Fatty Liver Disease and Non-Alcoholic Fatty Liver Disease Worsen the Outcome in Acute Pancreatitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods and Materials
2.1. Literature Search
2.2. Study Selection and Data Collection
2.3. Statistical Analysis
2.4. Risk of Bias and Quality Assessment of the Individual Studies
2.5. Details of Ethical Approval
3. Results
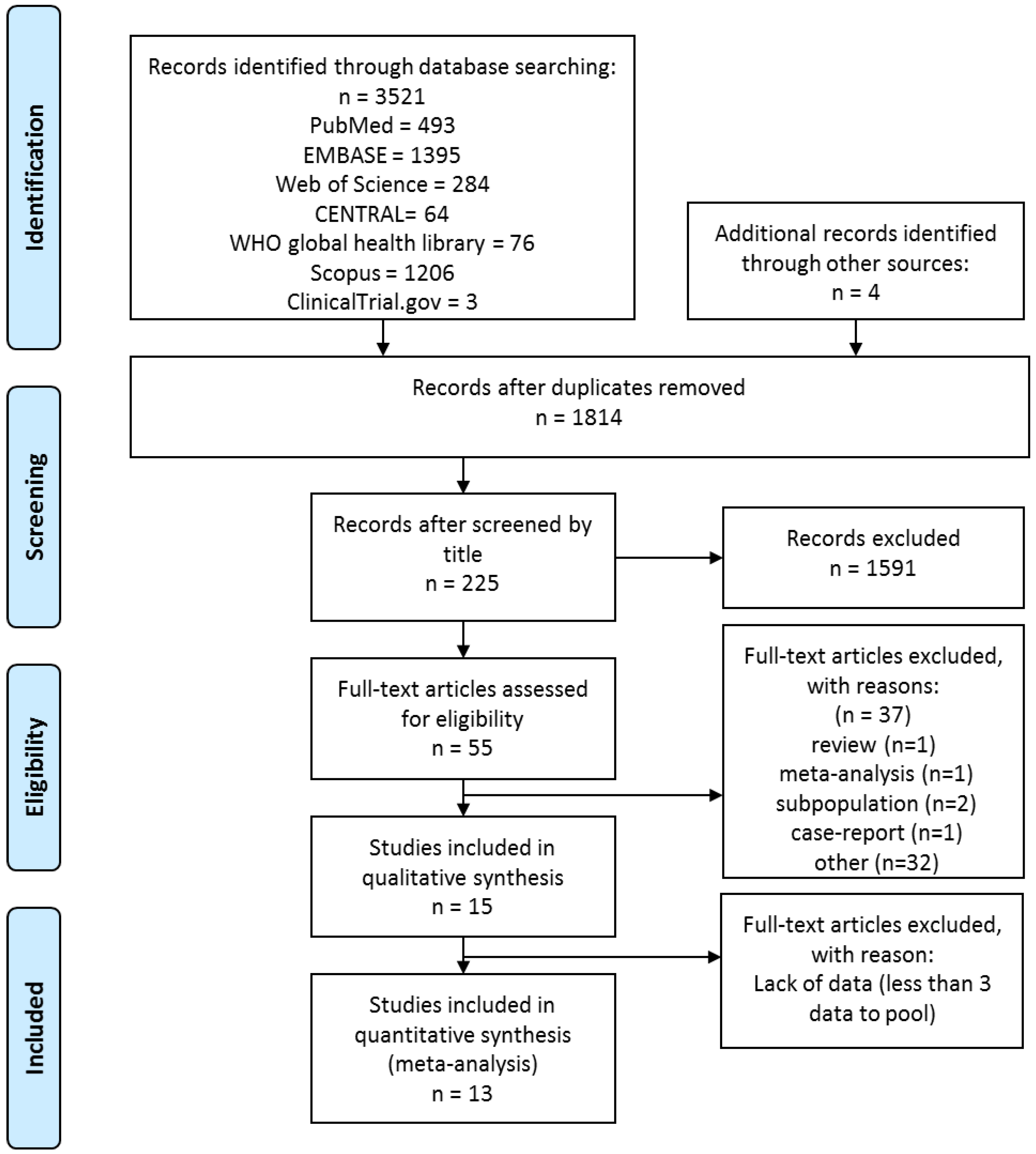
3.1. Search and Selection
3.2. Characteristics of the Studies Included in the Meta-Analysis
3.3. Findings of Meta-Analysis: FLD vs. No FLD
3.4. Findings of Meta-Analysis: NAFLD vs. No NAFLD
3.5. Additional Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
5.1. Implication for Practice
5.2. Implication for Research
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- EASL–EASD–EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102. [Google Scholar] [CrossRef] [PubMed]
- Párniczky, A.; Kui, B.; Szentesi, A.; Balázs, A.; Szűcs, Á.; Mosztbacher, D.; Czimmer, J.; Sarlós, P.; Bajor, J.; Gódi, S.; et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS ONE 2016, 11, e0165309. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.; van Santvoort, H.; Freeman, M.; Gardner, T.; Mayerle, J.; Vege, S.S.; Werner, J.; Banks, P.; McKay, C.; Fernandez-del Castillo, C. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013, 13, E1–E15. [Google Scholar]
- Wu, B.; Johannes, R.; Sun, X.; Tabak, Y.; Conwell, D.; Banks, P. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698. [Google Scholar] [CrossRef]
- Khan, A.A.; Parekh, D.; Cho, Y.; Ruiz, R.; Selby, R.R.; Jabbour, N.; Genyk, Y.S.; Mateo, R. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. AMA Arch. Surg. 2002, 137, 1136–1140. [Google Scholar] [CrossRef]
- Ranson, J.H.; Rifkind, K.M.; Roses, D.F.; Fink, S.D.; Eng, K.; Spencer, F.C. Prognostic signs and the role of operative management in acute pancreatitis. Surg. Gynecol. Obstet. 1974, 139, 69–81. [Google Scholar]
