A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation
Abstract
1. Introduction
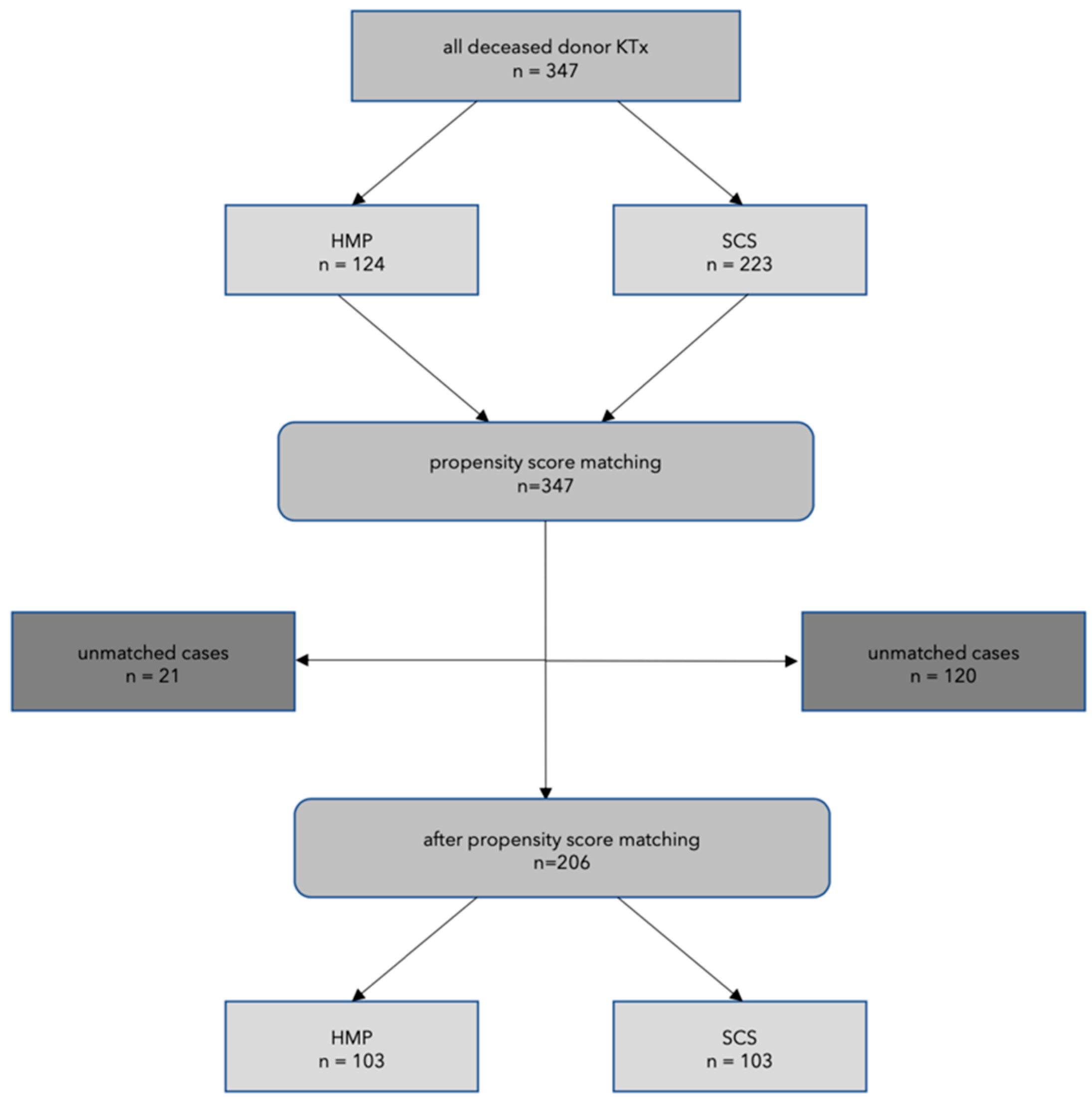
2. Material and Methods
Statistical Analysis
3. Results
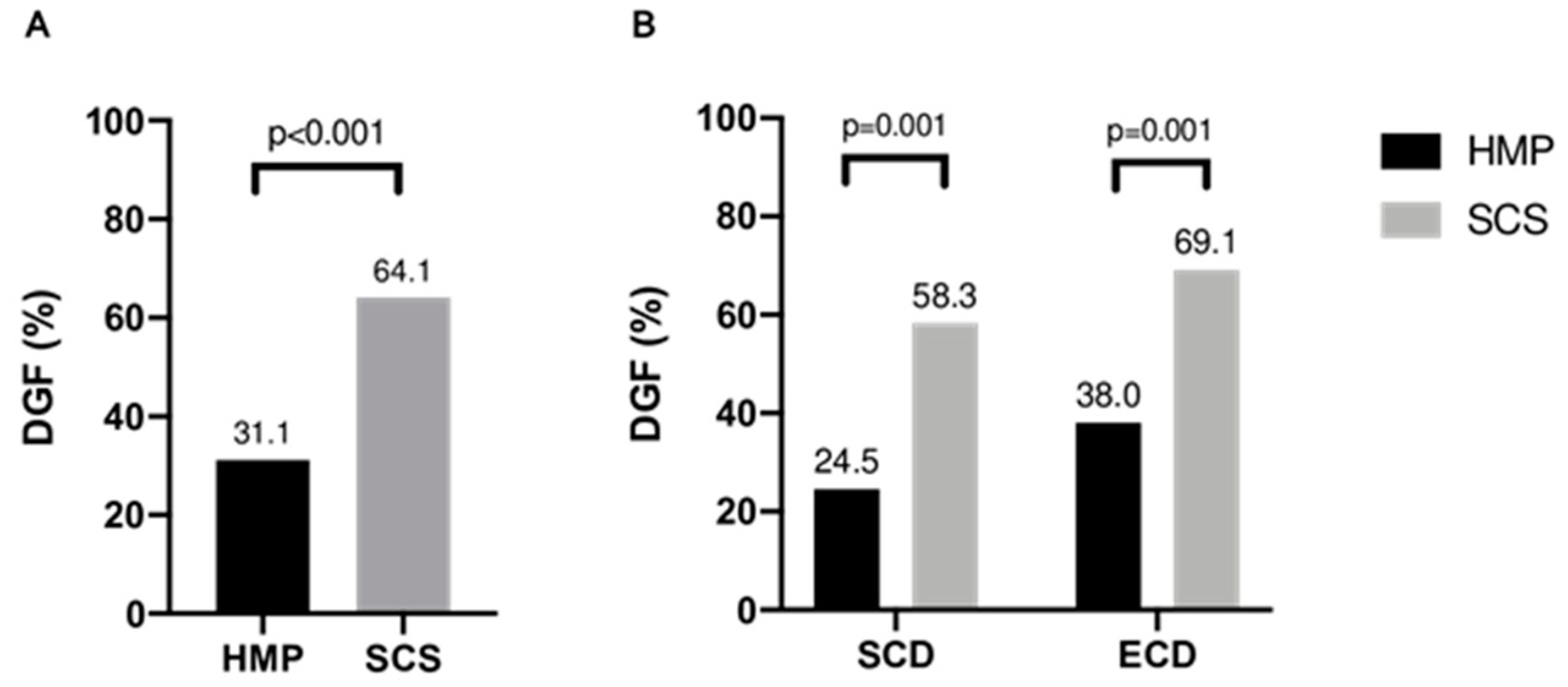
3.1. Incidence of DGF
3.2. Daytime vs. Nighttime Procedures
3.3. HMP Parameters
3.4. Complications
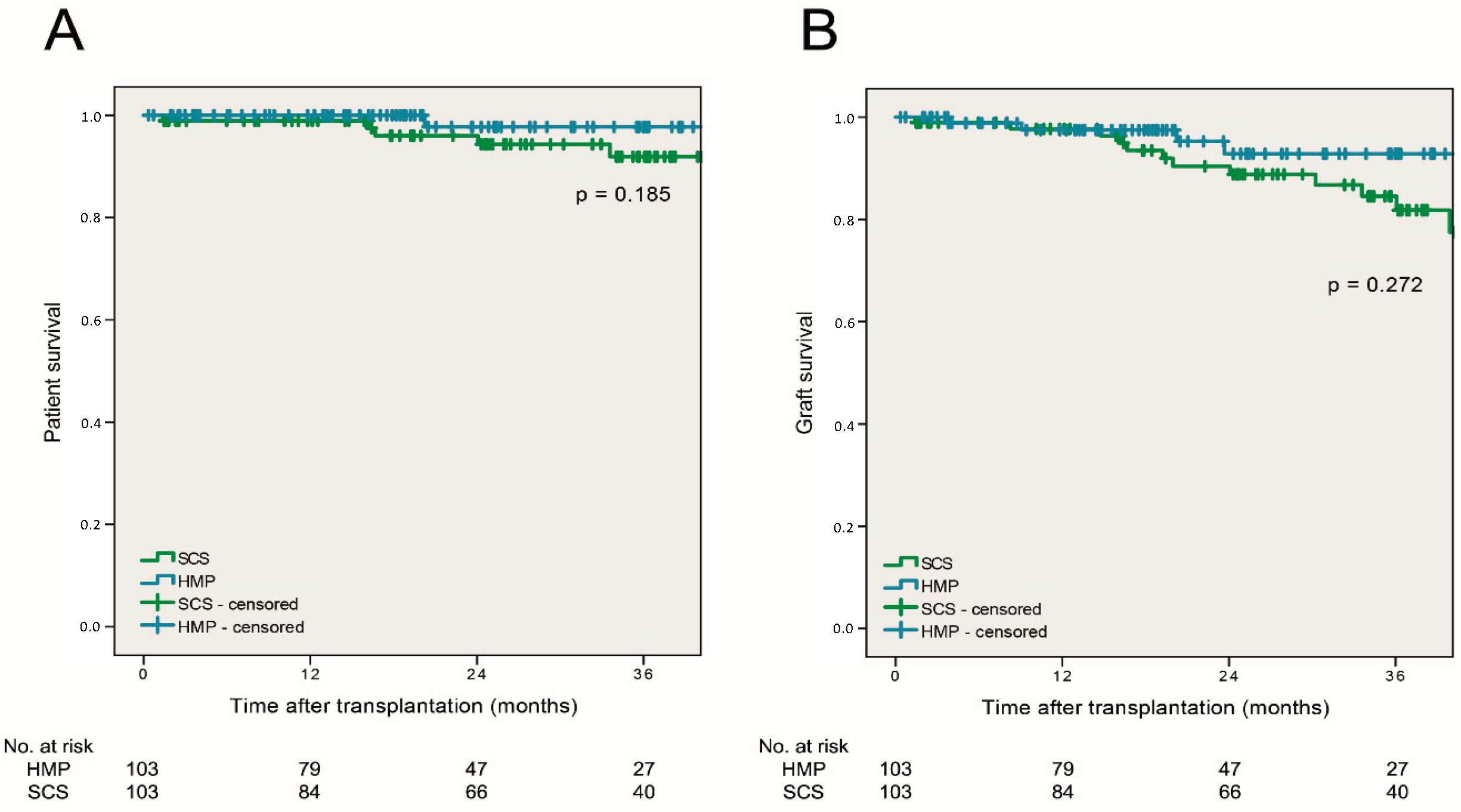
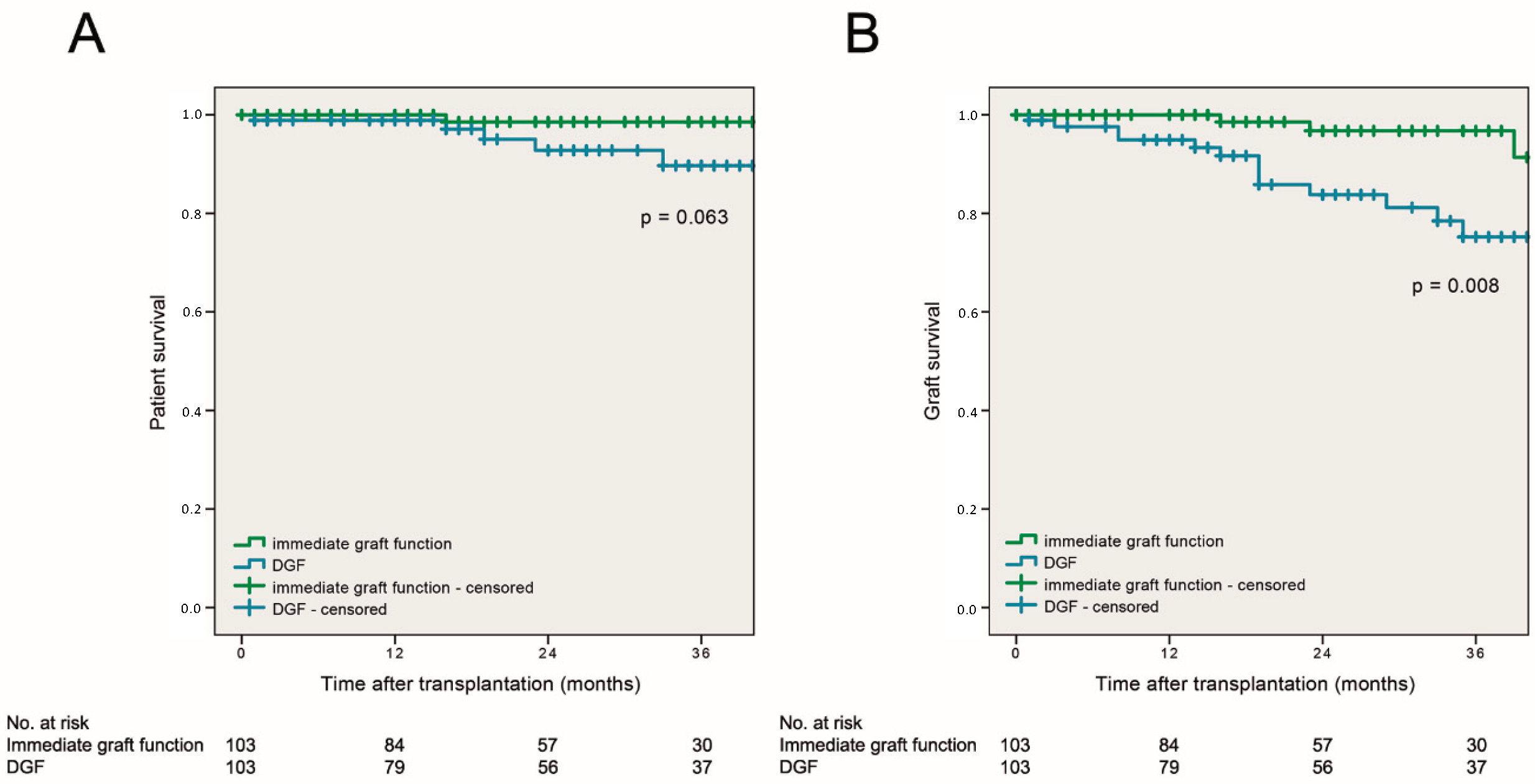
3.5. Patient and Graft Survival
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Aubert, O.; Reese, P.P.; Audry, B.; Bouatou, Y.; Raynaud, M.; Viglietti, D.; Legendre, C.; Glotz, D.; Empana, J.-P.; Jouven, X.; et al. Disparities in Acceptance of Deceased Donor Kidneys Between the United States and France and Estimated Effects of Increased US Acceptance. JAMA Intern. Med. 2019, 179, 1365. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; Van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef]
