Apelin Improves Prognostic Value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) Scales in Ambulatory Patients with End-Stage Heart Failure †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Laboratory Measurements
2.3. Analyzed Scales
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol. Pol. 2016, 74, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Inamdar, A.C. Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef]
- Alraies, M.C.; Eckman, P. Adult heart transplant: Indications and outcomes. J. Thorac. Dis. 2014, 6, 1120–1128. [Google Scholar]
- Szygula-Jurkiewicz, B.; Szczurek, W.; Skrzypek, M.; Zakliczyński, M.; Siedlecki, Ł.; Przybyłowski, P.; Zembala, M.; Gąsior, M. One-year survival of ambulatory patients with end-stage heart failure: The analysis of prognostic factors. Pol. Arch. Intern. Med. 2017, 127, 254–260. [Google Scholar] [CrossRef][Green Version]
- Kodziszewska, K.; Leszek, P.; Korewicki, J.; Piotrowski, W. Old markers, new approach to assessment of risk in heart failure. Kardiol. Pol. 2015, 73, 387–395. [Google Scholar] [CrossRef]
- Aaronson, K.D.; Cowger, J. Heart failure prognostic models: Why bother? Circ. Heart Fail. 2012, 5, 6–9. [Google Scholar] [CrossRef]
- Hsich, E.M. Matching the Market for Heart Transplantation. Circ. Heart Fail 2016, 9, e002679. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodward, M.; Patel, A.; McMurray, J.; MacMahon, S. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szyguła-Jurkiewicz, B.; Zakliczyński, M.W.; Król, B.; Gąsior, M.; Zembala, M. Prognostic value of selected risk scales in patients with end-stage heart failure. Kardiol. Pol. 2018, 76, 1320–1326. [Google Scholar] [CrossRef]
- Aaronson, K.D.; Schwartz, J.S.; Chen, T.M.; Wong, K.L.; Goin, J.E.; Mancini, D.M. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997, 95, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szyguła-Jurkiewicz, B.; Zakliczyński, M.; Król, B.; Gąsior, M.; Zembala, M. Prognostic utility of the N terminal prohormone of brain natriuretic peptide and the modified Model for End Stage Liver Disease in patients with end stage heart failure. Pol. Arch. Intern. Med. 2018, 128, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kato, T.S.; Farr, M.; Wu, C.; Givens, R.C.; Collado, E.; Mancini, D.M.; Schulze, P.C. Hepatic dysfunction in ambulatory patients with heart failure: Application of the MELD scoring system for outcome prediction. J. Am. Coll. Cardiol. 2013, 61, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.V.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Szokodi, I.; Tavi, P.; Foldes, G.; Voutilainen-Myllylä, S.; Ilves, M.; Tokola, H.; Pikkarainen, S.; Piuhola, J.; Rysä, J.; Tóth, M.; et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002, 91, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; You, T.; Guo, J.; Xu, R.; Guo, Q.; Lin, J.; Zhao, H. Effects of apelin on left ventricular-arterial coupling and mechanical efficiency in rats with ischemic heart failure. Dis. Markers 2019, 17, 4823156. [Google Scholar] [CrossRef]