- Balthazar, E.J. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology 2002, 223, 603–613. [Google Scholar] [CrossRef]
- Miko, A.; Vigh, E.; Matrai, P.; Soos, A.; Garami, A.; Balasko, M.; Czako, L.; Mosdosi, B.; Sarlos, P.; Eross, B.; et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: A predictive accuracy meta-analysis. Front. Physiol. 2019, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Milic, S.; Orlic, L.; Poropat, G.; Jakopcic, I.; Franjic, N.; Klanac, A.; Kristo, N.; Stimac, D. Metabolic syndrome and acute pancreatitis. Eur. J. Intern. Med. 2016, 32, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Szentesi, A.; Párniczky, A.; Vincze, Á.; Bajor, J.; Gódi, S.; Sarlós, P.; Gede, N.; Izbéki, F.; Halász, A.; Márta, K.; et al. Multiple hits in acute pancreatitis: Components of metabolic syndrome synergize each other’s deteriorating effects. Front. Physiol. 2019, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Miko, A.; Farkas, N.; Garami, A.; Szabo, I.; Vincze, A.; Veres, G.; Bajor, J.; Alizadeh, H.; Rakonczay, Z., Jr.; Vigh, E.; et al. Preexisting diabetes elevates risk of local and systemic complications in acute pancreatitis: Systematic review and meta-analysis. Pancreas 2018, 47, 917–923. [Google Scholar] [CrossRef]
- Dobszai, D.; Matrai, P.; Gyongyi, Z.; Csupor, D.; Bajor, J.; Eross, B.; Miko, A.; Szako, L.; Meczker, A.; Hagendorn, R.; et al. Body-mass index correlates with severity and mortality in acute pancreatitis: A meta-analysis. World J. Gastroenterol. 2019, 25, 729–743. [Google Scholar] [CrossRef]
- Kiss, L.; Fűr, G.; Mátrai, P.; Hegyi, P.; Ivány, E.; Cazacu, I.M.; Szabó, I.; Habon, T.; Alizadeh, H.; Gyöngyi, Z.; et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: Systematic review and meta-analysis. Sci. Rep. 2018, 8, 14096. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, H.; Wang, G.; Qiu, Z.; Bai, B.; Wang, S.; Yu, P.; Feng, Q.; Zhao, Q.; He, X.; et al. RNA sequence analysis of rat acute experimental pancreatitis with and without fatty liver: A gene expression profiling comparative study. Sci. Rep. 2017, 7, 734. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, Z.; Lu, Y.; Zhang, D.; Jia, Z.; Zhuang, X.; Shi, Y.; Xu, T.; Xing, L.; Shen, J. Influence of fatty liver on the severity and clinical outcome in acute pancreatitis. PLoS ONE 2015, 10, e0142278. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, S.; Wu, K.; Wang, N.; Wang, Y.; Wu, J.; Lu, G.; Gong, W.; Ding, Y.; et al. Nonalcoholic fatty liver disease aggravated the severity of acute pancreatitis in patients. BioMed Res. Int. 2019, 2019, 9583790. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Statistical Methods Group and Cochrane Handbook for Systematic Reviews of Interventions; CochraneConsumer Network: London, UK, 2019; pp. 241–284. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch. Surg. 1993, 128, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Niu, C. The effect of nonalcoholic fatty liver disease on the severity of acute pancreatitis. J. Prac. Med. 2017, 33, 3563–3565. [Google Scholar] [CrossRef]
- Hao, Y.M.; Feng, Q.X.; Feng, X.Y.; Yu, P.F.; Bai, B.; Qiu, Z.Y.; Wang, Q.; Wang, S.Q.; Zhao, Q.C. Combine identifying hepatic steatosis with APACHE-II score improves the prediction of severe acute pancreatitis. J. Dig. Dis. 2015, 16, 60. [Google Scholar] [CrossRef]
- Jasdanwala, S. NAFLD Diagnosed with abdominal ultrasound is a marker of severity in acute Pancreatitis. J. Gastrointest. Dig. Syst. 2015, 5, 2. [Google Scholar] [CrossRef]
- Jia, J.; Wu, Q.; Kou, J.; Yang, M. Relationship between fatty liver and pancreatitis. Int. J. Clin. Med. 2018, 9, 243–248. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Poropat, G.; Jakopcic, I.; Stimac, D.; Klanac, A.; Carovic, F.; Milic, S. Nonalcoholic fatty liver and the severity of acute pancreatitis. Eur. J. Intern. Med. 2017, 38, 73–78. [Google Scholar] [CrossRef]
