Long-Term Follow-Up of Targeted Biopsy Yield (LOFTY Study) in Ulcerative Colitis Surveillance Colonoscopy
Abstract
1. Introduction
2. Methods
2.1. Initial Randomized Controlled Trial
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Mortality and Cause of Death
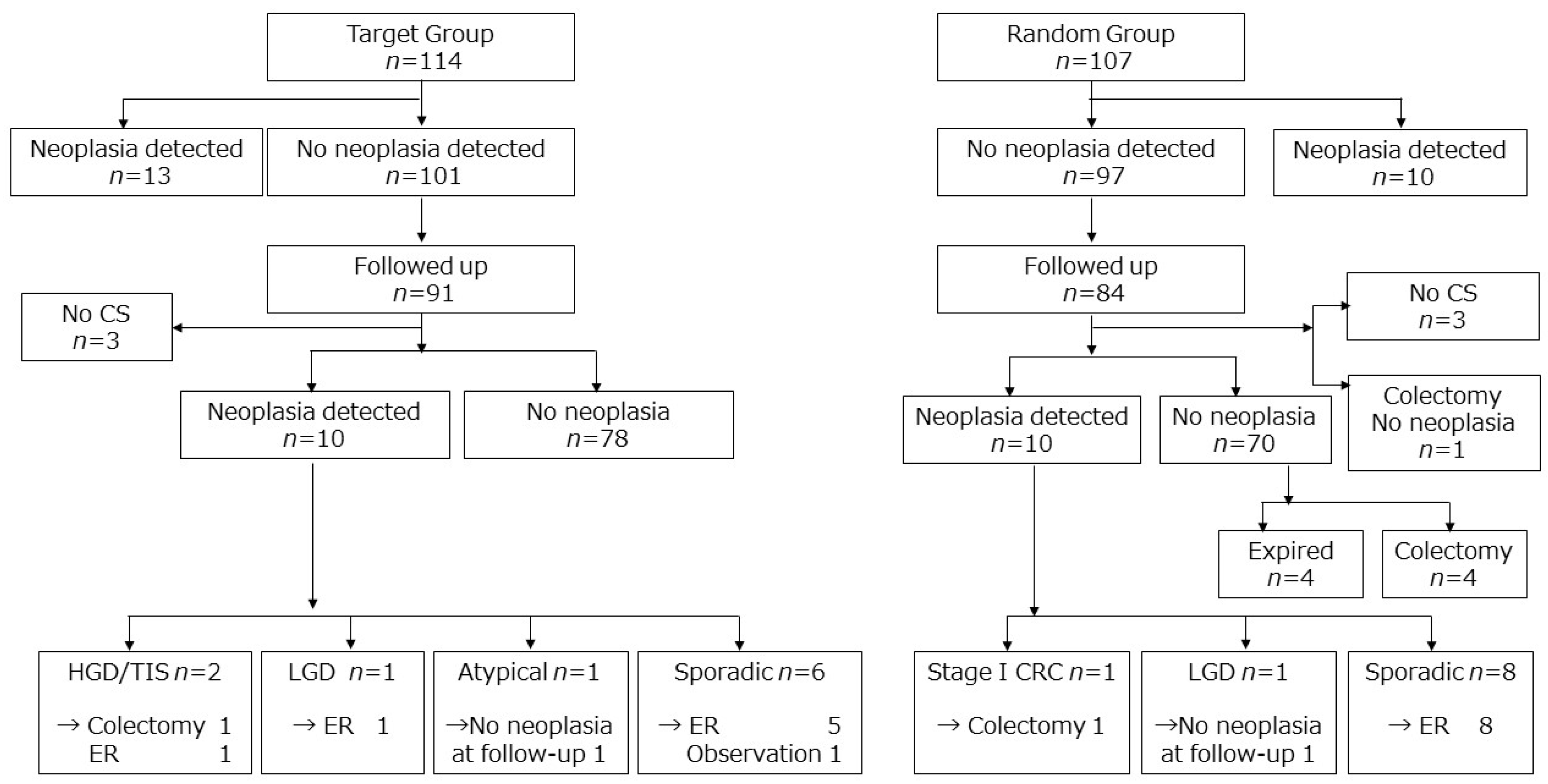
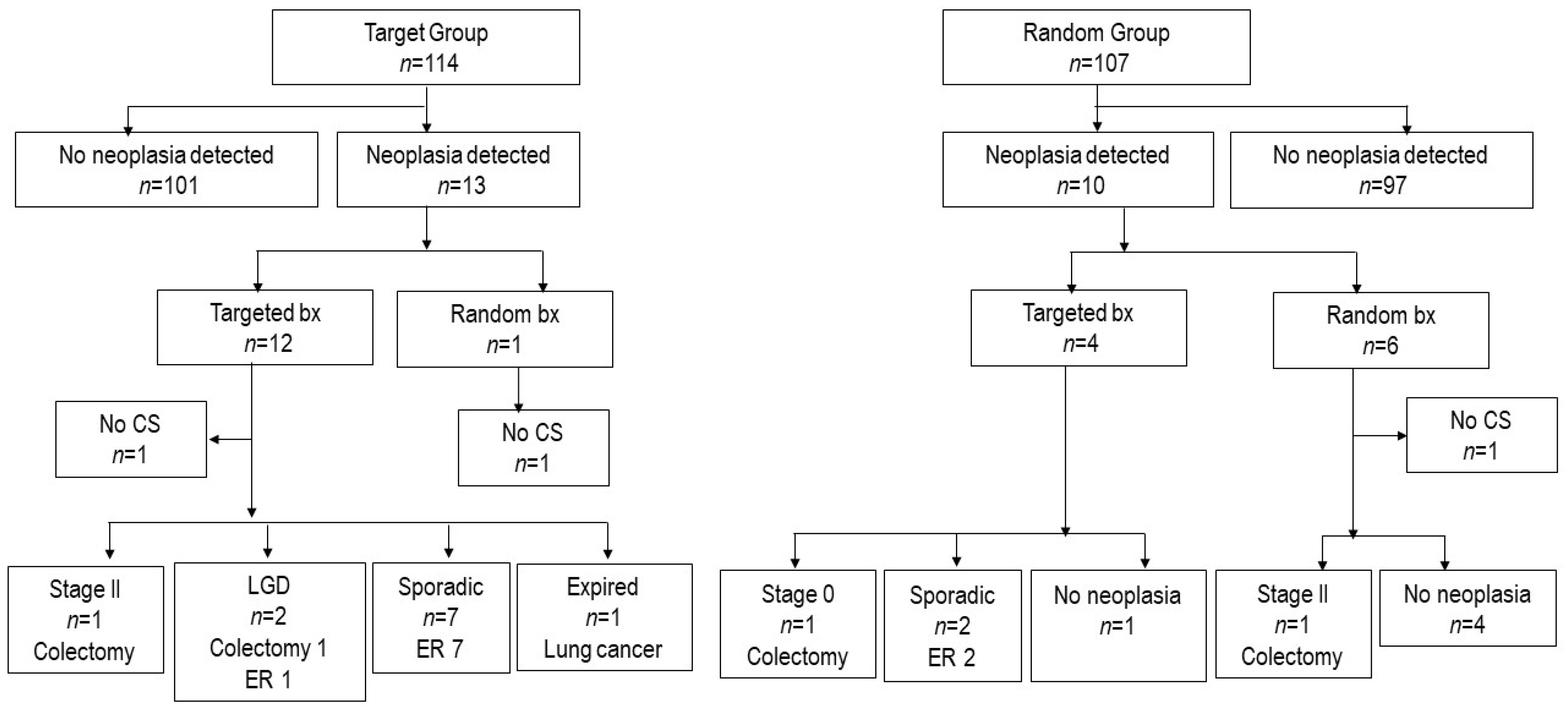
3.2. Colorectal Cancer Development and Fate of Dysplasia Detected in the RCT
3.3. Colectomy Rate
3.4. Extra-Colonic Cancer
3.5. Real-World Surveillance Method After the RCT
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosures
Abbreviations
| CI | confidence interval |
| CRC | colorectal cancer |
| CS | colonoscopy |
| HGD | high-grade dysplasia |
| LGD | low-grade dysplasia |
| LOFTY | LOng-term Follow-up of Targeted biopsy Yield |
| PSC | primary sclerosing cholangitis |
| RCT | randomized controlled trial |
| TNF | tumor necrosis factor |
| WLE | white light endoscopy |
References
- Jeong, D.Y.; Kim, S.; Son, M.J.; Son, C.Y.; Kim, J.Y.; Kronbichler, A.; Lee, K.H.; Shin, J.I. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun. Rev. 2019, 18, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Anzai, H.; Ikeuchi, H.; Futami, K.; Fukushima, K.; Sugita, A.; Uchino, M.; Higashi, D.; Itabashi, M.; Watanabe, K.; et al. Surveillance Colonoscopy for Ulcerative Colitis-Associated Colorectal Cancer Offers Better Overall Survival in Real-World Surgically Resected Cases. Am. J. Gastroenterol. 2019, 114, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.A.; Brown, G.R.; Weideman, R.A.; Crook, T.W.; Cipher, D.J.; Spechler, S.J.; Feagins, L.A. Incidence of Colorectal Cancer and Extracolonic Cancers in Veteran Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 617–623. [Google Scholar] [CrossRef]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef]
- Kopylov, U.; Vutcovici, M.; Kezouh, A.; Seidman, E.; Bitton, A.; Afif, W. Risk of Lymphoma, Colorectal and Skin Cancer in Patients with IBD Treated with Immunomodulators and Biologics: A Quebec Claims Database Study. Inflamm. Bowel Dis. 2015, 21, 1847–1853. [Google Scholar] [CrossRef]
- Fukata, N.; Okazaki, K.; Omiya, M.; Matsushita, M.; Watanabe, M. Hematologic malignancies in the Japanese patients with inflammatory bowel disease. J. Gastroenterol. 2014, 49, 1299–1306. [Google Scholar] [CrossRef]
