Hospitalization Rates and Comorbidities in Patients with Progressive Supranuclear Palsy in Germany from 2010 to 2017
Abstract
1. Introduction
2. Methods
Statistical Analyses
3. Results
3.1. Development of Inpatient Case Numbers with PSP (G23.1) between 2010 and 2017
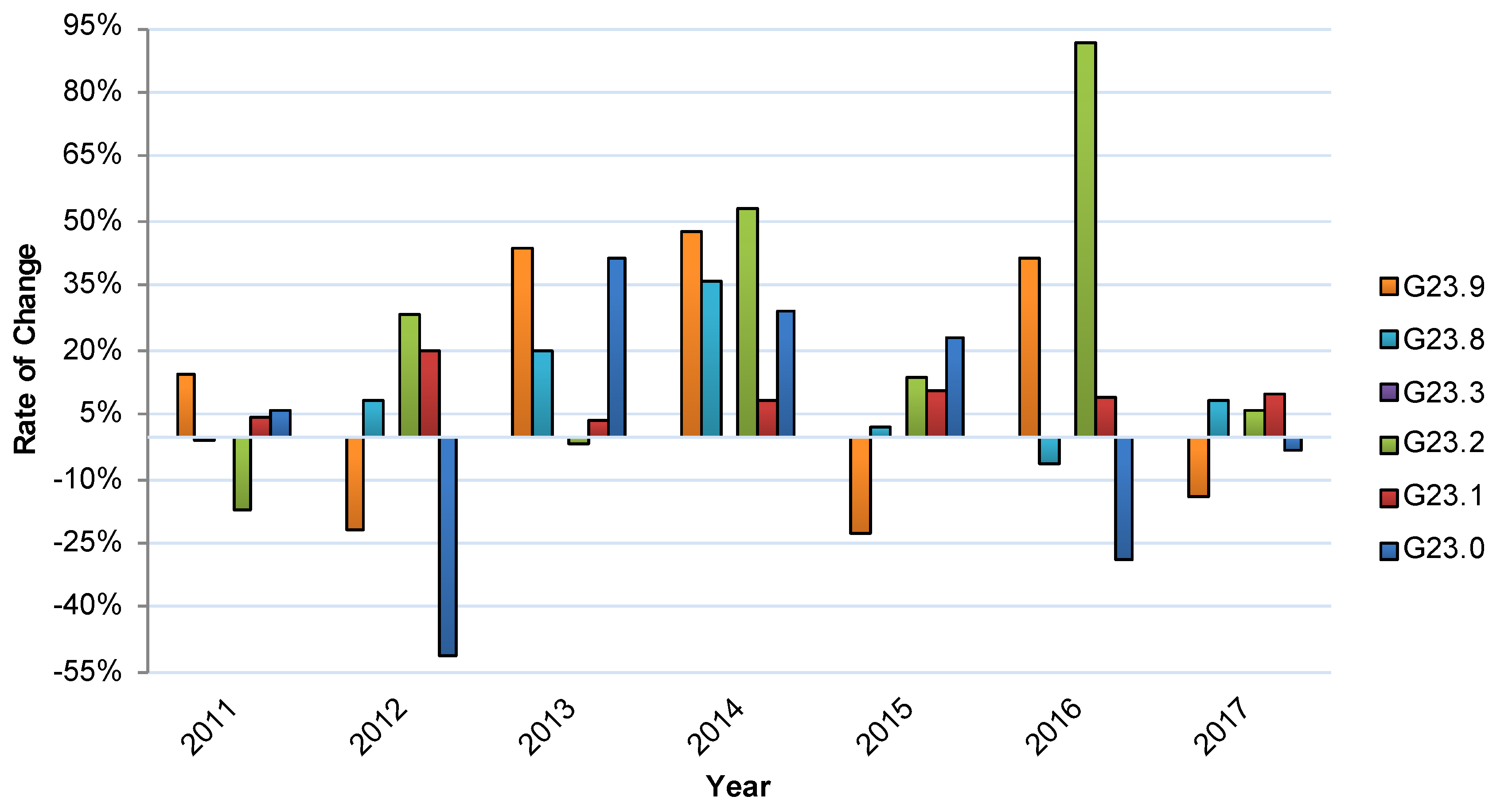
3.2. Relative Annual Changes in Inpatient Treatment for G23.- Disease Subgroups
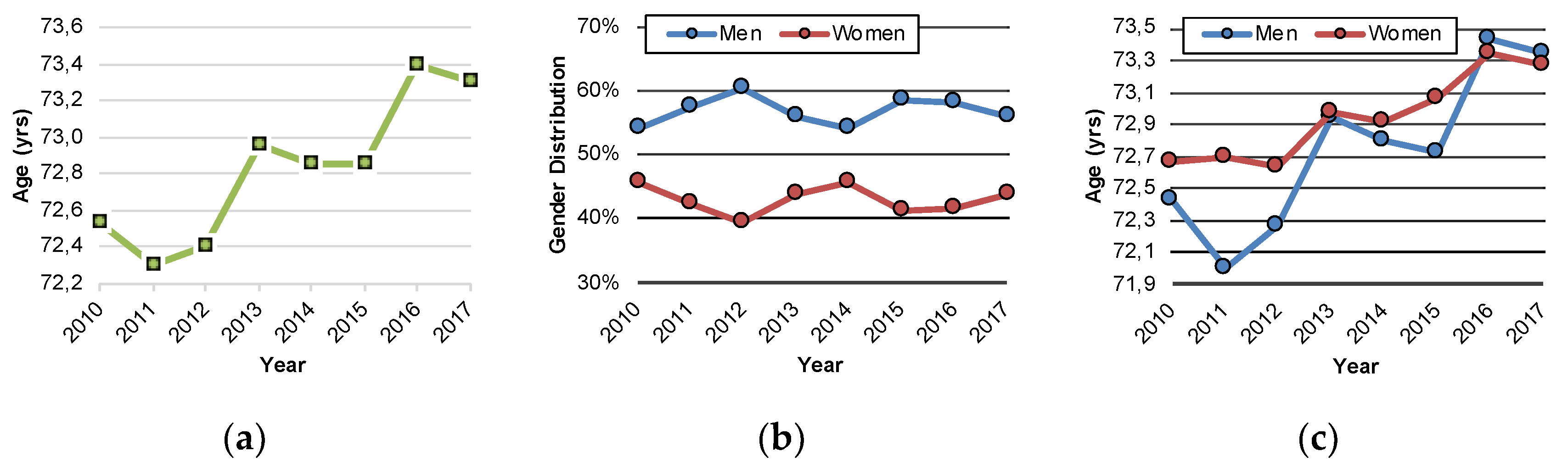
3.3. Characterization of PSP (G23.1) Inpatients
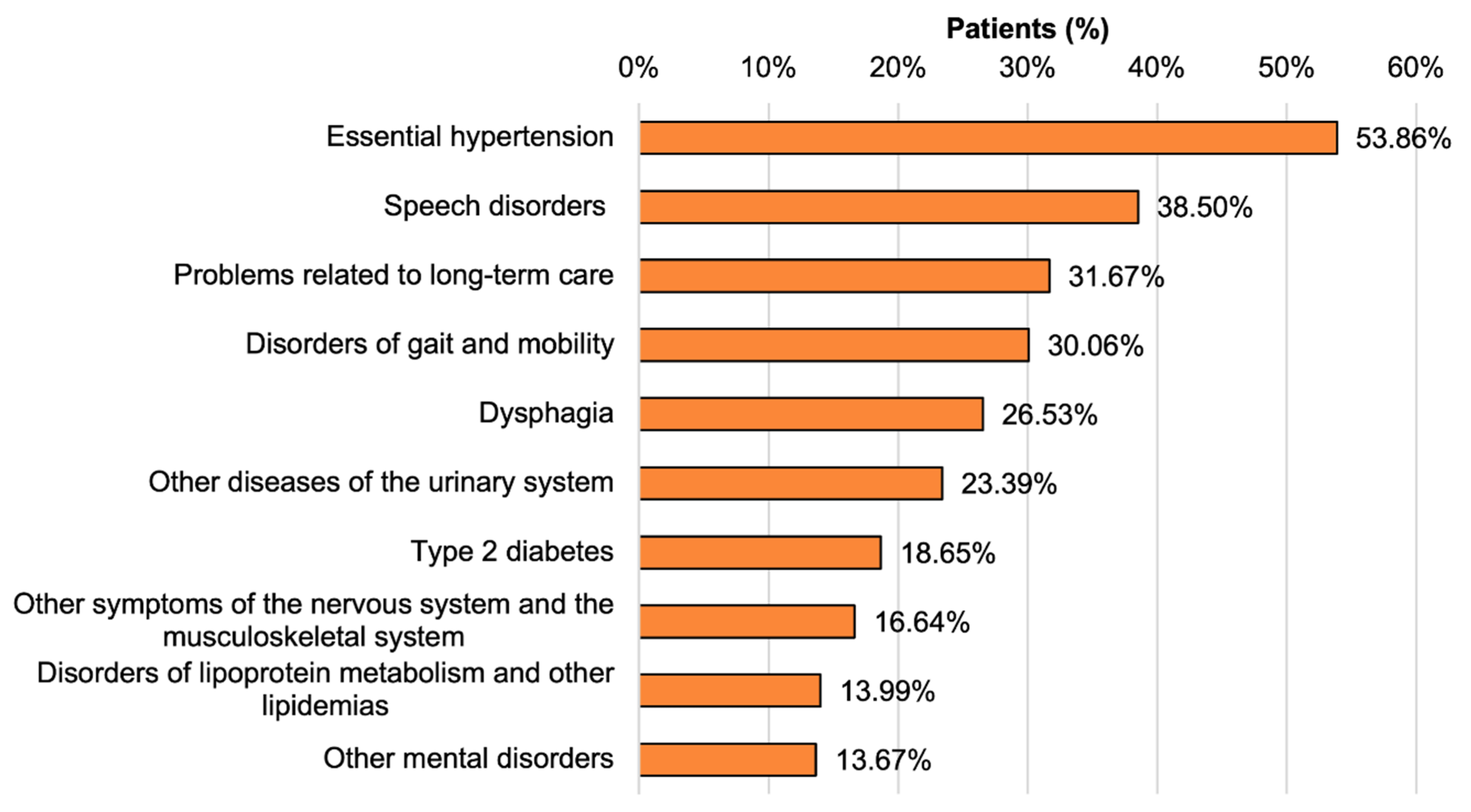
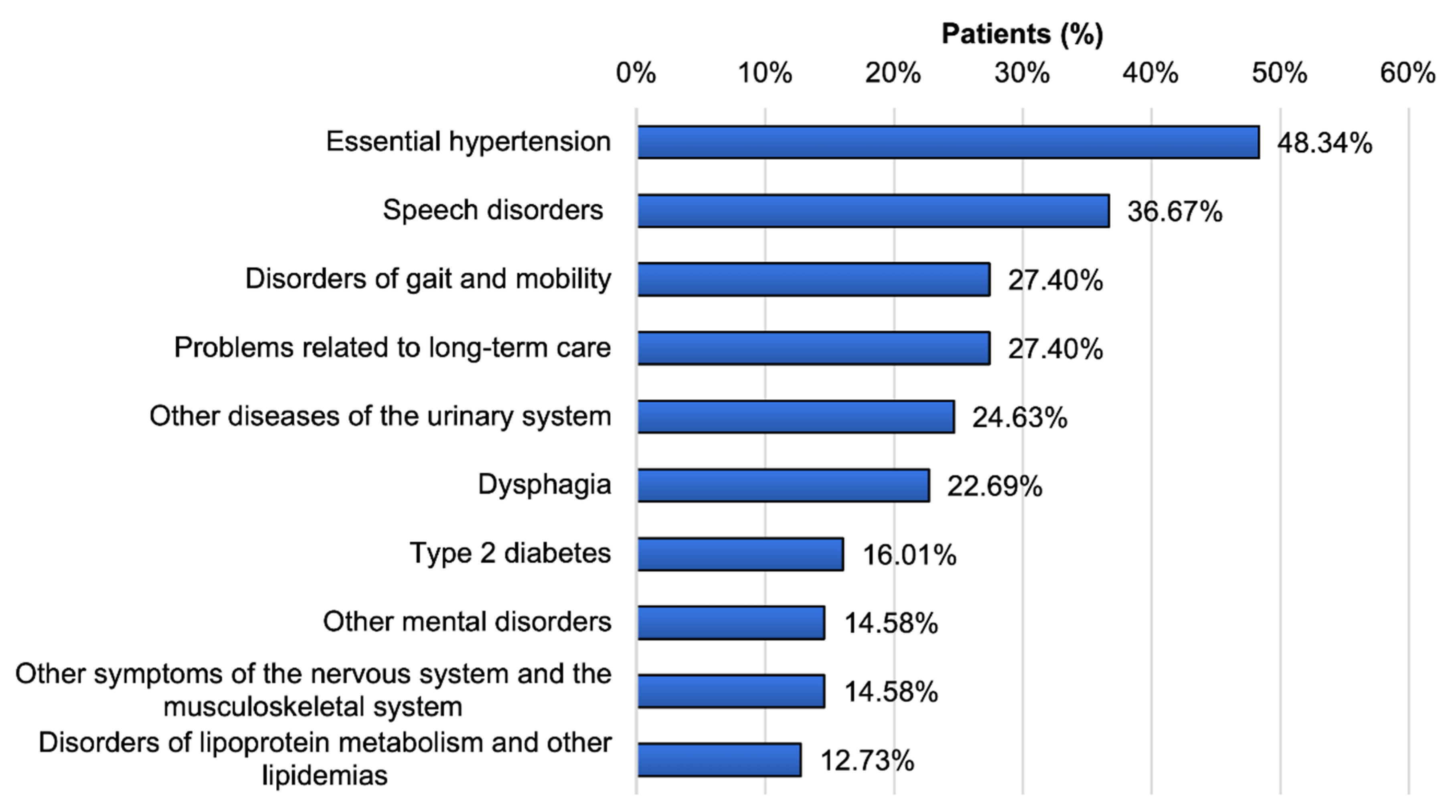
3.4. Comorbidities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nutt, J.G. Motor subtype in Parkinson’s disease: Different disorders or different stages of disease? Mov. Disord. 2016, 31, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.-M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.-J.; Respondek, G.; Stamelou, M.; Arzberger, T.; Ferguson, L.; Gelpi, E.; Giese, A.; Grossman, M.; Irwin, D.J.; Pantelyat, A.; et al. How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov. Disord. 2019, 34, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Respondek, G.; Levin, J.; Höglinger, G.U. Progressive supranuclear palsy and multiple system atrophy: Clinicopathological concepts and therapeutic challenges. Curr. Opin. Neurol. 2018, 31, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Tönges, L.; Zella, M.A.S. Antibody-based immunotherapies for Parkinsonian syndromes. Neural Regen. Res. 2019, 14, 1903–1904. [Google Scholar] [CrossRef]