- Morel-Cerda, E.C.; Velarde-Ruiz Velasco, J.A.; Álvarez-López, F.; García-Jiménez, E.S.; Rangel-Orozco, M.F.; González-Álvarez, R.; Flores-Mendoza, J.F.; Zaragoza-Scherman, C.F.; Velarde-Chávez, J.A.; Mora-Huerta, J.A. Prevalence of fatty liver in patients with acute pancreatitis. Rev. Méd. 2019, 9, 113–118. [Google Scholar]
- Ze-hua, P.; Lin, B.A.I.; Hong, P.U.; Long-lin, Y.I.N.; Jia-yuan, C.; Jin, J.; Ning, A.N. Abdominal CT scan in predicting complications of acute pancreatitis. Chin. J. Gen. Surg. 2012, 27, 789–793. [Google Scholar]
- Satapathy, S.; Friedman, B.; Bittman, M.; Aronson, S.; Kwak, N.; Novak, S.; Inamdar, S.; Cerulli, M.; David, B. Hepatic steatosis a novel marker for severe outcomes in patients with acute pancreatitis. Am. J. Gastroenterol. 2011, 106, S115–S116. [Google Scholar] [CrossRef]
- Suchsland, T.; Aghdassi, A.; Kühn, K.; Simon, P.; Lerch, M.M.; Mayerle, J.; Flessa, S. Predictive factors for and incidence of hospital readmissions of patients with acute and chronic pancreatitis. Pancreatology 2015, 15, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Li, S.; Feng, Q.; Feng, X.; Zhao, Q. Fatty liver indicates increased severity of acute pancreatitis. J. Gastroenterol. Hepatol. 2013, 28, 240. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, X.M.; Jiang, Z.Q.; Tang, W.; Huang, X.H.; Yang, L.; Feng, Z.S. Fatty liver in acute pancreatitis: Characteristics in magnetic resonance imaging. J. Comput. Assist. Tomogr. 2012, 36, 400–405. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, I.S.; Choi, M.H.; Lee, K.; Ham, H.; Oh, H.J.; Park, S.H.; Lim, C.H.; Choi, M.G. Impact of fatty liver on acute pancreatitis severity. Gastroenterol. Res. Pract. 2017, 2017, 4532320. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, M.; Huang, L.; Gao, Y.; Li, X. Risk factors of hyperglycemia in patients after a first episode of acute pancreatitis: A retrospective cohort. Pancreas 2017, 46, 209–218. [Google Scholar] [CrossRef]