- Watanabe, T.; Ajioka, Y.; Mitsuyama, K.; Watanabe, K.; Hanai, H.; Nakase, H.; Kunisaki, R.; Matsuda, K.; Iwakiri, R.; Hida, N.; et al. Comparison of Targeted vs Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer. Gastroenterology 2016, 151, 1122–1130. [Google Scholar] [CrossRef]
- Moussata, D.; Allez, M.; Cazals-Hatem, D.; Treton, X.; Laharie, D.; Reimund, J.M.; Bertheau, P.; Bourreille, A.; Lavergne-Slove, A.; Brixi, H.; et al. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut 2018, 67, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ten Hove, J.R.; Shah, S.C.; Shaffer, S.R.; Bernstein, C.N.; Castaneda, D.; Palmela, C.; Mooiweer, E.; Elman, J.; Kumar, A.; Glass, J.; et al. Consecutive negative findings on colonoscopy during surveillance predict a low risk of advanced neoplasia in patients with inflammatory bowel disease with long-standing colitis: Results of a 15-year multicentre, multinational cohort study. Gut 2019, 68, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Ignjatovic-Wilson, A.; Askari, A.; Lee, G.H.; Warusavitarne, J.; Moorghen, M.; Thomas-Gibson, S.; Saunders, B.P.; Rutter, M.D.; Graham, T.A.; et al. Low-grade dysplasia in ulcerative colitis: Risk factors for developing high-grade dysplasia or colorectal cancer. Am. J. Gastroenterol. 2015, 110, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Kazama, S.; Nozawa, H.; Kawai, K.; Kiyomatsu, T.; Tanaka, J.; Tanaka, T.; Nishikawa, T.; Yamaguchi, H.; Ishihara, S.; et al. Laparoscopic surgery for ulcerative colitis: A review of the literature. Surg. Today 2015, 45, 933–938. [Google Scholar] [CrossRef]
- Hata, K.; Kishikawa, J.; Anzai, H.; Shinagawa, T.; Kazama, S.; Ishii, H.; Nozawa, H.; Kawai, K.; Kiyomatsu, T.; Tanaka, J.; et al. Surveillance colonoscopy for colitis-associated dysplasia and cancer in ulcerative colitis patients. Dig. Endosc. 2016, 28, 260–265. [Google Scholar] [CrossRef]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R.; Panel, S.G.D. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015, 148, 639–651. [Google Scholar] [CrossRef]
- Choi, C.H.; Rutter, M.D.; Askari, A.; Lee, G.H.; Warusavitarne, J.; Moorghen, M.; Thomas-Gibson, S.; Saunders, B.P.; Graham, T.A.; Hart, A.L. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am. J. Gastroenterol. 2015, 110, 1022–1034. [Google Scholar] [CrossRef]
