell. Pediatr. 2016, 3, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussbaum, C.; Gloning, A.; Pruenster, M.; Frommhold, D.; Bierschenk, S.; Genzel-Boroviczény, O.; Von Andrian, U.H.; Quackenbush, E.; Sperandio, M. Neutrophil and endothelial adhesive function during human fetal ontogeny. J. Leukoc. Biol. 2013, 93, 175. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, C.; Sperandio, M. Innate immune cell recruitment in the fetus and neonate. J. Reprod. Immunol. 2011, 90, 74–81. [Google Scholar] [CrossRef]
- Tcharmtchi, M.H.; Smith, C.W.; Mariscalco, M.M. Neonatal neutrophil interaction with P-selectin: Contribution of P-selectin glycoprotein ligand-1 and sialic acid. J. Leukoc. Biol. 2000, 67, 73–80. [Google Scholar] [CrossRef]
- Buschmann, K.; Tschada, R.; Metzger, M.-S.; Braach, N.; Kuss, N.; Hudalla, H.; Poeschl, J.; Frommhold, D. RAGE controls leukocyte adhesion in preterm and term infants. BMC Immunol. 2014, 15, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mieulet, V.; Yan, L.; Choisy, C.; Sully, K.; Procter, J.; Kouroumalis, A.; Krywawych, S.; Pende, M.; Ley, S.C.; Moinard, C.; et al. TPL-2-Mediated Activation of MAPK Downstream of TLR4 Signaling Is Coupled to Arginine Availability. Sci. Signal. 2010, 3, ra61. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.; Schneider, H.; Luckner, C.; Giese, T.; Langhans, C.-D.; Fuentes, J.M.; Kropf, P.; Mueller, I.; Kolb, A.; Modolell, M.; et al. Suppression of T-cell functions by human granulocyte arginase. Blood 2006, 108, 1627–1634. [Google Scholar] [CrossRef] [Green Version]
- Oberlies, J.; Watzl, C.; Giese, T.; Luckner, C.; Kropf, P.; Müller, I.; Ho, A.D.; Munder, M. Regulation of NK Cell Function by Human Granulocyte Arginase. J. Immunol. 2009, 182, 5259–5267. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Zhao, Z.-Q.; Vinten-Johansen, J. L-Arginine inhibits neutrophil adherence and coronary artery dysfunction. Cardiovasc. Res. 1996, 31, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.R.; Jessup, W.; Hailstones, D.L.; Celermajer, D.S. L-Arginine Reduces Human Monocyte Adhesion to Vascular Endothelium and Endothelial Expression of Cell Adhesion Molecules. Circulation 1997, 95, 662–668. [Google Scholar] [CrossRef]
- Wilson, K.; Hawken, S.; Ducharme, R.; Potter, B.K.; Little, J.; Thébaud, B.; Chakraborty, P. Metabolomics of prematurity: Analysis of patterns of amino acids, enzymes, and endocrine markers by categories of gestational age. Pediatr. Res. 2013, 75, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Jaeger, L.A.; Bazer, F.W.; Rhoads, J. Arginine deficiency in preterm infants: Biochemical mechanisms and nutritional implications. J. Nutr. Biochem. 2004, 15, 442–451. [Google Scholar] [CrossRef]
- Davis, J.S.; Anstey, N.M. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis. Crit. Care Med. 2011, 39, 380–385. [Google Scholar] [CrossRef]
- Robinson, J.L.; Smith, V.; Stoll, B.; Agarwal, U.; Premkumar, M.H.; Lau, P.; Cruz, S.M.; Manjarin, R.; Olutoye, O.O.; Burrin, D.G.; et al. Prematurity reduces citrulline-arginine-nitric oxide production and precedes the onset of necrotizing enterocolitis in piglets. Am. J. Physiol. Liver Physiol. 2018, 315, G638–G649. [Google Scholar] [CrossRef] [Green Version]