- Hou, S.; Tang, X.; Cui, H.; Liu, C.; Bai, X.; Shi, L.; Shi, Y. Fatty liver disease is associated with the severity of acute pancreatitis:A systematic review and meta-analysis. Int. J. Surg. 2019, 65, 147–153. [Google Scholar] [CrossRef]
- Younossi, Z.; Henry, L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology 2016, 150, 1778–1785. [Google Scholar] [CrossRef]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef]
- Wei, H.A.N.; Jun, Y.A.N.; Jian, W.; Tie, W.E.N.; Lijie, B.A.I.; Xiaoqi, H.; Xia, W.; Xing, J.I. The diagnostic and prognostic value of CT scans in patients with acute pancreatitis complications. J. Pract. Radiol. 2017, 33, 1205–1208. [Google Scholar]
- Wang, Q.; Du, J.; Yu, P.; Bai, B.; Zhao, Z.; Wang, S.; Zhu, J.; Feng, Q.; Gao, Y.; Zhao, Q.; et al. Hepatic steatosis depresses alpha-1-antitrypsin levels in human and rat acute pancreatitis. Sci. Rep. 2015, 5, 17833. [Google Scholar] [CrossRef] [PubMed]
- Simons-Linares, C.R.; Romero-Marrero, C.; Jang, S.; Bhatt, A.; Lopez, R.; Vargo, J.; Stevens, T.; Carey, W.; Chahal, P. Clinical outcomes of acute pancreatitis in patients with cirrhosis. Pancreatology 2019, 20, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yu, C.; Deng, F.; He, W.-H.; Xia, L.; Zhou, M.; Lan, G.-L.; Huang, X.; Lei, Y.-P.; Zhou, X.-J.; et al. New risk factors for infected pancreatic necrosis secondary to severe acute pancreatitis: The role of initial contrast-enhanced computed tomography. Dig. Dis. Sci. 2019, 64, 553–560. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Country (Centre) | Recruitment Period | Acute Pancreatitis Diagnosis | Leading Etiology of Acute Pancreatitis | Nr. of Acute Pancreatitis Cases | Fatty Liver Disease | Examined Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| Definition | Diagnostic Method (Cut-Off) | Nr. FLD Cases (%) | |||||||