- Pedersen, N.; Duricova, D.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Risk of extra-intestinal cancer in inflammatory bowel disease: Meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 2010, 105, 1480–1487. [Google Scholar] [CrossRef]
- Chaparro, M.; Ramas, M.; Benitez, J.M.; Lopez-Garcia, A.; Juan, A.; Guardiola, J.; Minguez, M.; Calvet, X.; Marquez, L.; Fernandez Salazar, L.I.; et al. Extracolonic Cancer in Inflammatory Bowel Disease: Data from the GETECCU Eneida Registry. Am. J. Gastroenterol. 2017, 112, 1135–1143. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Bouvier, A.M.; Colombel, J.F.; Lemann, M.; Cosnes, J.; Hebuterne, X.; Cortot, A.; Bouhnik, Y.; Gendre, J.P.; et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef]
- Kobayashi, T.; Uda, A.; Udagawa, E.; Hibi, T. Lack of Increased Risk of Lymphoma by Thiopurines or Biologics in Japanese Patients with Inflammatory Bowel Disease: A Large-Scale Administrative Database Analysis. J. Crohn’s Colitis 2020, 14, 617–623. [Google Scholar] [CrossRef]
- Shinozaki, M.; Kobayashi, K.; Kunisaki, R.; Hisamatsu, T.; Naganuma, M.; Takahashi, K.I.; Iwao, Y.; Suzuki, Y.; Watanabe, M.; Itabashi, M.; et al. Surveillance for dysplasia in patients with ulcerative colitis: Discrepancy between guidelines and practice. Dig. Endosc. 2017, 29, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Uraoka, T.; Watanabe, K.; Hata, K.; Kawasaki, K.; Mizuno, K.; Misawa, M.; Hosoe, N.; Moriyama, T.; Kawachi, H. Endoscopic diagnosis and treatment of ulcerative colitis-associated neoplasia. Dig. Endosc. 2019, 31, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Fritsch, J.; Holtmann, M.; Koehler, H.H.; Stolte, M.; Kanzler, S.; Nafe, B.; Jung, M.; Galle, P.R.; Neurath, M.F. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003, 124, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; Saunders, B.P.; Schofield, G.; Forbes, A.; Price, A.B.; Talbot, I.C. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 2004, 53, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mooiweer, E.; van der Meulen-de Jong, A.E.; Ponsioen, C.Y.; Fidder, H.H.; Siersema, P.D.; Dekker, E.; Oldenburg, B. Chromoendoscopy for Surveillance in Inflammatory Bowel Disease Does Not Increase Neoplasia Detection Compared With Conventional Colonoscopy With Random Biopsies: Results From a Large Retrospective Study. Am. J. Gastroenterol. 2015, 110, 1014–1021. [Google Scholar] [CrossRef]