- Dalzell, J.R.; Rocchiccioli, J.P.; Weir, R.A.P.; Jackson, C.E.; Padmanabhan, N.; Gardner, R.S.; Petrie, M.C.; McMurray, J.J.V. The Emerging Potential of the Apelin APJ System in Heart Failure. J. Card Fail. 2015, 21, 489–498. [Google Scholar] [CrossRef]
- Goidescu, C.M.; Vida-Simiti, L.A. The Apelin-APJ system in the evolution of heart failure. Clujul. Med. 2015, 88, 3–8. [Google Scholar] [CrossRef]
- Dai, T.; Ramirez-Correa, G.; Gao, W.D. Apelin increases contractility in failing cardiac muscle. Eur. J. Pharmacol. 2006, 553, 222–228. [Google Scholar] [CrossRef]
- Alba, A.C.; Agoritsas, T.; Jankowski, M.; Courvoisier, D.; Walter, S.D.; Guyatt, G.H.; Ross, H.J. Risk prediction models for mortality in ambulatory patients with heart failure: A systematic review. Circ. Heart Fail. 2013, 6, 881–889. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Adams, K., Jr.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.F.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Rørth, R.; Jhund, P.S.; Yilmaz, M.B.; Kristensen, S.L.; Welsh, P.; Desai, A.S.; Køber, L.; Prescott, M.F.; Rouleau, J.L.; Solomon, S.D.; et al. Comparison of BNP and NT-proBNP in Patients With Heart Failure and Reduced Ejection Fraction. Circ. Heart Fail. 2020, 13, e006541. [Google Scholar] [CrossRef]
- MacGowan, G.A.; Neely, D.; Peaston, R.; Wrightson, N.; Parry, G. Evaluation of NT-proBNP to predict outcomes in advanced heart failure. Int. J. Clin. Pract. 2010, 64, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pellicori, P.; Pan, D.; Dierckx, R.; Clark, A.L.; Cleland, J.G.F. Dynamic risk stratification using serial measurements of plasma concentrations of natriuretic peptides in patients with heart failure. Int. J. Cardiol. 2018, 269, 196–200. [Google Scholar] [CrossRef] [PubMed]
| All Included (n = 240) # | Survivors (n = 165) | Non-Survivors (n = 75) | p * | |
|---|---|---|---|---|
| HFSS components | ||||
| Ischemic etiology of HF, % | 118 (49.2) | 78 (47.3) | 40 (53.3) | 0.3840 |
| Rest HR, beats per min | 72.00 (65.00–79.00) | 70.00 (65.00–78.00) | 75.00 (67.00–80.00) | 0.3917 |
| Rest mBP, mmHg | 74.81 (9.36) | 75.68 (9.13) | 72.91 (9.64) | 0.0334 * |
| Sodium, mmol/L | 139 (137–141) | 140 (138–141) | 138 (135–139) | <0.0001 * |
| VO2 max, mL/kg/min | 11.20 (10.30–12.20) | 11.30 (10.40–12.30) | 10.90 (9.70–12.20) | 0.1544 |
| Presence of IVCD, % | 105 (43.8) | 70 (42.4) | 35 (46.7) | 0.5392 |