- van der Vliet, J.A.; Warlé, M.C.; Cheung, C.L.; Teerenstra, S.; Hoitsma, A.J. Influence of prolonged cold ischemia in renal transplantation. Clin. Transplant. 2011, 25, E612–E616. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; O′Callaghan, J.M.; Pirenne, J.; Ploeg, R.J. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors. Transpl. Int. 2015, 28, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Helfer, M.S.; Pompeo, J.C.; Costa, O.R.S.; Vicari, A.R.; Ribeiro, A.R.; Manfro, R.C. Long-term effects of delayed graft function duration on function and survival of deceased donor kidney transplants. J. Bras. Nefrol. 2019, 41, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Metzger, R.A.; Delmonico, F.L.; Feng, S.; Port, F.K.; Wynn, J.J.; Merion, R.M. Expanded criteria donors for kidney transplantation. Am. J. Transplant. 2003, 3 (Suppl. 4), 114–125. [Google Scholar] [CrossRef]
- Querard, A.H.; Le Borgne, F.; Dion, A.; Giral, M.; Mourad, G.; Garrigue, V.; Rostaing, L.; Kamar, N.; Loupy, A.; Legendre, C.; et al. Propensity score-based comparison of the graft failure risk between kidney transplant recipients of standard and expanded criteria donor grafts: Toward increasing the pool of marginal donors. Am. J. Transplant. 2018, 18, 1151–1157. [Google Scholar] [CrossRef]
- Ojo, A.O.; Hanson, J.A.; Meier-Kriesche, H.; Okechukwu, C.N.; Wolfe, R.A.; Leichtman, A.B.; Agodoa, L.Y.; Kaplan, B.; Port, F.K. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J. Am. Soc. Nephrol. 2001, 12, 589–597. [Google Scholar]
- Belzer, F.O.; Ashby, B.S.; Dunphy, J.E. 24-hour and 72-hour preservation of canine kidneys. Lancet 1967, 2, 536–538. [Google Scholar] [CrossRef]
- Belzer, F.O.; Ashby, B.S.; Gulyassy, P.F.; Powell, M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N. Engl. J. Med. 1968, 278, 608–610. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; Van Kasterop-Kutz, M.; Van Der Heide, J.J.H.; Squifflet, J.