- Yang, D.H.; Park, S.J.; Kim, H.S.; Park, Y.S.; Park, D.I.; Lee, K.M.; Jung, S.A.; Choi, C.H.; Koo, J.S.; Cheon, J.H.; et al. High-Definition Chromoendoscopy Versus High-Definition White Light Colonoscopy for Neoplasia Surveillance in Ulcerative Colitis: A Randomized Controlled Trial. Am. J. Gastroenterol. 2019, 114, 1642–1648. [Google Scholar] [CrossRef]
- Iannone, A.; Ruospo, M.; Palmer, S.C.; Principi, M.; Barone, M.; Di Leo, A.; Strippoli, G.F.M. Systematic review with network meta-analysis: Endoscopic techniques for dysplasia surveillance in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; East, J.E.; Hassan, C.; Hazewinkel, Y.; Kaminski, M.F.; Neumann, H.; Pellise, M.; Antonelli, G.; Bustamante Balen, M.; Coron, E.; et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 1155–1179. [Google Scholar] [CrossRef]
- Odze, R.D.; Goldblum, J.; Noffsinger, A.; Alsaigh, N.; Rybicki, L.A.; Fogt, F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod. Pathol. 2002, 15, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, F.D.; Offerhaus, G.J.; Schipper, M.E.; Siersema, P.D.; Vleggaar, F.P.; Oldenburg, B. Endoscopic and pathological aspects of colitis-associated dysplasia. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Shah, S.C.; Ten Hove, J.R.; Torres, J.; Mooiweer, E.; Castaneda, D.; Glass, J.; Elman, J.; Kumar, A.; Axelrad, J.; et al. No Association between Pseudopolyps and Colorectal Neoplasia in Patients with Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 1333–1344. [Google Scholar] [CrossRef]
- Tanaka, A.; Takikawa, H. Geoepidemiology of primary sclerosing cholangitis: A critical review. J. Autoimmun. 2013, 46, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; Saunders, B.P.; Wilkinson, K.H.; Rumbles, S.; Schofield, G.; Kamm, M.A.; Williams, C.B.; Price, A.B.; Talbot, I.C.; Forbes, A. Cancer surveillance in longstanding ulcerative colitis: Endoscopic appearances help predict cancer risk. Gut 2004, 53, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Saunders, B.; Wilkinson, K.; Rumbles, S.; Schofield, G.; Kamm, M.; Williams, C.; Price, A.; Talbot, I.; Forbes, A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004, 126, 451–459. [Google Scholar] [CrossRef]