- Amin, H.J.; Zamora, S.A.; McMillan, D.D.; Fick, G.H.; Butzner, J.; Parsons, H.G.; Scott, R. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J. Pediatr. 2002, 140, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Polycarpou, E.; Zachaki, S.; Tsolia, M.; Papaevangelou, V.; Polycarpou, N.; Briana, D.D.; Gavrili, S.; Kostalos, C.; Kafetzis, D. Enteral L-Arginine Supplementation for Prevention of Necrotizing Enterocolitis in Very Low Birth Weight Neonates. J. Parenter. Enter. Nutr. 2013, 37, 617–622. [Google Scholar] [CrossRef]
- Shah, P.S.; Shah, V.S.; Kelly, L.E. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 2017, CD004339. [Google Scholar] [CrossRef] [PubMed]
- El-Shimi, M.S.; Awad, H.A.; Abdelwahed, M.A.; Mohamed, M.H.; Khafagy, S.; Saleh, G. Enteral L-Arginine and Glutamine Supplementation for Prevention of NEC in Preterm Neonates. Int. J. Pediatr. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marißen, J.; Haiß, A.; Meyer, C.; Van Rossum, T.; Bünte, L.M.; Frommhold, D.; Gille, C.; Goedicke-Fritz, S.; Göpel, W.; Hudalla, H.; et al. Efficacy of Bifidobacterium longum, B. infantis and Lactobacillus acidophilus probiotics to prevent gut dysbiosis in preterm infants of 28+0-32+6 weeks of gestation: A randomised, placebo-controlled, double-blind, multicentre trial: The PRIMAL Clinical Study protocol. BMJ Open 2019, 9, e032617. [Google Scholar] [CrossRef] [Green Version]
- Ruef, P.; Gehm, J.; Gehm, L.; Felbinger, C.; Pöschl, J.; Kuss, N. Determination of whole blood and plasma viscosity by means of flow curve analysis. Gen. Physiol. Biophys. 2014, 33, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Boghossian, N.S.; Page, G.P.; Bell, E.F.; Stoll, B.J.; Murray, J.C.; Cotten, C.M.; Shankaran, S.; Walsh, M.C.; Laptook, A.R.; Newman, N.S.; et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J. Pediatr. 2013, 162, 1120. [Google Scholar] [CrossRef] [Green Version]
- Burgess, L.; Morgan, C.; Mayes, K.; Tan, M. Plasma Arginine Levels and Blood Glucose Control in Very Preterm Infants Receiving 2 Different Parenteral Nutrition Regimens. J. Parenter. Enter. Nutr. 2013, 38, 243–253. [Google Scholar] [CrossRef]
- Bulbul, A.; Okan, F.; Bulbul, L.; Nuhoglu, A.; Nuhoǧlu, A. Effect of low versus high early parenteral nutrition on plasma amino acid profiles in very low birth-weight infants. J. Matern. Neonatal Med. 2011, 25, 770–776. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [Green Version]
- Müller, I.; Munder, M.; Kropf, P.; Hänsch, G.M. Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol. 2009, 30, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Mathias, B.J.; Murphy, T.J.; Rincon, J.C.; López, M.C.; Ungaro, R.; Ellett, F.; Jorgensen, J.A.; Wynn, J.L.; Baker, H.V.; et al. Neutrophil chemotaxis and transcriptomics in term and preterm neonates. Transl. Res. 2017, 190, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Hathaway, L.; Mincemoyer, R.; Schenke, W.H.; Kirby, M.; Csako, G.; Waclawiw, M.A.; Panza, J.A.; Cannon, R.O. Oral L-arginine in patients with coronary artery disease on medical management. Circulation 2000, 101, 2160–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtmann, A.; Germena, G.; Block, H.; Boras, M.; Rossaint, J.; Sundd, P.; Lefort, C.; Fisher, C.I.; Buscher, K.; Gelschefarth, B.; et al. The PSGL-1–L-selectin signaling complex regulates neutrophil adhesion under flow. J. Cell Biol. 2013, 203, 2032OIA125. [Google Scholar] [CrossRef]
- Badurdeen, S.; Mulongo, M.M.; Berkley, J.A. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr. Res. 2014, 77, 290–297. [Google Scholar] [CrossRef]