- Enders, D.; Balzer-Geldsetzer, M.; Riedel, O.; Dodel, R.; Wittchen, H.-U.; Sensken, S.-C.; Wolff, B.; Reese, J.-P. Prevalence, Duration and Severity of Parkinson’s Disease in Germany: A Combined Meta-Analysis from Literature Data and Outpatient Samples. Eur. Neurol. 2017, 78, 128–136. [Google Scholar] [CrossRef]
- Heinzel, S.; Berg, D.; Binder, S.; Ebersbach, G.; Hickstein, L.; Herbst, H.; Lorrain, M.; Wellach, I.; Maetzler, W.; Petersen, G.; et al. Do We Need to Rethink the Epidemiology and Healthcare Utilization of Parkinson’s Disease in Germany? Front. Neurol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Tönges, L.; Bartig, D.; Muhlack, S.; Jost, W.; Gold, R.; Krogias, C. Characteristics and dynamics of inpatient treatment of patients with Parkinson’s disease in Germany: Analysis of 1.5 million patient cases from 2010 to 2015. Nervenarzt 2019, 90, 167–174. (In German) [Google Scholar] [CrossRef]
- Stangl, S.; Haas, K.; Eggers, C.; Reese, J.-P.; Tönges, L.; Volkmann, J. Care of patients with Parkinson’s disease in Germany. Nervenarzt 2020, 91, 493–502. (In German) [Google Scholar] [CrossRef]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Fleury, V.; Brindel, P.; Nicastro, N.; Burkhard, P.R. Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland. Park. Relat. Disord. 2018, 54, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Golbe, L.I.; Davis, P.H.; Schoenberg, B.S.; Duvoisin, R.C. Prevalence and natural history of progressive supranuclear palsy. Neurology 1988, 38, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Miyake, M.; Kusumi, M.; Adachi, Y.; Nakashima, K. Prevalence of progressive supranuclear palsy in Yonago, Japan. Mov. Disord. 2004, 19, 1239–1240. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, H.; Kitayama, M.; Wada-Isoe, K.; Kowa, H.; Nakashima, K. Prevalence of progressive supranuclear palsy in Yonago: Change throughout a decade. Brain Behav. 2016, 6, e00557. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Ben-Shlomo, Y.; Quinn, N. Prevalence of progressive supranuclear palsy and multiple system atrophy: A cross-sectional study. Lancet 1999, 354, 1771–1775. [Google Scholar] [CrossRef]
- Coyle-Gilchrist, I.T.S.; Dick, K.M.; Patterson, K.; Rodríquez, P.V.; Wehmann, E.; Wilcox, A.; Lansdall, C.J.; Dawson, K.E.; Wiggins, J.; Mead, S.; et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 2016, 86, 1736–1743. [Google Scholar] [CrossRef]
- Jabbari, E.; Holland, N.; Chelban, V.; Jones, P.S.; Lamb, R.; Rawlinson, C.; Guo, T.; Costantini, A.A.; Tan, M.M.X.; Heslegrave, A.J.; et al. Diagnosis Across the Spectrum of Progressive Supranuclear Palsy and Corticobasal Syndrome. JAMA Neurol. 2020, 77, 377–387. [Google Scholar] [CrossRef]
- Dell’Aquila, C.; Zoccolella, S.; Cardinali, V.; De Mari, M.; Iliceto, G.; Tartaglione, B.; Lamberti, P.; Logroscino, G. Predictors of survival in a series of clinically diagnosed progressive supranuclear palsy patients. Park. Relat. Disord. 2013, 19, 980–985. [Google Scholar] [CrossRef]
- Santacruz, P.; Uttl, B.; Litvan, I.; Grafman, J. Progressive supranuclear palsy: A survey of the disease course. Neurology 1998, 50, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Balkau, B.; Charles, M.A.; Drivsholm, T.; Borch-Johnsen, K.; Wareham, N.; Yudkin, J.S.; Morris, R.; Zavaroni, I.; Van Dam, R.; Feskins, E.; et al. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002, 28, 364–376. [Google Scholar] [PubMed]
- Assunção, N.; Sudo, F.K.; Drummond, C.; De Felice, F.G.; Mattos, P. Metabolic Syndrome and cognitive decline in the elderly: A systematic review. PLoS ONE 2018, 13, e0194990. [Google Scholar] [CrossRef] [PubMed]
- Piot, I.; Schweyer, K.; Respondek, G.; Stamelou, M.; Sckopke, P.; Schenk, T.; Goetz, C.G.; Stebbins, G.T.; Höglinger, G.U.; Gasser, T.; et al. The Progressive Supranuclear Palsy Clinical Deficits Scale. Mov. Disord. 2020, 35, 650–661. [Google Scholar] [CrossRef]
