Oxidative Stress Markers and the Retinopathy of Prematurity
Abstract
1. Introduction
2. Epidemiology and Risk Factors
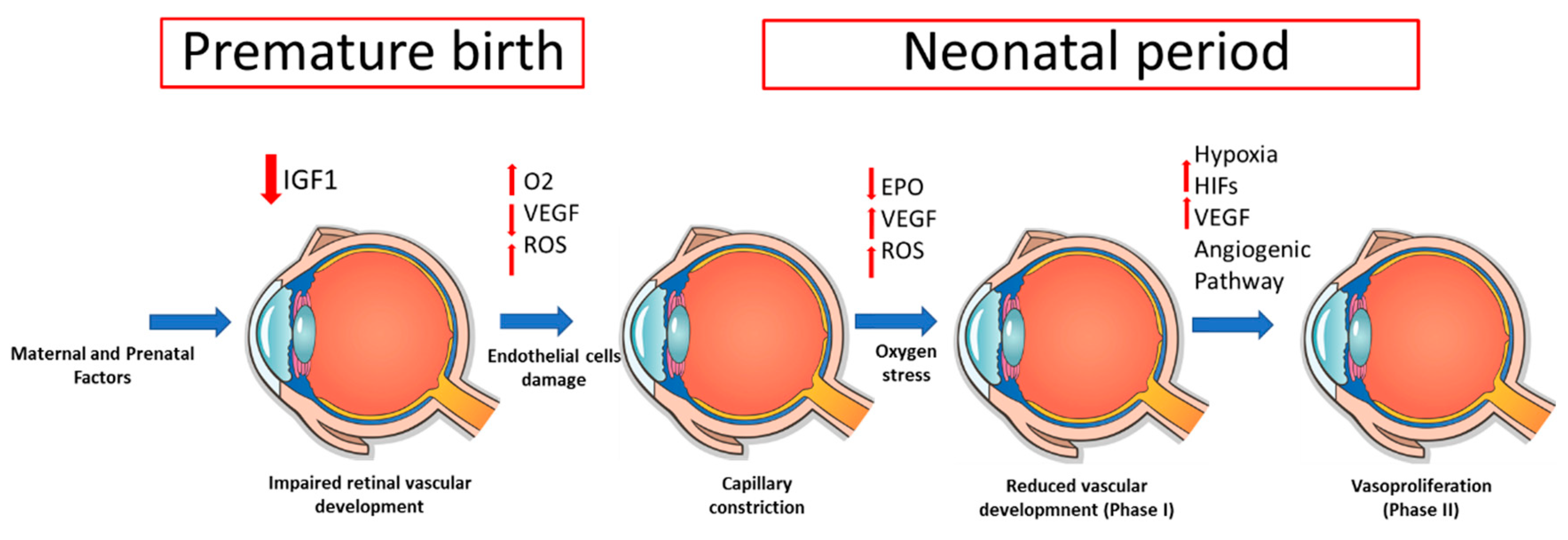
2.1. Prenatal and Perinatal Factors
2.2. Maternal Factors
2.3. Postnatal Factors
3. ROP Stages
4. Pathophysiology
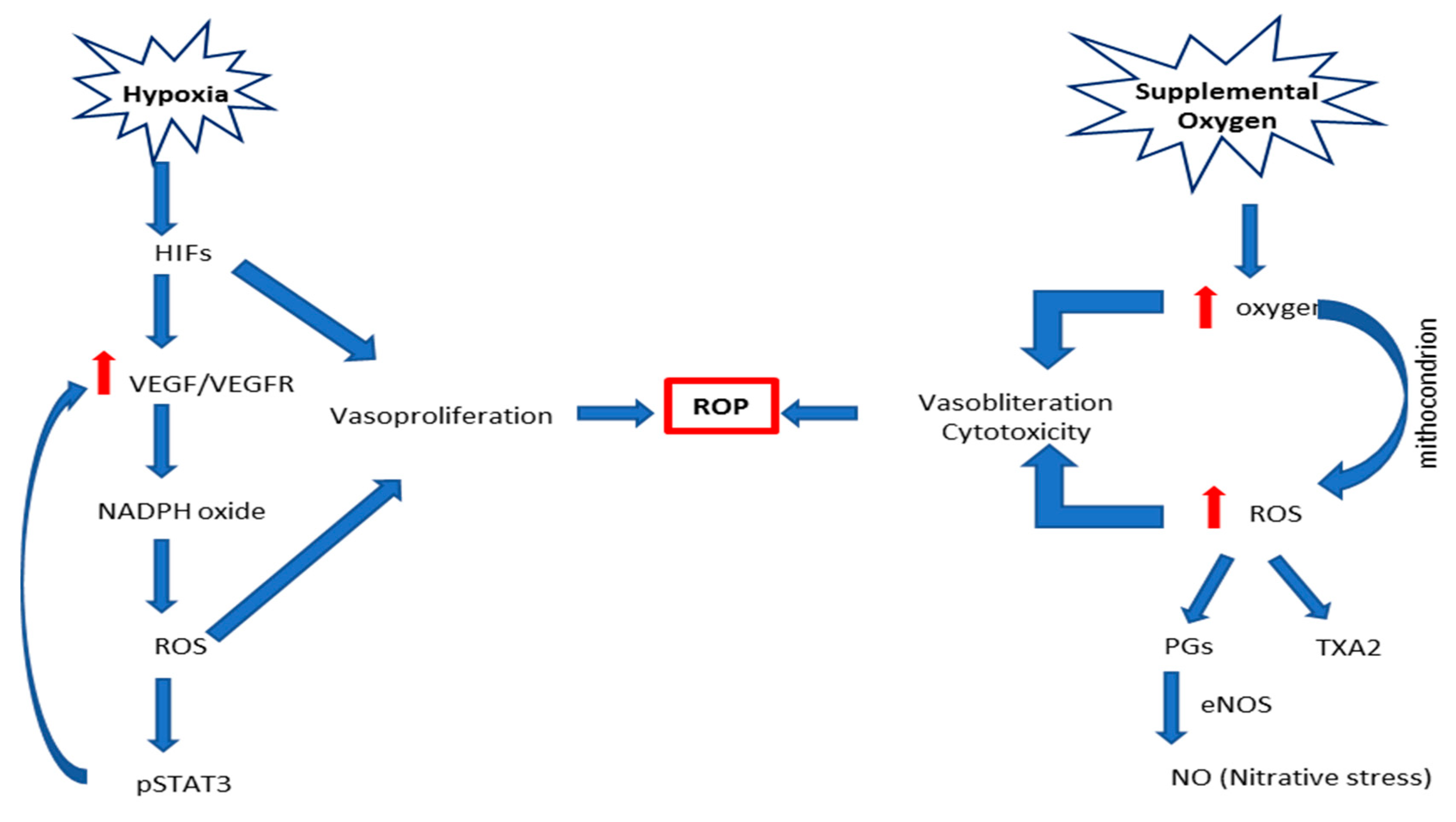
5. Oxidative Stress
5.1. Main Pathophysiological Mechanisms and Mediators
5.1.1. Hyperoxia/Hypoxia
5.1.2. Nitro-Oxidative Stress
5.1.3. Lipid Peroxidation
6. Biomarkers
6.1. Malondialdehyde
6.2. 8-Hydroxy 2-Deoxyguanosine
6.3. GSH/GSSG Ratio
6.4. Protein Oxidation
6.5. Lipids
7. The Role of Scavenging Systems in ROP
7.1. Glutathione
7.2. Nrf2/Keap1
7.3. Paraoxonase
7.4. Stanniocalcin-1
8. Nonenzymatic Scavenging Systems
8.1. Vitamins E and C
8.2. Omega-3 and PUFA
8.3. Lutein
8.4. LT and Biological Fluids
9. Discussion
10. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.J.; Port, A.D.; Swan, R.J.; Campbell, P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- Alajbegovic-Halimic, J.; Zvizdic, D.; Alimanovic-Halilovic, E.; Dodik, I.; Duvnjak, S. Risk factors for retinopathy of prematurity in premature born children. Med. Arch. 2015, 69, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Fierson, W.M. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [CrossRef] [PubMed]
- Beligere, N.; Perumalswamy, V.; Tandon, M.; Mittal, A.; Floora, J.; Vijayakumar, B.; Miller, M.T. Retinopathy of prematurity and neurodevelopmental disabilities in premature infants. Semin. Fetal Neonatal Med. 2015, 20, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 2008, 84, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.D.; Cernichiaro-Espinosa, L.A.; Berrocal, A.M. Management of retinopathy of prematurity-use of anti-VEGF therapy. Asia-Pacific J. Ophthalmol. 2018, 7, 56–62. [Google Scholar] [CrossRef]
- Bashinsky, A.L. Retinopathy of prematurity. N. C. Med. J. 2017, 78, 124–128. [Google Scholar] [CrossRef]
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Fierson, W.M. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013, 13, 189–195. [Google Scholar] [CrossRef]
- Ludwig, C.A.; Chen, T.A.; Hernandez-Boussard, T.; Moshfeghi, A.A.; Moshfeghi, D.M. The epidemiology of retinopathy of prematurity in the United States. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 553–562. [Google Scholar] [CrossRef]
- Borroni, C.; Carlevaro, C.; Morzenti, S.; De Ponti, E.; Bozzetti, V.; Console, V.; Capobianco, S.; Tagliabue, P.E. Survey on retinopathy of prematurity (ROP) in Italy. Ital. J Pediatr. 2013, 39, 43. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.S.; Bell, E.F.; Donohue, P.; Tomlinson, L.A.; Binenbaum, G. Perinatal risk factors for the retinopathy of prematurity in postnatal growth and rop study. Ophthalmic. Epidemiol. 2019, 26, 270–278. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sapiehab, P.; Joyala, J.S.; Duhamela, F.; Shaoa, Z.; Sitarasa, N.; Picarda, E.; Zhoua, E.; Lachapellec, P.; Chemto, S. Understanding retinopathy of prematurity: Update on pathogenesis. Neonatology 2011, 100, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Smith, L.E. Retinopathy of prematurity. Angiogenesis 2007, 10, 133–140. [Google Scholar] [CrossRef]