| Characteristics | Random Group | Target Group | p-Value |
|---|---|---|---|
| Followed-up cases | n = 93 | n = 102 | |
| Neoplasia at RCT | 11 | 9 | |
| No neoplasia at RCT | 82 | 93 | |
| Age, y, mean (SD) | 48.3 (13.4) | 49.8 (14.0) | 0.447 |
| Sex | |||
| Female | 29 (31.2%) | 39 (38.2%) | 0.367 |
| Male | 64 (68.8%) | 63 (61.8%) | |
| Extent of UC | |||
| Total colitis | 63 (67.7%) | 57 (55.9%) | 0.207 |
| Left-sided colitis | 25 (26.9%) | 39 (38.2%) | |
| Others | 5 (5.4%) | 6 (5.9%) | |
| Primary sclerosing cholangitis | 0 (0%) | 0 (0%) | |
| UC duration at RCT | 16.4 ± 6.9 | 15.8 ± 6.5 | 0.523 |
| Smoking history | |||
| Never smoked | 52 (55.9%) | 62 (60.8%) | 0.19 |
| Current smoker | 8 (8.6%) | 4 (3.9%) | |
| Ex-smoker | 9 (9.7%) | 17 (16.7%) | |
| Unknown | 24 (25.8%) | 19 (18.6%) | |
| Medication at RCT | |||
| 5 ASA | 86 (92.5%) | 101 (99.0%) | 0.029 |
| Steroid | 13 (14.1%) | 15 (14.7%) | 1 |
| Apheresis | 12 (12.9% | 20 (19.6%) | 0.247 |
| Immunomodulator | 29 (31.2%) | 26 (25.5%) | 0.427 |
| Anti-TNFα | 5 (5.4%) | 2 (2.0%) | 0.261 |
| Number of biopsies, median (IQR) | |||
| At RCT | 36 (29,37) | 3 (2, 8) | <0.001 |
| After RCT | 3 (1, 6) | 3 (1, 6) | 0.204 |
| Follow-up, y, mean (range) | 8.7 (0.14–10.0) | 8.8 (0.98–10.1) | 0.902 |
| Age | Sex | Smoking | Anti-TNF * | Thiopurine * | Primary Site | Status |
|---|---|---|---|---|---|---|
| 60s | M | Current | − | − | Unknown primary | Dead |
| 50s | M | Current | − | − | Bile duct | Dead |
| 60s | M | Ex | − | − | Parotid gland | Dead |
| 70s | M | Ex | − | − | Lung | Dead |
| 80s | M | Ex | − | − | Lung | Alive |
| 70s | M | Never | − | − | Pancreas | Alive |
| 50s | F | Never | − | + | Breast | Alive |
| 60s | M | Never | − | − | Breast | Alive |
| 70s | M | Never | − | − | Kidney | Alive |
| 80s | F | Unknown | − | − | Kidney | Alive |
| Group | Sex | Age at RCT | Pathology at RCT | Final Pathology | Interval after RCT (years) | Procedure | Location $ and Morphology at CS | Remarks |
|---|---|---|---|---|---|---|---|---|
| Random | Female | 50 s | LGD | CRC (T4N0M0) | 5.6 | Colectomy after ESD | R, 0-IIa | Same location † |
| Random | Female | 40 s | LGD | Intramucosal Ca | 1.5 | Colectomy after EMR | R, 0-IIa+Is | Additional surgery ‡ |
| Random | Male | 60 s | neg | CRC (T1bN0M0) | 4.9 | Colectomy after EMR | T, 0-Isp | Additional surgery ‡ |