- Scherbaum, R.; Hartelt, E.; Kinkel, M.; Gold, R.; Muhlack, S.; Tönges, L. Parkinson’s Disease Multimodal Complex Treatment improves motor symptoms, depression and quality of life. J. Neurol. 2020, 267, 954–965. [Google Scholar] [CrossRef]

| ICD-10 | Diagnosis |
|---|---|
| G23.- | Other degenerative diseases of the basal ganglia |
| G23.0 | Neurodegeneration with Brain Iron Accumulation |
| G23.1 | Steele–Richardson–Olzewski syndrome (PSP) |
| G23.2 | Multiple system atrophy of parkinsonian type (MSA-P) |
| G23.3 | Multiple system atrophy of cerebellar type (MSA-C) |
| G23.8 | Other specified degenerative diseases of the basal ganglia |
| G23.9 | Other degenerative diseases of the basal ganglia, not further defined |
| ICD-10 | Diagnosis | Cases, n | Distribution, % |
|---|---|---|---|
| G23.- | Other degenerative diseases of the basal ganglia | 4120 | 100 |
| G23.0 | Neurodegeneration with Brain Iron Accumulation | 26 | 0.6 |
| G23.1 | Steele–Richardson–Olszewski syndrome (PSP) | 1780 | 43.2 |
| G23.2 | Multiple system atrophy of parkinsonian type (MSA-P) | 1071 | 26.0 |
| G23.3 | Multiple system atrophy of cerebellar type (MSA-C) | 407 | 9.9 |
| G23.8 | Other specified degenerative diseases of the basal ganglia | 786 | 19.1 |