- Sapieha, P.; Hamel, D.; Shao, Z.; Rivera, J.C.; Zaniolo, K.; Joyal, J.S.; Chemtob, S. Proliferative retinopathies: Angiogenesis that blinds. Int. J. Biochem. Cell. Biol. 2010, 42, 5–12. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Penn, J.S. Mechanisms and Management of Retinopathy of Prematurity. N. Engl. J Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef]
- Banjac, L.; Banjac, G.; Kotur-Stevuljević, J.; Spasojević-Kalimanovska, V.; Gojković, T.; Bogavac-Stanojević, N.; Jelić-Ivanović, Z.; Banjac, G. Pro-oxidants and antioxidants in retinopathy of prematurity. Acta Clin. Croat. 2018, 57, 458–463. [Google Scholar] [CrossRef]
- Stone, W.L.; Shah, D.; Hollinger, S.M. Retinopathy of prematurity: An oxidative stress neonatal disease. Front. Biosci. 2016, 21, 165–177. [Google Scholar] [CrossRef]
- Rivera, J.C.; Dabouz, R.; Noueihed, B.; Omri, S.; Tahiri Chemtob, S. Ischemic retinopathies: Oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2017, 3940241. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Longini, M.; Vezzosi, P.; Marzocchi, B.; Paffetti, P.; Bracci, L. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr. Res. 2002, 52, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, A.; Perruzzi, C.; Ju, M.; Engström, E.; Hard, A.L.; Liu, J.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Sjodell, L.; et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: Direct correlation with clinical retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2001, 98, 5804–5808. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.X.; Hartnett, M.E. Signaling Pathways Triggered by Oxidative Stress That Mediate Features of Severe Retinopathy of Prematurity. JAMA Ophthalmol. 2013, 131, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Shabrawey, A.; Bartoli, M.; El-Remessy, A.B.; Platt, D.H.; Matragoon, S.; Behzadian, M.A.; Caldwell, R.W.; Caldwell, R.B. Inhibition of NAD(P)H Oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am. J. Path. 2005, 167, 599–607. [Google Scholar] [CrossRef]
- Brooks, S.E.; Gu, X.; Samuel, S.; Marcus, D.M.; Bartoli, M.; Huang, P.L.; Caldwell, R.B. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Investig. Ophthalmol. Vis. Sci. 2001, 42, 222–228. [Google Scholar]
- Beauchamp, M.H.; Sennlaub, F.; Speranza, G.; Gobeil, F.; Checchin, D.; Kermorvant-Duchemin, E.; Abran, D.; Hardy, P.; Lachapelle, P.; Varma, D.R.; et al. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic. Biol. Med. 2004, 37, 1885–1894. [Google Scholar] [CrossRef]
- Gu, X.; El-Remessy, A.B.; Brooks, S.E.; Al-Shabrawey, M.; Tsai, N.T.; Caldwell, R.B. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am. J. Physiol. Cell Physiol. 2003, 285, 546–554. [Google Scholar] [CrossRef]
