Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Trial Registration
2.3. Patients
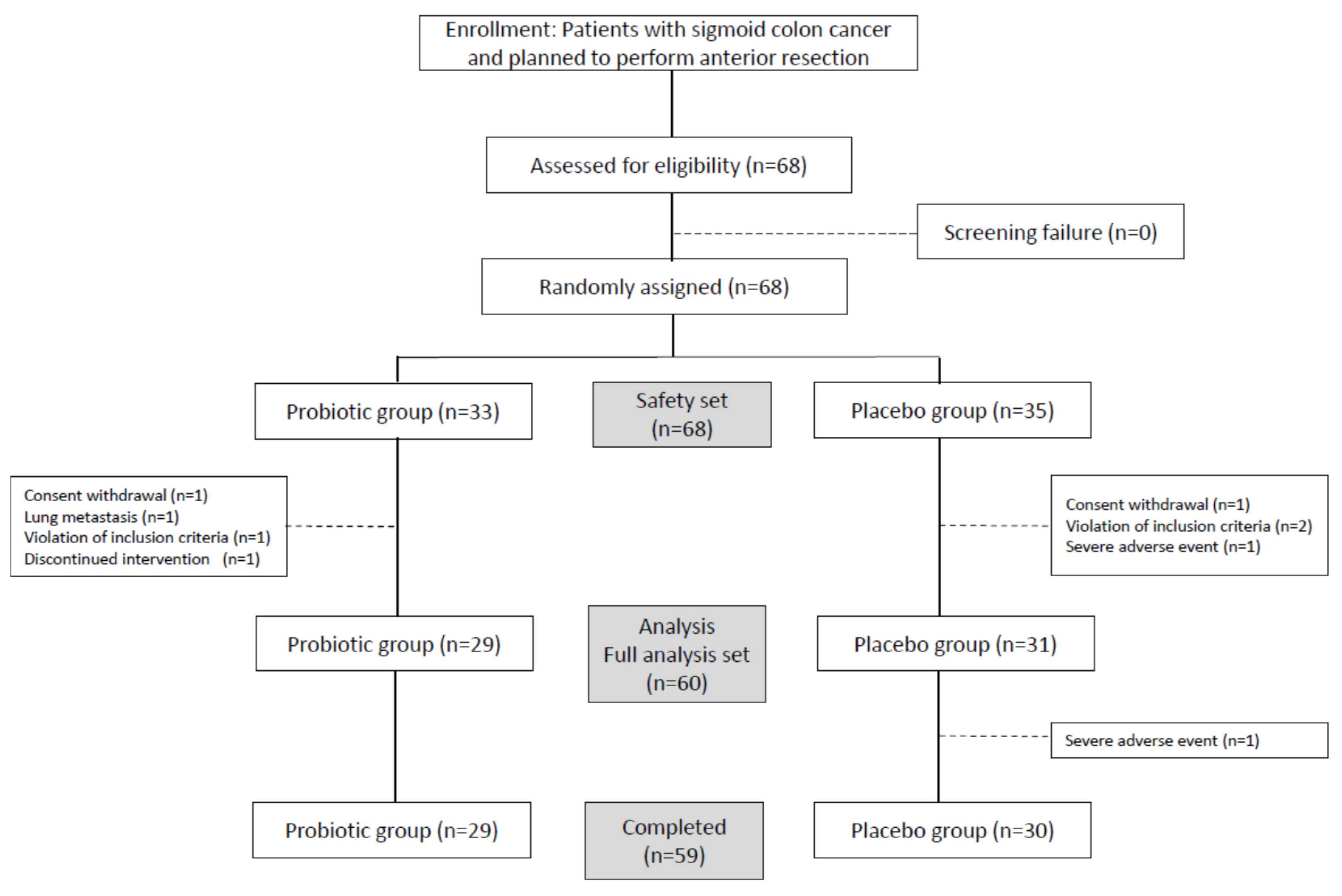
2.4. Randomization and Masking
2.5. Procedure
2.5.1. Intervention and Surgical Resection
2.5.2. Fecal Sample Collection and Total Fecal DNA Extraction
2.5.3. Bacterial Composition Analysis, Next-Generation Sequencing, and Bioinformatics Analysis
2.6. Outcomes
2.7. Statistical Analysis
2.8. Patient and Public Involvement
3. Results