| G23.9 | Other degenerative diseases of the basal ganglia, not further defined | 50 | 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zella, M.A.S.; Bartig, D.; Herrmann, L.; Respondek, G.; Höglinger, G.; Gold, R.; Woitalla, D.; Krogias, C.; Tönges, L. Hospitalization Rates and Comorbidities in Patients with Progressive Supranuclear Palsy in Germany from 2010 to 2017. J. Clin. Med. 2020, 9, 2454. https://doi.org/10.3390/jcm9082454
Zella MAS, Bartig D, Herrmann L, Respondek G, Höglinger G, Gold R, Woitalla D, Krogias C, Tönges L. Hospitalization Rates and Comorbidities in Patients with Progressive Supranuclear Palsy in Germany from 2010 to 2017. Journal of Clinical Medicine. 2020; 9(8):2454. https://doi.org/10.3390/jcm9082454
Chicago/Turabian StyleZella, Maria Angela Samis, Dirk Bartig, Lennard Herrmann, Gesine Respondek, Günter Höglinger, Ralf Gold, Dirk Woitalla, Christos Krogias, and Lars Tönges. 2020. "Hospitalization Rates and Comorbidities in Patients with Progressive Supranuclear Palsy in Germany from 2010 to 2017" Journal of Clinical Medicine 9, no. 8: 2454. https://doi.org/10.3390/jcm9082454
APA StyleZella, M. A. S., Bartig, D., Herrmann, L., Respondek, G., Höglinger, G., Gold, R., Woitalla, D., Krogias, C., & Tönges, L. (2020). Hospitalization Rates and Comorbidities in Patients with Progressive Supranuclear Palsy in Germany from 2010 to 2017. Journal of Clinical Medicine, 9(8), 2454. https://doi.org/10.3390/jcm9082454