- Saini, R.; Singh, S. Inducible nitric oxide synthase: An asset to neutrophils. J. Leukoc. Biol. 2018, 105, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Berchner-Pfannschmidt, U.; Yamac, H.; Trinidad, B.; Fandrey, J. Nitric Oxide Modulates Oxygen Sensing by Hypoxia-inducible Factor 1-dependent Induction of Prolyl Hydroxylase 2. J. Biol. Chem. 2006, 282, 1788–1796. [Google Scholar] [CrossRef] [Green Version]
- Caplan, M.S.; Hedlund, E.; Hill, N.; MacKendrick, W. The role of endogenous nitric oxide and platelet-activating factor in hypoxia-induced intestinal injury in rats. Gastroenterology 1994, 106, 346–352. [Google Scholar] [CrossRef]
- Dekaney, C.M.; Wu, G.; Jaeger, L.A. Ornithine Aminotransferase Messenger RNA Expression and Enzymatic Activity in Fetal Porcine Intestine. Pediatr. Res. 2001, 50, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Dekaney, C.M.; Wu, A.G.; Jaeger, L.A. Gene Expression and Activity of Enzymes in the Arginine Biosynthetic Pathway in Porcine Fetal Small Intestine. Pediatr. Res. 2003, 53, 274–280. [Google Scholar] [CrossRef]
- Hoang, A.N.; Jones, C.N.; Dimisko, L.; Hamza, B.; Martel, J.; Kojic, N.; Irimia, D. Measuring neutrophil speed and directionality during chemotaxis, directly from a droplet of whole blood. Technology 2013, 1, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.N.; Hoang, A.N.; Martel, J.M.; Dimisko, L.; Mikkola, A.; Inoue, Y.; Kuriyama, N.; Yamada, M.; Hamza, B.; Kaneki, M.; et al. Microfluidic assay for precise measurements of mouse, rat, and human neutrophil chemotaxis in whole-blood droplets. J. Leukoc. Biol. 2016, 100, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luiking, Y.C.; Poeze, M.; Ramsay, G.; Deutz, N. The Role of Arginine in Infection and Sepsis. J. Parenter. Enter. Nutr. 2005, 29, S70–S74. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Poeze, M.; Deutz, N. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin. Sci. 2014, 128, 57–67. [Google Scholar] [CrossRef]
- Lopez, A.; Lorente, J.A.; Steingrub, J.; Bakker, J.; McLuckie, A.; Willatts, S.; Brockway, M.; Anzueto, A.; Holzapfel, L.; Breen, D.; et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: Effect on survival in patients with septic shock. Crit. Care Med. 2004, 32, 21–30. [Google Scholar] [CrossRef]


| Clinical Characteristics | Preterm Infants | Term Infants | p-Value |
|---|---|---|---|
| Day 1 | Day 1 | ||
| n = 44 | n = 17 | ||
| GA in weeks + days, mean ± SD | 30 + 6 ± 1 + 3 | 38 + 3 ± 1 + 5 | <0.0001 |
| 5 min APGAR, mean ± SD | 8.0 ± 0.9 | 9.4 ± 0.9 | <0.0001 |
| 10 min APGAR, mean ± SD | 8.6 ± 0.7 | 9.8 ± 0.6 | <0.0001 |
| Male gender, n (%) | 29 (66) | 11 (65) | 0.714 |
| Weight in g, mean ± SD | 1569 ± 355 | 3045 ± 464 | <0.0001 |
| Head circumference in cm, mean ± SD | 28.8 ± 1.5 | 34.4 ± 1.5 | <0.0001 |
| Body length in cm, mean ± SD | 41 ± 3 | 51 ± 3 | <0.0001 |
| Nutrition, n (%) | |||
| Breast milk exclusively | 23 (52) | 17 (100) | 0.0004 |
| Formula supplementation | 21(48) | 0 | |
| PROM, n (%) | 10 (23) | 0 | 0.032 |
| Antibiotics, n (%) | 39 (89) | 0 | <0.0001 |
| Laboratory parameters on admission | |||
| Arterial pH, mean ± SD | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.483 |
| CRP > 2 mg/L, n (%) | 1 (2) | 2 (12) | 0.124 |
| WBC count/nL, mean ± SD | 10.1 ± 3.9 | 13.7 ± 4.9 | 0.004 |