| MAGGIC components | ||||
| Age, years | 58.0 (51.50.0–64.0) | 57.00 (50.00–63.00) | 60.00 (54.00–65.00) | 0.0517 |
| Male, % | 212 (88.3) | 142 (86.1) | 70 (93.3) | 0.1038 |
| NYHA III, % | 208 (86.7) | 149 (90.3) | 59 (78.7) | 0.014 * |
| NYHA IV, % | 32 (13.3) | 16 (9.7) | 16 (21.3) | 0.014 * |
| HF diagnosed within the last 18 months, % | 12 (5.0) | 7 (4.2) | 5 (6.7) | 0.4245 |
| Current smoker, % | 37 (15.4) | 25 (15.2) | 12 (16) | 0.8660 |
| Type 2 diabetes, % | 96 (40) | 62 (37.6) | 34 (45.3) | 0.2555 |
| COPD, % | 27 (11.3) | 18 (10.9) | 9 (12) | 0.8042 |
| BMI, kg/m2 | 27.02 (24.13–30.47) | 27.36 (24.69–31.18) | 25.66 (22.63–29.54) | 0.014 * |
| Resting SBP, mmHg | 100 (92–110) | 102 (97.–114) | 100 (90–103) | <0.0001 * |
| Creatinine, µmol/L | 107 (94.5–125.5) | 104 (88.6–110) | 127 (106–147) | <0.0001 * |
| B-blockers, % | 238 (99.2) | 163 (98.8) | 75 (100) | 0.8725 |
| ACEI/ARB, % | 234 (97.5) | 160 (97) | 74 (98.7) | 0.4351 |
| Common to HFSS and MAGGIC | ||||
| LVEF, % | 17.00 (15.00–20.00) | 18.00 (15.00–20.00) | 15.00 (13.00–18.00) | 0.0003 * |
| SCORES | ||||
| HFSS | 7.54 (7.16–8.00) | 7.73 (7.33–8.19) | 7.29 (6.92–7.62) | <0.0001 * |
| MAGGIC | 25.00 (22.00–27.50) | 24.00 (22.00–26.00) | 27.00 (25.00–30.00) | <0.0001 * |
| Apelin, pg/mL | 37.88 (29.65–52.30) | 42.01 (34.43–64.87) | 29.27 (21.04–36.55) | <0.0001 * |
| NT-proBNP, pg/mL | 2854.5 (1657–6189) | 2026 (1553–4674) | 5093 (2437–7856) | <0.0001 * |
| HFSS-apelin | 11.20 (10.35–12.24) | 11.62 (10.99–13.28) | 10.26 (9.48–10.77) | <0.0001 * |
| MAGGIC-apelin | 1.55 (0.49–2.15) | 1.09 (0.10–1.77) | 2.51 (1.75–3.20) | <0.0001 * |
| HFSS-NT-proBNP | 7.33 (6.82–7.75) | 7.48 (7.10–7.97) | 6.87 (6.63–7.32) | <0.0001 * |
| MAGGIC-NT-proBNP | 4.22 (3.80–4.79) | 3.99 (3.65–4.44) | 4.65 (4.15–5.17) | <0.0001 * |
| All Included # (n = 240) | Survivors (n = 165) | Non-Survivors (n = 75) | p | |
|---|---|---|---|---|
| Comorbidities | ||||
| Hypertension, % | 140 (58.3) | 88 (53.3) | 52 (69.3) | 0.0198 * |
| Persistent atrial fibrillation, % | 116 (48.3) | 84 (50.9) | 32 (42.7) | 0.2363 |
| Hypercholesterolemia, % | 155 (64.6) 0 0 | 105 (63.6) | 50 (66.7) | 0.6491 |
| Pulmonary hypertension, % | 65 (27.1) | 42 (25.5%) | 23 (30.7%) | 0.3997 |
| Laboratory parameters | ||||
| Leukocytes, ×109/L | 7.68 (6.08–8.89) | 7.33 (6.06–8.68) | 8.05 (6.56–9.14) | 0.0856 |
| Haemoglobin, mmol/L | 8.83 (0.97) | 8.81 (0.94) | 8.88 (1.05) | 0.6334 |
| Platelets, ×109/L | 186.00 (158.50–232.00) | 185.00 (158.00–232.00) | 191.00 (159.00–235.00) | 0.8326 |
| Total bilirubin, µmol/L | 15.85 (11.65–20.95) | 15.30 (11.50–19.60) | 18.50 (11.70–23.00) | 0.0212 * |
| Albumin, g/L | 43.00 (41.00–46.00) | 44.00 (42.00–46.00) | 42.00 (38.00–44.00) | <0.0001 * |