-P.; Van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Zhong, Z.; Lan, J.; Ye, S.; Liu, Z.; Fan, L.; Zhang, Y.; Fu, Z.; Qiao, B.; Ko, D.S.; Wang, Y.; et al. Outcome Improvement for Hypothermic Machine Perfusion Versus Cold Storage for Kidneys From Cardiac Death Donors. Artif. Organs. 2017, 41, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Savoye, E.; Macher, M.A.; Videcoq, M.; Gatault, P.; Hazzan, M.; Abboud, I.; Thierry, A.; Bertrand, D.; Drouin, S.; Sayegh, J.; et al. Evaluation of outcomes in renal transplantation with hypothermic machine perfusion for the preservation of kidneys from expanded-criteria donors. Clin. Transplant. 2019, 33, e13536. [Google Scholar] [CrossRef]
- Yuan, X.; Theruvath, A.J.; Ge, X.; Floerchinger, B.; Jurisch, A.; García-Cardeña, G.; Tullius, S.G. Machine perfusion or cold storage in organ transplantation: Indication, mechanisms, and future perspectives. Transpl. Int. 2010, 23, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kaths, J.M.; Paul, A.; Robinson, L.A.; Selzner, M. Ex vivo machine perfusion for renal graft preservation. Transplant. Rev. 2018, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Kienzl-Wagner, K.; Schneiderbauer, S.; Bösmüller, C.; Schneeberger, S.; Pratschke, J.; Ollinger, R. Nighttime procedures are not associated with adverse outcomes in kidney transplantation. Transpl. Int. 2013, 26, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, B.; Lohmann, D.; Sollinger, D.; Stein, R.; Lutz, J. Association of Kidney Donor Risk Index with the Outcome after Kidney Transplantation in the Eurotransplant Senior Program. Ann. Transplant. 2018, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Husen, P.C.B.; Davies, L.; Knight, S.; Paul, A.; Ploeg, R.J. End-Hypothermic Machine Perfusion with Oxygenation after Static Cold Storage versus Static Cold Storage alone in ECD Kidneys from Donation after Brain Death Donors: Results of a Prospective International Randomised Controlled Trial in Kidney Transplantation. Available online: https://www.esot.org/resources/consortium-organ-preservation-europe (accessed on 1 June 2020).