| Dou J. et al., 2017 [26] (article in Chinese) | China (single-center) | 2013–2016 | 2 out of 3 criteria | G 37% H 10% | 251 | NAFLD | US (NR) | 117 (47) | AP severity (Atlanta 2012) § |
| Hao Y.M. et al., 2015 [27] † | China (single-center) | 2011–2013 | NR | NR | 148 | FLD | NR | 41 (28) | AP severity (Atlanta 1992) |
| Jasdanwala S, 2015 [28] | USA (multicenter) | Not reported | 2 out of 3 criteria | NR | 574 | NAFLD | CT or US (NR) | 193 (34) | In-hospital mortality, AP severity (Atlanta 2012), LOH, ICU admission, BISAP |
| Jia J. et al., 2018 [29] | China (single-center) | 2016–2017 | 2 out of 3 criteria | NR | 128 | FLD | CT (HAI<1) | 56 (44) | AP severity (Atlanta 2012), ANC, APFC |
| Mikolasevic I. et al., 2016 [30] | Croatia (single-center) | 2008–2015 | 2 out of 3 criteria | G 84% H 1% | 822 | NAFLD | CT (HA > 10 HU, or LD < 40 HU) or US | 198 (24) | In-hospital mortality, AP severity (Atlanta 2012) §, ANC, APFC, PP, LOH, APACHE-II, CTSI |
| Morel C.E. et al., 2019 [31] (article in Spanish) | Mexico (single-center) | 2017–2018 | 2 out of 3 criteria | G 70% A 11% H 5% | 186 | FLD | US (NR) | 68 (37) | AP severity (Atlanta 2012), persistent SIRS |
| Peng Z.H. et al., 2012 [32] (article in Chinese) | China (single-center) | 2010–2011 | 2 out of 3 criteria | G 57% | 606 | FLD | CT (HAI < 1) | 498 (82) | In-hospital mortality, overall complications § |
| Satapathy S. et al., 2011 [33] † | USA (single-center) | 2002–2009 | NR | G 39% A 18% | 108 | FLD | CT (HAI < 0.8) | 23 (21) | In-hospital mortality, ANC, PP, LOH, ICU admission, need for antibiotics, CTSI, Ranson 48 h |
| Suchsland T. et al., 2015 [34] | Germany (single-center) | 2006–2011 | ICD-10 | NR | 373 | FLD | NR | NR | Risk of hyperglycemia after AP |
| Wang S. et al., 2013 [35] † | China (single-center) | 2010–2011 | NR | NR | 120 | FLD | NR | 35 (29) | AP severity (Atlanta 1992) §, SIRS, pulmonary failure, metabolic disturbances |