- Leduc, M.; Kermorvant-Duchemin, E.; Chechim, D.; Sennlaub, F.; Chemtob, S. Hypercapnia and Trans-Arachidonic acid-induced retinal microvascular degeneration: Implications in the genesis of retinopathy of prematurity. Med. Sci. 2007, 23, 939–943. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Pryor, W.A. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998, 25, 392–403. [Google Scholar] [CrossRef]
- Kroncke, K.D. Mechanisms and biological consequences of nitrosative stress. Biol. Chem. 2003, 384, 1341. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite reactions and diffusion in biology. Chem. Res. Toxicol. 1998, 11, 720–721. [Google Scholar] [CrossRef] [PubMed]
- Checchin, D.; Sennlaub, F.; Sirinyan, M.; Brault, S.; Zhu, T.; Kermorvant-Duchemin, E.; Hardy, P.; Balazy, M.; Chemtob, S. Hypercapnia prevents neovascularization via nitrative stress. Free Radic. Biol. Med. 2006, 40, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.C.; Bornstein, P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix. Biol. 2003, 22, 63–71. [Google Scholar] [CrossRef]
- Guo, N.; Krutzsch, H.C.; Inman, J.K.; Roberts, D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997, 57, 1735–1742. [Google Scholar] [PubMed]
- Nor, J.E.; Mitra, R.S.; Sutorik, M.M.; Mooney, D.J.; Castle, V.P.; Polverini, P.J. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J. Vasc. Res. 2000, 37, 209–218. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019, 266, 121–129. [Google Scholar] [CrossRef]
- Bersani, I.; Pluchinotta, F.; Dotta, A.; Savarese, I.; Campi, F.; Auriti, C.; Chuklantseva, N.; Piersigilli, F.; Gazzolo, F.; Varrica, A.; et al. Early predictors of perinatal brain damage: The role of neurobiomarkers. Clin. Chem. Lab. Med. 2020, 58, 471–486. [Google Scholar] [CrossRef]
- Garg, U.; Jain, A.; Singla, P.; Sarita Beri, S.; Garg, R.; Saili, A. Free radical status in retinopathy of prematurity. Indian J. Clin. Bio Chem. 2012, 27, 196–199. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Ates, O.; Alp, H.H.; Caner, I.; Yildirim, A.; Tastekin, A.; Kocer, I.; Baykal, O. Oxidative DNA damage in retinopathy of prematurity. Eur. J. Ophthalmol. 2009, 19, 80–85. [Google Scholar] [CrossRef]
- Loft, S.; Fischer-Nielsen, A.; Jeding, I.B.; Vistisen, K.; Pulsen, H.E. 8- Hydroxydeoxyguanosine as a urine biormarker of oxidative DNA damage. J. Toxicol. Environ. Health 1993, 40, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A. The role of 8- hydroxyguanine in carcinogenesis. Carcionogenesis 1990, 11, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.Y.; Cui, Y.; Chen, L.; Song, J.; Sun, L. Urine 8-deoxyguanosine levels in diabetic retinopathy patients. Eur. J. Ophtalmol. 2008, 18, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S.; et al. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CC14 poisoning? Free Radic. Biol. Med. 2005, 15, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Bakunowicz-Lazarczyk, A.; Dzienis, K.; Skrzydlewska, E.; Szczepański, M.; Waszkiewiczz, E. The estimation of selected parameters in antioxidant system in red blood cells in ROP screening of premature infants. Klin. Oczna. 2006, 108, 413–415. [Google Scholar]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995, 1, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Narag, M.; Banerjee, B.D.; Basu, S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: A case control study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef]
- Deliyanti, D.; Lee, J.Y.; Petratos, S.; Meyer, C.J.; Ward, K.W.; Wilkinson-Berka, J.L.; de Haan, J.B. A potent Nrf2 activator, dh404, bolsters antioxidant capacity in glial cells and attenuates ischaemic retinopathy. Clin. Sci. 2016, 130, 1375–1387. [Google Scholar] [CrossRef]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Mishra, M.; Kowluru, R.A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Prow, T.W.; Bhutto, I.A.; Yerrapureddy, A.; McLeod, D.S.; Yamamoto, M.; Reddy, S.P.; Lutty, G.A. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp. Eye. Res. 2010, 90, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Sporn, M.B. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Hartnett, M.E.; Bretz, C.A.; Hann, C.R.; Cui, R.Z.; Marmorstein, A.D.; Sheikh-Hamad, D.; Fautsch, M.P.; Roddy, G.W. Stanniocalcin-1 is a modifier of oxygen induced retinopathy severity. Curr. Eye Res. 2020, 45, 46–51. [Google Scholar] [CrossRef]
- Huang, L.; Garcia, G.; Lou, Y.; Zhou, Q.; Truong, L.D.; Di Mattia, G.; Lan, X.R.; Lan, H.Y.; Wang, Y.; Sheikh-Hamad, D. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am. J. Pathol. 2009, 174, 1368–1378. [Google Scholar] [CrossRef]
- Ito, Y.; Zemans, R.; Correll, K.; Yang, I.V.; Ahmad, A.; Gao, B.; Mason, R.J. Stanniocalcin-1 is induced by hypoxia inducible factor in rat alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2014, 452, 1091–1097. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Qin, Y. Recombinant adeno-associated virus-delivered hypoxia-inducible stanniocalcin-1 expression effectively inhibits hypoxia-induced cell apoptosis in cardiomyocytes. J. Cardiovasc. Pharmacol. 2014, 64, 522–529. [Google Scholar] [CrossRef]
- Durukan Tolvanen, A.; Westberg, J.A.; Serlachius, M.; Chang, A.C.; Reddel, R.R.; Andersson, L.C.; Tatlisumak, T. Stanniocalcin 1 is important for poststroke functionality, but dispensable for ischemic tolerance. Neuroscience 2013, 229, 49–54. [Google Scholar] [CrossRef]
- Zhang, K.; Lindsberg, P.J.; Tatlisumak, T.; Kaste, M.; Olsen, H.S.; Andersson, L.C. Stanniocalcin: A molecular guard of neurons during cerebral ischemia. Proc. Natl. Acad. Sci. USA 2000, 97, 3637–3642. [Google Scholar] [CrossRef]