| I/T ratio > 0.25, n (%) | 2 (4) | 0 | 0.371 |
| Flow Chamber, Adherence/mm2, Mean ± SD | Preterm Infants Day 1 n = 17 | Preterm Infants Day 28 n = 17 | ||||
|---|---|---|---|---|---|---|
| L-Arginine Responder n = 9 | L-Arginine Non-Responder n = 8 | p-Value | L-Arginine Responder n = 8 | L-Arginine Non-Responder n = 9 | p-Value | |
| Uncoated | 4.3 ± 2.7 | 2.0 ± 1.9 | 0.990 | 2.1 ± 1.6 | 3.0 ± 2.1 | 0.999 |
| Coated − L-arginin | 33.5 ± 9.4 | 14.3 ± 8.7 | 0.0001 | 36.7 ± 13.4 | 17.2 ± 12.0 | 0.001 |
| Coated + L-arginin | 18.3 ± 9.6 | 16.4 ± 10.1 | 0.996 | 11.3 ± 8.8 | 15.5 ± 10.7 | 0.938 |
| Clinical characteristics | ||||||
| GA in weeks + days, mean ± SD | 216.4 ± 9.8 | 215.0 ± 9.1 | 0.759 | 222.1 ± 5.5 | 216.3 ± 9.6 | 0.154 |
| APGAR 5′, mean ± SD | 6.4 ± 1.7 | 6.0 ± 1.6 | 0.947 | 8.4 ± 1.1 | 8.1 ± 0.8 | 0.565 |
| APGAR 10′, mean ± SD | 8.6 ± 0.9 | 8.5 ± 0.8 | 0.892 | 8.9 ± 0.6 | 8.8 ± 0.7 | 0.764 |
| Male gender, n (%) | 6 (66.7) | 6 (75.0) | 0.707 | 4 (50.0) | 7 (77.8) | 0.232 |
| Weight in g, mean ± SD | 1584.4 ± 367.9 | 1673.6 ± 173.5 | 0.541 | 2465.6 ± 263.8 | 2452.2 ± 315.9 | 0.926 |
| Head circumference in cm, mean ± SD | 29.1 ± 1.7 | 28.9 ± 0.8 | 0.751 | 31.5 ± 1.5 | 33.3 ± 1.4 | 0.020 |
| Body length in cm, mean ± SD | 41.4 ± 2.8 | 41.0 ± 2.3 | 0.735 | 45.0 ± 1.39 | 46.2 ± 2.6 | 0.247 |
| Breast milk feeds, n (%) | 9 (100.0) | 7 (87.5) | 0.274 | 8 (100.0.) | 7 (77.8) | 0.156 |
| PROM, n (%) | 2 (22.2) | 2 (25.0) | 0.893 | 3 (37.5) | 1 (11.1) | 0.200 |
| Antibiotics, n (%) | 8 (89) | 7 (88) | 0.929 | 2 (25) | 2 (22) | 0.893 |
| Birth mode, n (%) | ||||||
| Vaginal spontanous | 2 (22.2) | 2 (25.0) | 0.893 | 2 (25.0) | 1 (11.1) | 0.453 |
| Caesarean Section | 7 (77.8) | 6 (75.0) | 6 (75.0) | 8 (88.9) | ||
| Laboratory parameters | ||||||
| Arterial pH, mean ± SD | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.954 | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.203 |
| CRP > 2 mg/L, n (%) | 0 | 0 | 3 (37.5) | 4 (44.4) | 0.772 | |
| WBC count/nL, mean ± SD | 11.4 ± 4.2 | 10.5 ± 5.0 | 0.573 | 9.3 ± 1.9 | 11.0 ± 2.7 | 0.159 |
| I/T ratio > 0.25, n (%) | 0 | 0 | 0 | 0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitterer, R.; Lajqi, T.; Kranig, S.A.; Braun, M.; Theissig, N.; Kuss, N.; Pöschl, J.; Frommhold, D.; Hudalla, H. L-Arginine Modulates Neonatal Leukocyte Recruitment in a Gestational Age-Dependent Manner. J. Clin. Med. 2020, 9, 2772. https://doi.org/10.3390/jcm9092772
Fitterer R, Lajqi T, Kranig SA, Braun M, Theissig N, Kuss N, Pöschl J, Frommhold D, Hudalla H. L-Arginine Modulates Neonatal Leukocyte Recruitment in a Gestational Age-Dependent Manner. Journal of Clinical Medicine. 2020; 9(9):2772. https://doi.org/10.3390/jcm9092772
Chicago/Turabian StyleFitterer, Raphaela, Trim Lajqi, Simon Alexander Kranig, Maylis Braun, Nicole Theissig, Navina Kuss, Johannes Pöschl, David Frommhold, and Hannes Hudalla. 2020. "L-Arginine Modulates Neonatal Leukocyte Recruitment in a Gestational Age-Dependent Manner" Journal of Clinical Medicine 9, no. 9: 2772. https://doi.org/10.3390/jcm9092772
APA StyleFitterer, R., Lajqi, T., Kranig, S. A., Braun, M., Theissig, N., Kuss, N., Pöschl, J., Frommhold, D., & Hudalla, H. (2020). L-Arginine Modulates Neonatal Leukocyte Recruitment in a Gestational Age-Dependent Manner. Journal of Clinical Medicine, 9(9), 2772. https://doi.org/10.3390/jcm9092772