| Wu D. et al., 2019 [19] | China (single-center) | 2012–2016 | 2 out of 3 criteria | G 32% H 48% | 656 | NAFLD | CT (HAI < 1) | 378 (58) | AP severity (Atlanta 2012) §, SIRS, BISAP, Ranson score |
| Xiao B. et al., 2012 [36] | China (single-center) | 2009–2011 | Pain and laboratory results ‡ | G 38% | 50 | FLD | MRI (HAI) | 33 (66) | In-hospital mortality, MRSI |
| Xu C. et al., 2015 [18] | China (single-center) | 2000–2014 | 2 out of 3 criteria | G 58% A 22% H 11% | 2671 | FLD/ NAFLD | CT (HAI < 1) | 480 (18) | In-hospital mortality, AP severity (Atlanta 2012), ANC, systemic and local complications, APACHE-II |
| Yoon S.B. et al., 2017 [37] | Korea (single-center) | 2009–2016 | 2 out of 3 criteria | G 36% A 34% H 3% | 200 | FLD | CT (HAI < 1) | 67 (34) | In-hospital mortality, AP severity (Atlanta 2012) §, ANC, PP, APFC, LOH |
| Yuan L. et al., 2017 [38] | China (single-center) | 2009–2013 | 2 out of 3 criteria | G 49% A 5% H 10% | 310 | FLD | NR | 119 (39) | hospital readmission after the first episode of AP |
| Outcome | N0 of Studies (N0 of PTS) | Odds Ratio (95% CI) | I2 (%) | Chi2 |
|---|---|---|---|---|
| FLD vs no-FLD | ||||
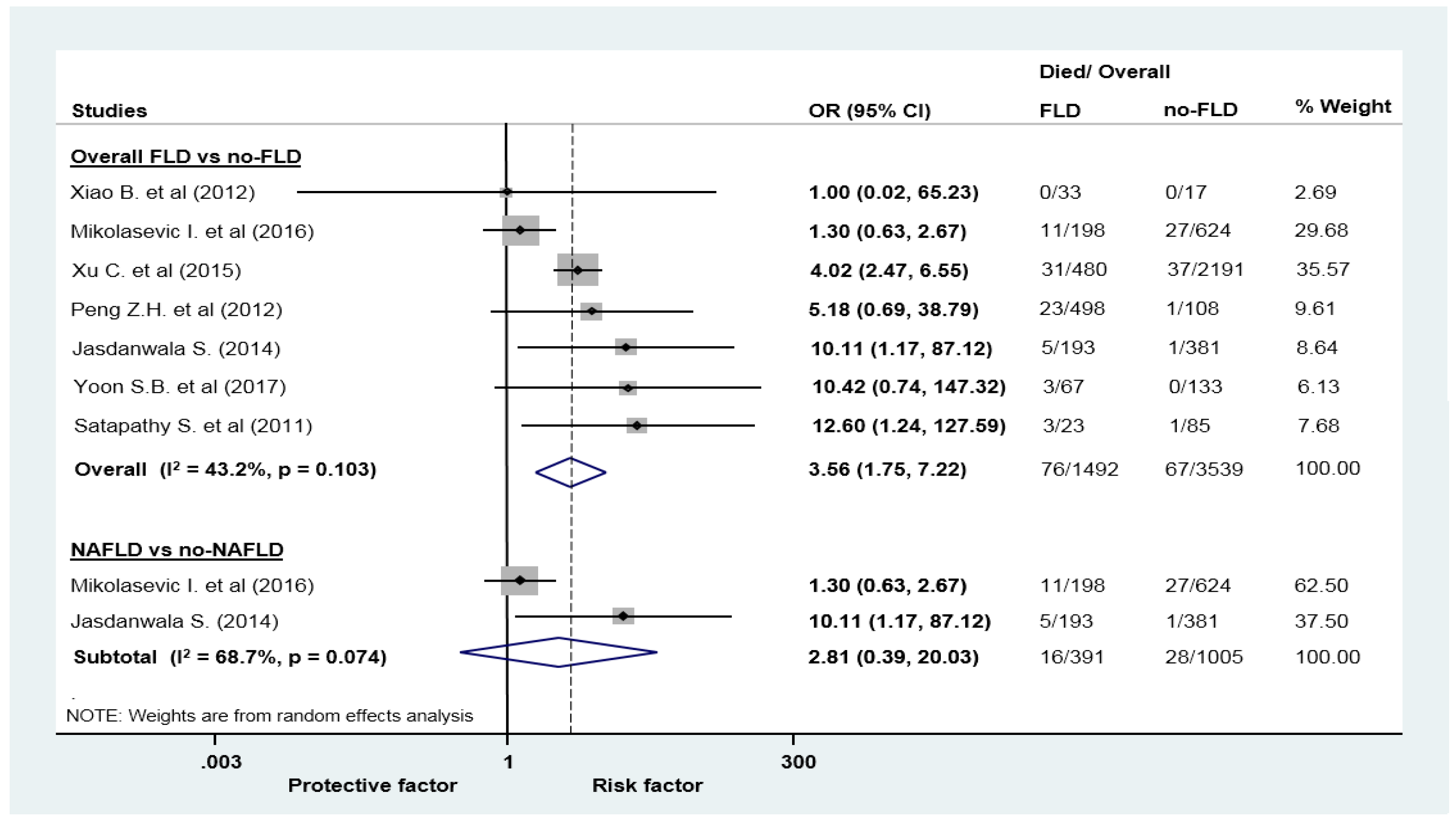
| Mortality | 7 (5031) | 3.56 (1.77–8.28) | 43.2 | 0.103 |
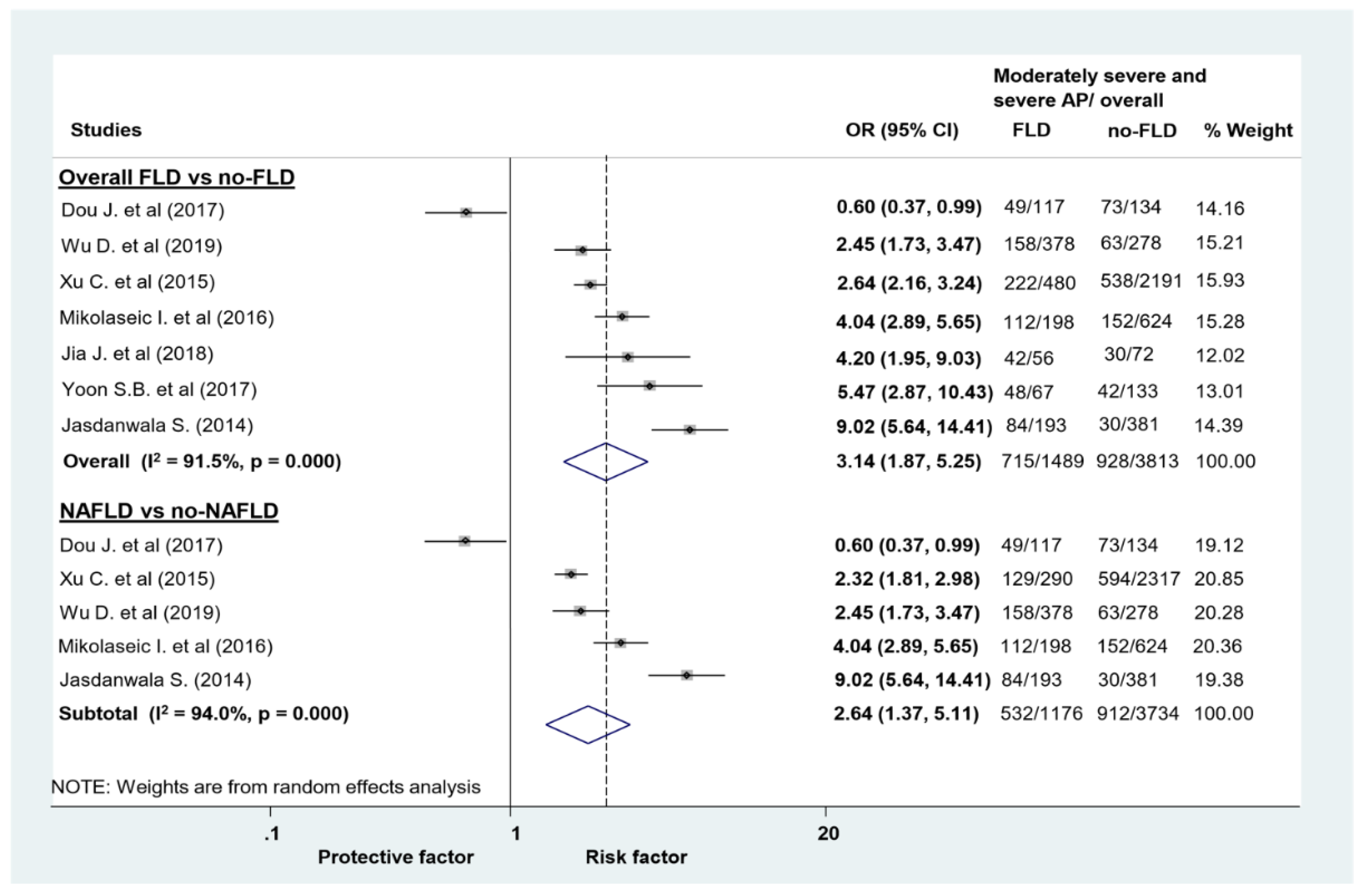
| Composite of MSAP and SAP (uni) | 7 (5302) | 3.14 (1.87–5.25) | 91.5 | 0 |
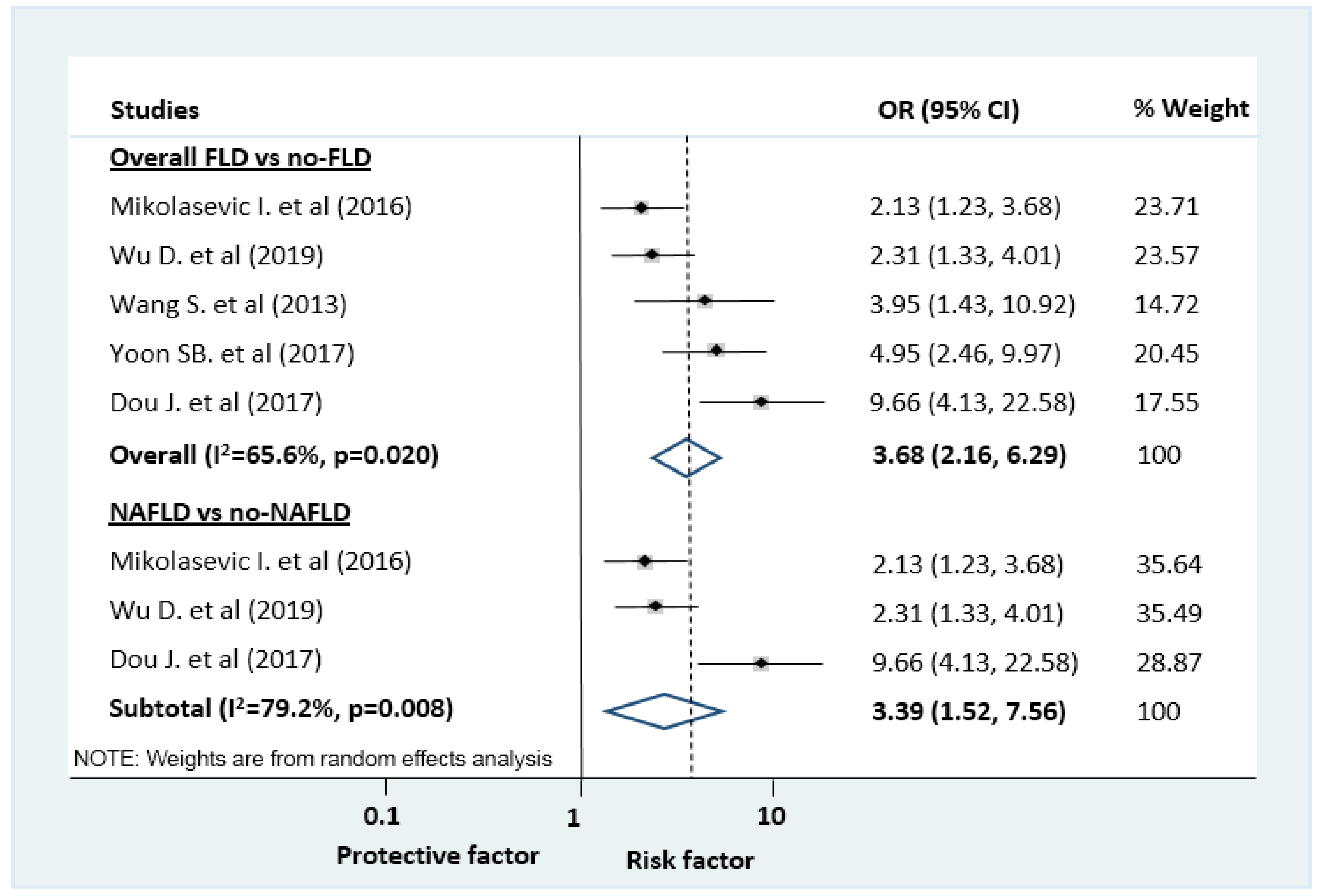
| Composite of MSAP and SAP (multi) ‡ | 5 (NR) | 3.68 (2.16–6.29) | 65.6 | 0.020 |
| SAP by Atlanta 2012 | 8 (4931) | 2.67 (2.01–3.56) | 32.0 | 0.173 |
| SAP by Atlanta 1992 | 2 (268) | 4.70 (2.65–8.32) | 0 | 0.634 |
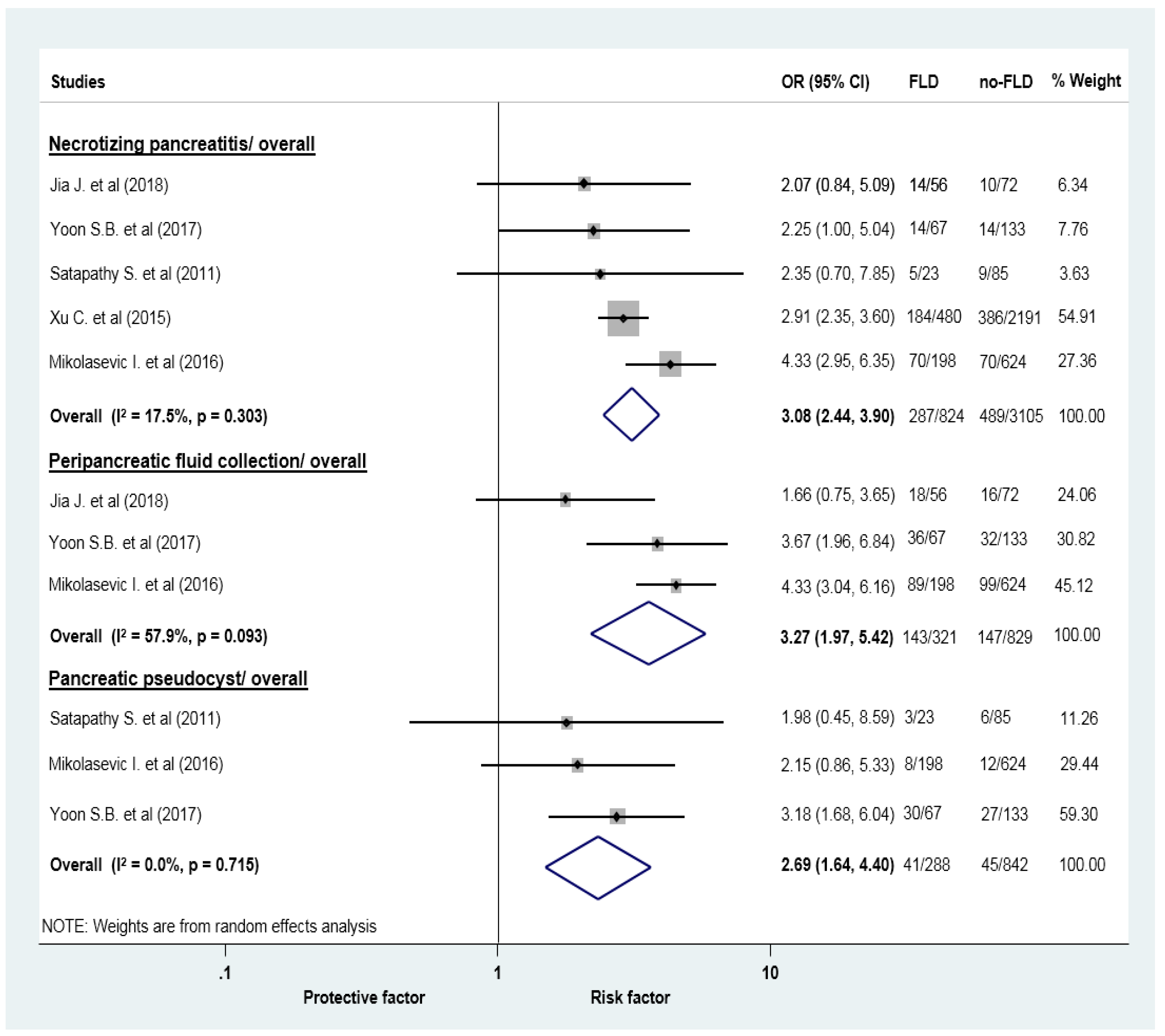
| Acute necrotic collection | 5 (3929) | 3.08 (2.44–3.90) | 17.5 | 0.303 |
| Acute peripancreatic fluid collection | 3 (1150) | 3.27 (1.97–5.42) | 57.9 | 0.093 |