- Roddy, G.W.; Rosa, R.H.; Oh, J.Y.; Ylostalo, J.H.; Bartosh, T.J., Jr.; Choi, H.; Lee, R.H.; Yasumura, D.; Ahern, K.; Nielsen, G.; et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol. Ther. 2012, 20, 788–797. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, J.H.; Yun, J.H.; Kim, J.A.; Kim, T.E.; Lee, H.J.; Kim, S.H.; Park, K.H.; Oh, J.Y. Stanniocalcin-1 protects retinalganglion cells by inhibiting apoptosis and oxidative damage. PLoS ONE 2013, 8, e63749. [Google Scholar] [CrossRef]
- Roddy, G.W.; Viker, K.B.; Winkler, N.S.; Bahler, C.K.; Holman, B.H.; Sheikh-Hamad, D.; Roy Chowdhury, U.; Stamer, W.D.; Fautsch, M.P. Stanniocalcin-1 is an ocular hypotensive agent and a downstream effector molecule that is necessary for the intraocular pressure-lowering effects of latanoprost. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Law, A.Y.; Ching, L.Y.; Lai, K.P.; Wong, C.K. Identification and characterization of the hypoxia-responsive element in human stanniocalcin-1 gene. Mol. Cell. Endocrinol. 2010, 314, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.Y. Vascular and neuronal protection in the developing retina: Potential therapeutic targets for retinopathy of prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, F.E. Vitamin C: An Antioxidant Agent, Vitamin C; IntechOpen: London, UK, 2017; p. 69660. [Google Scholar] [CrossRef]
- Hacışevki, A. An overview of ascorbic acid biochemistry. J. Fac. Pharm. 2009, 38, 233–255. [Google Scholar]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Rouhier, N.; Lemaire, S.D.; Jacquot, J.P. The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annu. Rev. Plant Biol. 2008, 59, 143–166. [Google Scholar] [CrossRef]
- Qawasmi, A.; Landeros-Weisenberger, A.; Bloch, M.H. Meta-analysis of LCPUFA supplementation of infant for- mula and visual acuity. Pediatrics 2013, 131, 262–272. [Google Scholar] [CrossRef]
- Fu, Z.; Lofqvist, C.A.; Shao, Z.; Sun, Y.; Joyal, J.S.; Hurst, C.G.; Cui, R.Z.; Evans, L.P.; Tian, K.; San Giovanni, J.P.; et al. Dietary v-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose–endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 2015, 101, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Stahl, A.; Chen, J.; Seaward, M.R.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Connor, K.M.; Aderman, C.M.; Liclican, E.; et al. 5-Lipoxygenase Metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 Polyunsaturated Fatty Acids. Sci. Transl. Med. 2011, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gong, Y.; Löfqvist, C.; Hellström, A.; Smith, L.E.H. Adiponectin in retinopathy. Biochim. Biophys. Acta. 2016, 1862, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Malamas, A.; Chranioti, A.; Tsakalidis, C.; Dimitrakos, S.A.; Mataftsi, A. The omega-3 and retinopathy of prematurity relationship. Int. J. Ophthalmol. 2017, 10, 18. [Google Scholar] [CrossRef]
- Löfqvist, C.A.; Najm, S.; Hellgren, G.; Engström, E.; Sävman, K.; Nilsson, A.K.; Andersso, M.X.; Hård, A.L.; Smith, L.E.H.; Hellström, A. Association of retinopathy of prematurity with low levels of arachidonic acid. A secondary analysis of a randomized clinical trial. JAMA Ophthalmology 2018, 136, 271–277. [Google Scholar] [CrossRef]