- Bahl, D.; Haddad, Z.; Datoo, A.; Qazi, Y.A. Delayed graft function in kidney transplantation. Curr. Opin. Organ Transplant. 2019, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Melih, K.V.; Boynuegri, B.; Mustafa, C.; Nilgun, A. Incidence, Risk Factors, and Outcomes of Delayed Graft Function in Deceased Donor Kidney Transplantation. Transplant. Proc. 2019, 51, 1096–1100. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic Machine Perfusion is Superior to Static Cold Storage in Deceased Donor Kidney Transplantation: A Meta-analysis. Clin. Transplant. 2020, e13814. [Google Scholar] [CrossRef]
- Gill, J.; Dong, J.; Eng, M.; Landsberg, D.; Gill, J.S. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation 2014, 97, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.W.; Laurence, J.M.; Richardson, A.J.; Pleass, H.C.; Allen, R.D. Hypothermic machine perfusion in deceased donor kidney transplantation: A systematic review. J. Surg. Res. 2013, 180, 176–182. [Google Scholar] [CrossRef]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.G.D.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef]
- Fechner, G.; Pezold, C.; Hauser, S.; Gerhardt, T.; Müller, S.C. Kidney’s nightshift, kidney’s nightmare? Comparison of daylight and nighttime kidney transplantation: Impact on complications and graft survival. Transplant. Proc. 2008, 40, 1341–1344. [Google Scholar] [CrossRef]
- Seow, Y.Y.; Alkari, B.; Dyer, P.; Riad, H. Cold ischemia time, surgeon, time of day, and surgical complications. Transplantation 2004, 77, 1386–1389. [Google Scholar] [CrossRef][Green Version]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlaeger, J.; Rauen, U.; Paul, A.; Minor, T. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014, 98, 944–950. [Google Scholar] [CrossRef]
- Thuillier, R.; Allain, G.; Celhay, O.; Hebrard, W.; Barrou, B.; Badet, L.; Leuvenink, H.G.; Hauet, T. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J. Surg. Res. 2013, 184, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Hofker, H.S.; Davies, L.; Knight, S.; Pirenne, J.; Ploeg, R.J. Oxygenated Hypothermic Machine Perfusion of Kidneys Donated after Circulatory Death: An International Randomised Controlled Trial [abstract]. Am. J. Transplant. 2019, 19 (Suppl. 3), 312. Available online: https://atcmeetingabstracts.com/abstract/oxygenated-hypothermic-machine-perfusion-of-kidneys-donated-after-circulatory-death-an-international-randomised-controlled-trial/ (accessed on 20 August 2019).

| HMP, n = 124 | SCS, n = 223 | p-Value | SMD | |
|---|---|---|---|---|
| CIT (hours) * | 17.5 (± 5.1) | 13.3 (± 4.6) | p < 0.001 | 0.865 |
| ECD (n, %) | 61 (49.2%) | 95 (42.6%) | p = 0.237 | 0.108 |
| SCD (n, %) | 63 (50.8%) | 128 (57.4%) | p = 0.237 | 0.108 |
| Gender MM (n, %) | 66 (53.2%) | 110 (49.3%) | p = 0.486 | 0.064 |
| CMV MM (n, %) | 21 (16.9%) | 38 (17.1%) | p = 0.966 | 0.004 |
| Re-TX (n, %) | 31 (25.0%) | 44 (19.7%) | p = 0.253 | 0.103 |
| ET-Senior (n, %) | 25 (20.2%) | 43 (19.3%) | p = 0.843 | 0.018 |
| HMP, n = 103 | SCS, n = 103 | SMD | |
|---|---|---|---|
| CIT (hours) * | 16.32 (± 4.3) | 15.97 (± 4.4) | 0.059 |
| ECD (n, %) | 50 (48.5%) | 55 (53.4%) | 0.080 |
| SCD (n, %) | 53 (51.5%) | 48 (46.6%) | 0.080 |
| Gender MM (n, %) | 54 (52.4%) | 49 (47.6%) | 0.078 |
| CMV MM (n, %) | 17 (16.5%) | 13 (12.6%) | 0.092 |
| Re-TX (n, %) | 23 (22.3%) | 25 (24.3%) | 0.019 |
| ET-Senior (n, %) | 19 (18.4%) | 16 (15.5%) | 0.062 |
| HMP, n = 103 | SCS, n = 103 | |
|---|---|---|
| Recipient age, median (range) | 57 (28–79) | 58 (26–76) |