| Uric acid, µmol/L | 424.50 (360.00–512.50) | 421.00 (353.00–506.00) | 447.00 (366.00–515.00) | 0.3751 |
| Urea, µmol/L | 8.25 (5.90–13.15) | 8.00 (5.70–10.30) | 10.60 (6.60–17.80) | 0.0014 * |
| Fibrinogen, mg/dl | 379.00 (312.50–442.00) | 366.00 (309.00–432.00) | 396.00 (324.00–459.00) | 0.0471 * |
| AST, U/L | 26.00 (20.00–31.50) | 26.00 (20.00–32.00) | 25.00 (19.00–31.00) | 0.7384 |
| ALT, U/L | 22.00 (15.00–33.00) | 22.00 (17.00–33.00) | 20.00 (14.00–33.00) | 0.1711 |
| ALP, U/L | 77.00 (62.00–100.00) | 75.00 (61.00–97.00) | 86.00 (64.00–104.00) | 0.0567 |
| GGTP, U/L | 69.00 (34.00–125.00) | 69.00 (32.00–125.00) | 69.00 (40.00–123.00) | 0.3524 |
| Cholesterol, mmol/L | 4.01 (3.28–4.79) | 4.03 (3.36–4.75) | 3.83 (3.14–4.84) | 0.3442 |
| LDL, mmol/L | 2.13 (1.64–2.71) | 2.14 (1.66–2.79) | 2.10 (1.62–2.67) | 0.6594 |
| hs-CRP, mg/L | 4.12 (1.93–6.88) | 3.40 (1.68–5.43) | 6.74 (2.79–9.33) | <0.0001 * |
| ESR, mm/h | 14.00 (8.00–21.00) | 11.00 (7.00–19.00) | 19.00 (12.00–25.00) | <0.0001 * |
| Glucose, mmol/L | 5.69 (0.66) | 5.67 (0.66) | 5.71 (0.66) | 0.6962 |
| HBA1c, % | 5.70 (5.35–6.30) | 5.80 (5.40–6.30) | 5.60 (5.30–6.20) | 0.1424 |
| Hemodynamic parameters | ||||
| PAPm, mmHg | 25.00 (19.00–32.00) | 25.00 (19.00–31.00) | 25.00 (19.00–35.00) | 0.5449 |
| PAWPm, mmHg | 17.00 (11.50–21.00) | 17.00 (12.00–20.00) | 17.00 (10.00–23.00) | 0.496 |
| PVR, Woods units | 1.86 (1.47–2.33) | 1.80 (1.46–2.28) | 2.00 (1.52–2.35) | 0.2938 |
| Cl, L/min/m2 | 1.93 (1.78–2.01) | 1.93 (1.80–2.00) | 1.94 (1.77–2.01) | 0.7835 |
| Echocardiographic parameters | ||||
| LA, mm | 52.00 (47.00–57.50) | 51.00 (47.00–58.00) | 54.00 (48.00–57.00) | 0.3655 |
| RVEDd, mm | 39.00 (35.00–40.00) | 38.00 (34.00–40.00) | 39.00 (37.00–42.00) | 0.0034 * |
| LVEDd, mm | 70.00 (63.50–80.00) | 70.00 (63.00–79.00) | 71.00 (64.00–81.00) | 0.3313 |
| Cardiac medication | ||||
| B-blockers, % | 238 (99.2) | 163 (98.8) | 75 (100) | 0.8725 |
| Loop diuretics, % | 238 (99.2) | 165 (100) | 75 (100) | |
| MRA, % | 240 (100) | 165 (100) | 75 (100) | |
| Digoxin, % | 70 (29.2) | 46 (27.9) | 24 (32) | 0.5150 |
| Amiodarone, % | 50 (20.8) | 39 (23.6) | 11 (14.7) | 0.1127 |
| Statin, % | 179 (74.6) | 122 (73.9) | 57 (76) | 0.7340 |
| Coumarin derivatives, % | 141 (58.8) | 98 (59.4) | 43 (57.3) | 0.7637 |
| Acetylsalicylic acid, % | 91 (37.9) | 61 (37) | 30 (40) | 0.6538 |
| Sildenafil, % | 65 (27.1) | 42 (25.5) | 23 (30.7) | 0.3997 |
| ICD/CRT-D, % | 240 (100) | 165 (100) | 75 (100) | |
| Allopurinol, % | 169 (70.4) | 121 (73.3) | 48 (64) | 0.1420 |
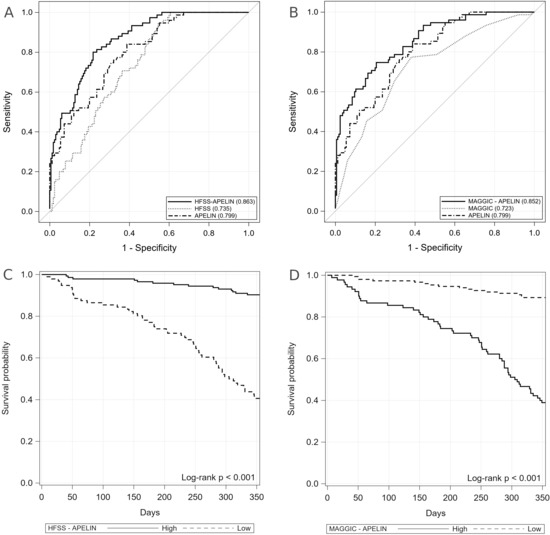
| AUC [±95% CI] | Cut-off | Sensitivity [±95% CI] | Specificity [±95% CI] | PPV [±95% CI] | NPV [±95% CI] | LR+ [±95% CI] | LR− [±95% CI] | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|