| Pancreatic pseudocyst | 3 (1130) | 2.69 (1.64–4.40) | 0 | 0.715 |
| SIRS | 4 (3634) | 2.39 (1.74–3.28) | 47 | 0.129 |
| Length of hospital stay | 5 (1955) | 1.46 (0.54–2.39) † | 40.7 | 0.150 |
| NAFLD vs no-NAFLD | ||||
| Mortality | 2 (1396) | 2.81 (0.39–20.03) | 68.7 | 0.074 |
| Composite of MSAP and SAP (uni) | 5 (4910) | 2.64 (1.37–5.11) | 94 | 0 |
| Composite of MSAP and SAP (multi) ‡ | 3 (NR) | 3.39 (1.52–7.56) | 79.2 | 0.008 |
| SAP by Atlanta 2012 | 3 (4085) | 2.21 (1.70–2.88) | 0 | 0.806 |
| Length of hospital stay | 3 (1647) | 1.41 (0.03–2.7) † | 68.5 | 0.042 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Váncsa, S.; Németh, D.; Hegyi, P.; Szakács, Z.; Hegyi, P.J.; Pécsi, D.; Mikó, A.; Erőss, B.; Erős, A.; Pár, G. Fatty Liver Disease and Non-Alcoholic Fatty Liver Disease Worsen the Outcome in Acute Pancreatitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2698. https://doi.org/10.3390/jcm9092698
Váncsa S, Németh D, Hegyi P, Szakács Z, Hegyi PJ, Pécsi D, Mikó A, Erőss B, Erős A, Pár G. Fatty Liver Disease and Non-Alcoholic Fatty Liver Disease Worsen the Outcome in Acute Pancreatitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(9):2698. https://doi.org/10.3390/jcm9092698
Chicago/Turabian StyleVáncsa, Szilárd, Dávid Németh, Péter Hegyi, Zsolt Szakács, Péter Jeno Hegyi, Dániel Pécsi, Alexandra Mikó, Bálint Erőss, Adrienn Erős, and Gabriella Pár. 2020. "Fatty Liver Disease and Non-Alcoholic Fatty Liver Disease Worsen the Outcome in Acute Pancreatitis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 9: 2698. https://doi.org/10.3390/jcm9092698
APA StyleVáncsa, S., Németh, D., Hegyi, P., Szakács, Z., Hegyi, P. J., Pécsi, D., Mikó, A., Erőss, B., Erős, A., & Pár, G. (2020). Fatty Liver Disease and Non-Alcoholic Fatty Liver Disease Worsen the Outcome in Acute Pancreatitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(9), 2698. https://doi.org/10.3390/jcm9092698