| Recipient male gender, n (%) | 74 (71.8%) | 71 (68.9%) |
| Recipient BMI kg/m2, mean ± SD | 25.5 ± 4.6 | 26.4 ± 4.2 |
| Prior TX, n (%) | 23 (22.3%) | 25 24.3% |
| Double kidney TX, n (%) | 6 (5.8%) | 9 (8.7%) |
| Donor age, median (range) | 55 (18–82) | 55 (18–84) |
| Donor male gender, n (%) | 52 (50.5%) | 64 (62.1%) |
| Donor BMI kg/m2, mean ± SD | 26.8 ± 5.2 | 27.2 ± 4.4 |
| Extended criteria donor, n (%) | 50 (48.5%) | 55 (53.4%) |
| Kidney donor risk index (KDRI), mean ± SD | 1.29 ± 0.42 | 1.31 ± 0.42 |
| Kidney donor profile index (KDPI), mean ± SD | 66.7 ± 23.9 | 67.3 ± 25.3 |
| Cause of end stage renal disease | ||
| Glomerulonephritis | 37 (35.9%) | 40 (38.8%) |
| Diabetic nephropathy | 19 (18.5%) | 16 (15.5%) |
| Hereditary renal disease | 12 (11.7) | 15 (14.6%) |
| Vascular nephropathy | 12 (11.7%) | 13 (12.6%) |
| Others | 23 (22.3%) | 19 (18.4%) |
| Cold ischemia time in hours, mean ± SD | 16.32 ± 4.3 | 15.97 ± 4.4 |
| Anastomosis time in minutes, mean ± SD | 33 ± 10 | 31 ± 9 |
| HLA A mm, mean ± SD | 1.02 ± 0.61 | 0.98 ± 0.69 |
| 0 and 1 | 83 (80.6%) | 80 (77.7%) |
| 2 | 20 (19.4%) | 22 (21.3%) |
| HLA B mm, mean ± SD | 1.29 ± 0.69 | 1.15 ± 0.66 |
| 0 and 1 | 59 (57.3%) | 72 (69.9%) |
| 2 | 44 (42.7%) | 31 (30.1%) |
| HLA DR mm, mean ± SD | 1.20 ± 0.63 | 1.02 ± 0.71 |
| 0 and 1 | 70 (68.0%) | 76 (73.8%) |
| 2 | 33 (32.0%) | 27 (26.2%) |
| HMP, n = 103 | SCS, n = 103 | p-Value | |
|---|---|---|---|
| overall complications | 27 (26.2%) | 29 (28.2%) | 0.438 |
| hematoma | 6 (5.8%) | 8 (7.8%) | 0.392 |
| lymphocele | 6 (5.8%) | 13 (12.6%) | 0.074 |
| urological complications | 8 (7.8%) | 7 (6.8%) | 0.500 |
| urinary leakage | 4 (3.9%) | 3 (2.9%) | |
| ureteral stenosis | 2 (1.9%) | 4 (3.9%) | |
| vesicoureteral reflux | 2 (1.9%) | 0 | |
| wound infections | 3 (2.9%) | 10 (9.7%) | 0.041 |
| vascular complications | 4 (3.8%) | 3 (2.9%) | 0.500 |
| arterial stenosis | 1 (1.0%) | 2 (1.9%) | |
| pseudoaneurysm | 1 (1.0%) | 0 |
| DGF | No DGF | p-Value | |
|---|---|---|---|
| HMP | |||
| serum creatinine | 2.0 (0.9–4.1) | 1.42 (0.5–3.9) | 0.001 |
| GFR | 32.9 (11.3–76.3) | 46.8 (15.6–122.4) | 0.004 |
| SCS | |||
| serum creatinine | 1.84 (0.92–3.8) | 1.5 (0.8–3.63) | 0.009 |
| GFR | 33.9 (16.7–82.0) | 42.2 (19.6–98.8) | 0.015 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasteiger, S.; Berchtold, V.; Bösmüller, C.; Dostal, L.; Ulmer, H.; Bogensperger, C.; Resch, T.; Rudnicki, M.; Neuwirt, H.; Oberhuber, R.; et al. A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation. J. Clin. Med. 2020, 9, 2311. https://doi.org/10.3390/jcm9072311
Gasteiger S, Berchtold V, Bösmüller C, Dostal L, Ulmer H, Bogensperger C, Resch T, Rudnicki M, Neuwirt H, Oberhuber R, et al. A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation. Journal of Clinical Medicine. 2020; 9(7):2311. https://doi.org/10.3390/jcm9072311
Chicago/Turabian StyleGasteiger, Silvia, Valeria Berchtold, Claudia Bösmüller, Lucie Dostal, Hanno Ulmer, Christina Bogensperger, Thomas Resch, Michael Rudnicki, Hannes Neuwirt, Rupert Oberhuber, and et al. 2020. "A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation" Journal of Clinical Medicine 9, no. 7: 2311. https://doi.org/10.3390/jcm9072311
APA StyleGasteiger, S., Berchtold, V., Bösmüller, C., Dostal, L., Ulmer, H., Bogensperger, C., Resch, T., Rudnicki, M., Neuwirt, H., Oberhuber, R., Cardini, B., Scheidl, S., Mayer, G., Öfner, D., Weissenbacher, A., & Schneeberger, S. (2020). A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation. Journal of Clinical Medicine, 9(7), 2311. https://doi.org/10.3390/jcm9072311