| HFSS | 0.7350 [0.6730–0.7971] | ≤7.8 | 0.95 [0.87–0.99] | 0.45 [0.38–0.54] | 0.44 [0.36–0.52] | 0.95 [0.88–0.99] | 1.74 [1.47–1.99] | 0.12 [0.003–0.23] | 0.61 [0.54–0.67] |
| MAGGIC | 0.7230 [0.6538–0.7923] | ≥25 | 0.77 [0.66–0.86] | 0.62 [0.54–0.69] | 0.48 [0.39–0.57] | 0.86 [0.78–0.92] | 2.03 [1.56–2.49] | 0.37 [0.21–0.53] | 0.67 [0.60–0.73] |
| Apelin | 0.7992 [0.7421–0.8562] | ≤39.66 | 0.84 [0.74–0.91] | 0.61 [0.53–0.69] | 0.50 [0.41–0.59] | 0.89 [0.82–0.94] | 2.17 [1.70–2.64] | 0.26 [0.12–0.40] | 0.68 [0.62–0.74] |
| HFSS-apelin | 0.8633 [0.8176–0.9090] | ≤10.84 | 0.80 [0.69–0.88] | 0.78 [0.71–0.84] | 0.63 [0.52–0.72] | 0.90 [0.83–0.94] | 3.67 [2.52–4.81] | 0.26 [0.14–0.37] | 0.78 [0.73–0.84] |
| MAGGIC-apelin | 0.8523 [0.8016–0.9029] | ≥1.885 | 0.75 [0.63–0.84] | 0.79 [0.72–0.84] | 0.62 [0.52–0.72] | 0.87 [0.81–0.92] | 3.62 [2.43–4.82] | 0.32 [0.19–0.45] | 0.78 [0.72–0.83] |
| NT-proBNP | 0.7028 [0.6338–0.7718] | ≥2138 | 0.81 [0.71–0.89] | 0.53 [0.45–0.61] | 0.44 [0.35–0.53 | 0.86 [0.78–0.92] | 1.72 [1.38–2.06] | 0.35 [0.18–0.53] | 0.62 [0.55–0.68] |
| HFSS-NT-proBNP | 0.7665 [0.7076–0.8255] | ≤7.371 | 0.83 [0.71–0.89] | 0.60 [0.52–0.68] | 0.48 [0.39–0.57] | 0.88 [0.80–0.93] | 2.03 [1.59–2.48] | 0.31 [0.16–0.46] | 0.67 [0.60–0.73] |
| MAGGIC-NT-proBNP | 0.7380 [0.6705–0.8061] | ≥ 4.3 | 0.71 [0.59–0.81] | 0.69 [0.61–0.76] | 0.51 [0.41–0.61] | 0.84 [0.77–0.90] | 2.29 [1.66–2.91] | 0.43 [0.27–0.58] | 0.70 [0.63–0.75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurek, W.; Gąsior, M.; Skrzypek, M.; Szyguła-Jurkiewicz, B. Apelin Improves Prognostic Value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) Scales in Ambulatory Patients with End-Stage Heart Failure. J. Clin. Med. 2020, 9, 2300. https://doi.org/10.3390/jcm9072300
Szczurek W, Gąsior M, Skrzypek M, Szyguła-Jurkiewicz B. Apelin Improves Prognostic Value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) Scales in Ambulatory Patients with End-Stage Heart Failure. Journal of Clinical Medicine. 2020; 9(7):2300. https://doi.org/10.3390/jcm9072300
Chicago/Turabian StyleSzczurek, Wioletta, Mariusz Gąsior, Michał Skrzypek, and Bożena Szyguła-Jurkiewicz. 2020. "Apelin Improves Prognostic Value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) Scales in Ambulatory Patients with End-Stage Heart Failure" Journal of Clinical Medicine 9, no. 7: 2300. https://doi.org/10.3390/jcm9072300
APA StyleSzczurek, W., Gąsior, M., Skrzypek, M., & Szyguła-Jurkiewicz, B. (2020). Apelin Improves Prognostic Value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) Scales in Ambulatory Patients with End-Stage Heart Failure. Journal of Clinical Medicine, 9(7), 2300. https://doi.org/10.3390/jcm9072300