- Stahl, A.; Sapieha, P.; Connor, K.M.; Sangiovanni, J.P.; Chen, J.; Aderman, C.M.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Seaward, M.R.; et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ. Res. 2010, 107, 495–500. [Google Scholar] [CrossRef]
- Rotstein, N.P.; Politi, L.E.; German, O.L.; Girotti, R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2252–2259. [Google Scholar] [CrossRef]
- Li, S.Y.; Fu, Z.J.; Lo, A.C.Y. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxi. Med. Cell. Longev. 2012. [Google Scholar] [CrossRef]
- Sommerburg, O.; Keunen, J.E.E.; Bird, A.C.; Van Kuijk, F.J.G.M. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 8, 907–910. [Google Scholar] [CrossRef]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J Am. Diet. Assoc. 1993, 93, 284–296. [Google Scholar] [CrossRef]
- Picone, S.; Ritieni, A.; Fabiano, A.; Troise, A.D.; Graziani, G.; Paolillo, P.; Li Volti, G.; D’Orazio, N.; Galvano, F.; Gazzolo, D. Arterial cord blood lutein levels in preterm and term healthy newborns are sex and gestational age dependent. Clin. Biochem. 2012, 45, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Picone, S.; Ritieni, A.; Fabiano, A.; Graziani, G.; Paolillo, P.; Livolti, G.; Galvano, F.; Gazzolo, D. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: A trophic role for lutein. Clin. Biochem. 2017, 52, 80–84. [Google Scholar] [CrossRef]
- Manzoni, P.; Guardione, R.; Bonetti, P.; Priolo, C.; Maestri, A.; Mansoldo, C.; Mostert, M.; Anselmetti, G.; Sardei, D.; Biban, P.; et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: A multicenter randomized controlled trial. Am. J. Perinatol. 2013, 30, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Lori, I.; Favelli, F.; Frosini, S.; Messner, H.; Wanker, P.; De Marini, S.; Oretti, C.; Boldrini, A.; Massimiliano, C.; et al. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: A randomized controlled study. J. Matern. Fetal Neonatal. Med. 2012, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Giannantonio, C.; Cota, F.; Papacci, P.; Vento, G.; Valente, E.; Purcaro, V.; Costa, S. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. J. Matern. Fetal Neonatal Med. 2011, 24, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic. Biol. Med. 2006, 6, 979–984. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Akesson, B. Antioxidative factors in milk. Br. J Nutr. 2000, 84, 103–110. [Google Scholar] [CrossRef]
- Sommerburg, O.; Meissner, K.; Nelle, M.; Lenhartz, H.; Leichsenring, M. Carotenoid supply in breast-fed and formula-fed neonates. Eur. J. Pediatr. 2000, 159, 86–90. [Google Scholar] [CrossRef]
- Bettler, J.; Zimmer, J.P.; Neuringer, M.; DeRusso, P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010, 49, 45–51. [Google Scholar] [CrossRef]