| Target | Female | 40 s | HGD | LGD | 0.6 | ESD | D, 0-IIa, 0-IIa | Two synchronous lesions § |
| Target | Male | 40 s | LGD | CRC (T3N0M0) | 7.2 | Colectomy | S, 0-IIa | Progression? §§ |
| Target | Male | 50 s | neg | HGD | 1.8 | Colectomy | R, 0-IIb | |
| Target | Female | 30 s | neg | Intramucosal Ca * | 8.6 | ESD | R, 0-IIb |
| Variable | n | % |
|---|---|---|
| Number of colonoscopies | 1085 | |
| Biopsy method | ||
| Targeted biopsy only | 581 | 59.1% |
| Targeted plus random biopsy | 402 | 40.9% |
| Number of biopsy specimens | ||
| <10 | 1002 | 92.3% |
| 10–19 | 80 | 7.4% |
| 20–33 | 2 | 0.2% |
| >34 | 1 | 0.1% |
| Chromoendoscopy | ||
| Panchromoendoscopy | 50 | 4.6% |
| Specific area | 295 | 27.4% |
| Targeted area only | 169 | 15.7% |
| No dye spray | 563 | 52.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata, K.; Ishihara, S.; Ajioka, Y.; Mitsuyama, K.; Watanabe, K.; Hanai, H.; Kunisaki, R.; Nakase, H.; Matsuda, K.; Iwakiri, R.; et al. Long-Term Follow-Up of Targeted Biopsy Yield (LOFTY Study) in Ulcerative Colitis Surveillance Colonoscopy. J. Clin. Med. 2020, 9, 2286. https://doi.org/10.3390/jcm9072286
Hata K, Ishihara S, Ajioka Y, Mitsuyama K, Watanabe K, Hanai H, Kunisaki R, Nakase H, Matsuda K, Iwakiri R, et al. Long-Term Follow-Up of Targeted Biopsy Yield (LOFTY Study) in Ulcerative Colitis Surveillance Colonoscopy. Journal of Clinical Medicine. 2020; 9(7):2286. https://doi.org/10.3390/jcm9072286
Chicago/Turabian StyleHata, Keisuke, Soichiro Ishihara, Yoichi Ajioka, Keiichi Mitsuyama, Kenji Watanabe, Hiroyuki Hanai, Reiko Kunisaki, Hiroshi Nakase, Keiji Matsuda, Ryuichi Iwakiri, and et al. 2020. "Long-Term Follow-Up of Targeted Biopsy Yield (LOFTY Study) in Ulcerative Colitis Surveillance Colonoscopy" Journal of Clinical Medicine 9, no. 7: 2286. https://doi.org/10.3390/jcm9072286
APA StyleHata, K., Ishihara, S., Ajioka, Y., Mitsuyama, K., Watanabe, K., Hanai, H., Kunisaki, R., Nakase, H., Matsuda, K., Iwakiri, R., Hida, N., Tanaka, S., Takeuchi, Y., Shinozaki, M., Ogata, N., Moriichi, K., Hirai, F., Sugihara, K., Hisamatsu, T., ... Hibi, T. (2020). Long-Term Follow-Up of Targeted Biopsy Yield (LOFTY Study) in Ulcerative Colitis Surveillance Colonoscopy. Journal of Clinical Medicine, 9(7), 2286. https://doi.org/10.3390/jcm9072286