- VanderVeen, D.K.; Martin, C.R.; Mehendale, R.; Allred, E.N.; Dammann, O. Early nutrition and weight gain in preterm newborns and the risk of retinopathy of prematurity. PLoS ONE 2013, 8, 64325. [Google Scholar] [CrossRef]
- Lu, F.; Liu, Y.; Guo, Y.; Gao, Y.; Piao, Y.; Tan, S.; Tang, Y. Metabolomic changes of blood plasma associated with two phases of rat OIR. Exp. Eye Res. 2020, 190, 107855. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Z.; Li, S.; Yang, M.; Xiao, X.; Lian, C.; Wen, W.; He, H.; Zeng, J.; Wang, J.; et al. Targeted blood metabolomic study on retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef] [PubMed]
- Mantagos, I.S.; Vanderveen, D.K.; Smith, L.E.H. Emerging Treatments for Retinopathy of Prematurity. Semi. Ophthalmol. 2009, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Katargina, L.A.; Chesnokova, N.B.; Beznos, O.V.; Osipova, N.A. Melatonin as a new promising agent for the treatment and prevention of retinopathy of prematurity. Vestn. Oftalmol. 2016, 132, 59–63. [Google Scholar] [CrossRef] [PubMed]
| BM | RD | DI | LE | LM | Ak | RC | BF | Ref |
|---|---|---|---|---|---|---|---|---|
| MDA | Y | Y | Y | Y | SP, HPLC | N | B | [18,38,39] |
| 8-OHdG | Y | Y | Y | Y | HPLC | N | U, B | [40,41,42,43,44] |
| GSH/GSSG | Y | N | N | Y | HPLC, MS/MS | N | B | [19,36,45,46] |
| SOD | Y | Y | Y | Y | HPLC | N | B | [38,47] |
| GPX | Y | Y | Y | Y | SP | N | B | [38,47] |
| Nrf2/Keap1 | Y | N | N | Y | RT-PCR, ELISA | N | B | [48,49,50,51,52,53,54,55] |
| PON1 | Y | Y | Y | Y | SP | N | B | [18] |
| STC-1 | Y | Y | Y | Y | qPCR, ELISA | N | B | [56,57,58,59,60,61,62,63,64,65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziosi, A.; Perrotta, M.; Russo, D.; Gasparroni, G.; D’Egidio, C.; Marinelli, B.; Di Marzio, G.; Falconio, G.; Mastropasqua, L.; Li Volti, G.; et al. Oxidative Stress Markers and the Retinopathy of Prematurity. J. Clin. Med. 2020, 9, 2711. https://doi.org/10.3390/jcm9092711
Graziosi A, Perrotta M, Russo D, Gasparroni G, D’Egidio C, Marinelli B, Di Marzio G, Falconio G, Mastropasqua L, Li Volti G, et al. Oxidative Stress Markers and the Retinopathy of Prematurity. Journal of Clinical Medicine. 2020; 9(9):2711. https://doi.org/10.3390/jcm9092711
Chicago/Turabian StyleGraziosi, Alessandro, Marika Perrotta, Daniele Russo, Giorgia Gasparroni, Claudia D’Egidio, Benedetta Marinelli, Guido Di Marzio, Gennaro Falconio, Leonardo Mastropasqua, Giovanni Li Volti, and et al. 2020. "Oxidative Stress Markers and the Retinopathy of Prematurity" Journal of Clinical Medicine 9, no. 9: 2711. https://doi.org/10.3390/jcm9092711
APA StyleGraziosi, A., Perrotta, M., Russo, D., Gasparroni, G., D’Egidio, C., Marinelli, B., Di Marzio, G., Falconio, G., Mastropasqua, L., Li Volti, G., Mangifesta, R., & Gazzolo, D. (2020). Oxidative Stress Markers and the Retinopathy of Prematurity. Journal of Clinical Medicine, 9(9), 2711. https://doi.org/10.3390/jcm9092711