3.1. Patient Characteristics and Postoperative Complications
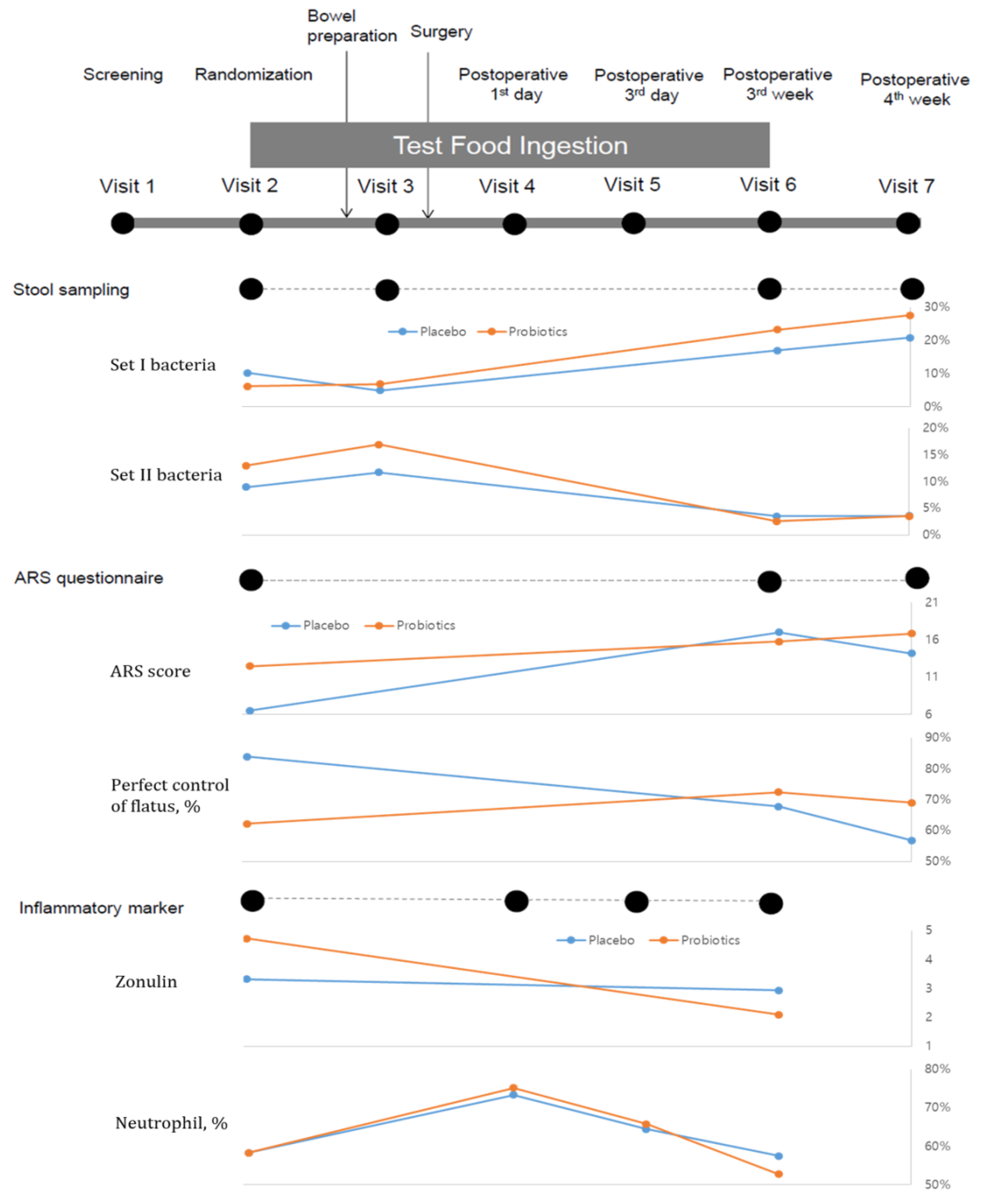
3.2. Changes in Anterior Resection Syndrome
3.3. Changes in Inflammatory Markers
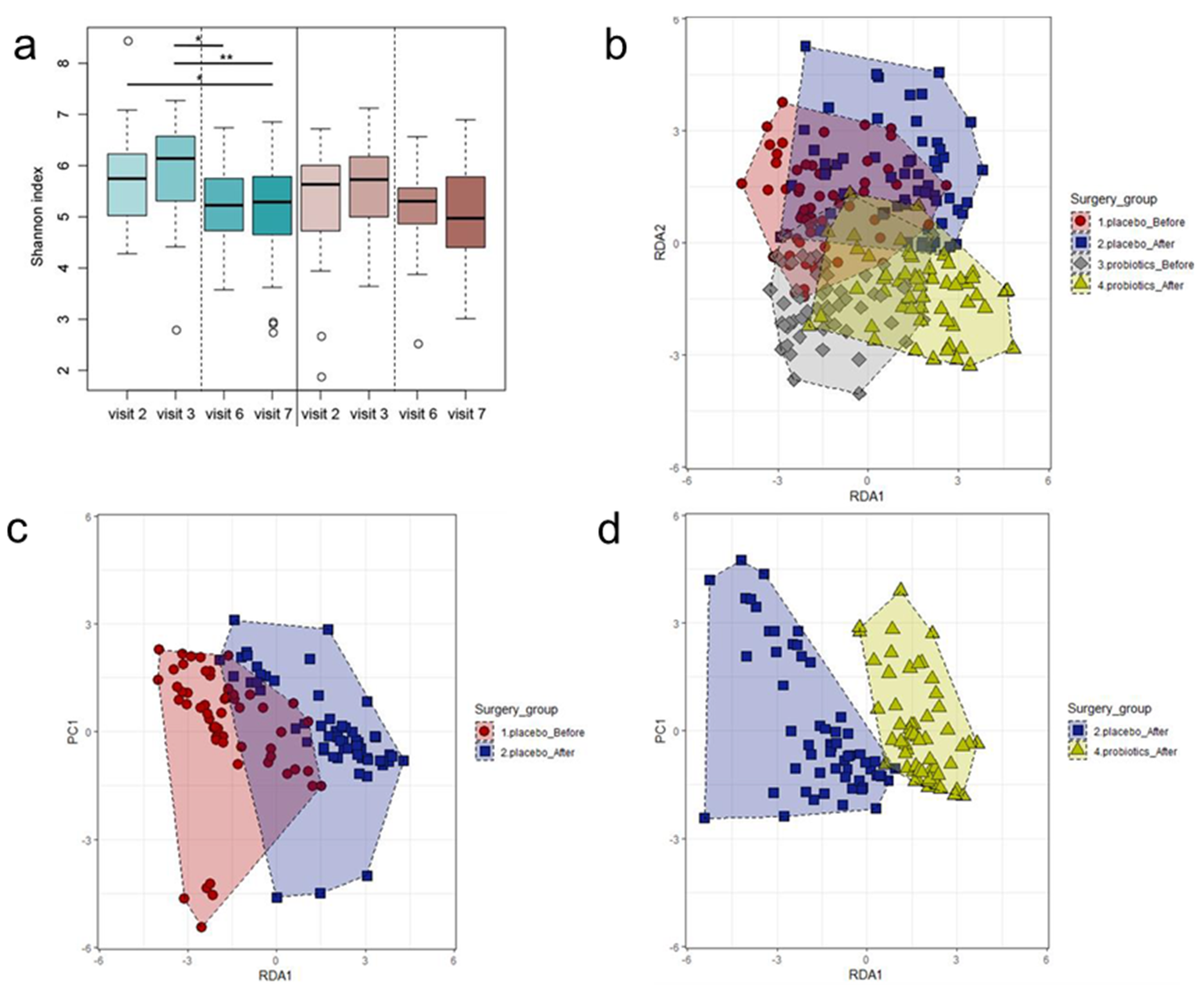
3.4. Changes in Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bryant, C.L.; Lunniss, P.J.; Knowles, C.H.; Thaha, M.A.; Chan, C.L. Anterior resection syndrome. Lancet Oncol. 2012, 13, e403–e408. [Google Scholar] [CrossRef]
- Aschard, H.; Laville, V.; Tchetgen, E.T.; Knights, D.; Imhann, F.; Seksik, P.; Zaitlen, N.; Silverberg, M.S.; Cosnes, J.; Weersma, R.K.; et al. Genetic effects on the commensal microbiota in inflammatory bowel disease patients. PLoS Genet. 2019, 15, e1008018. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Stephens, J.H.; Hewett, P.J. Clinical trial assessing VSL#3 for the treatment of anterior resection syndrome. Anz J. Surg. 2012, 82, 420–427. [Google Scholar]
- Flemer, B.; Lynch, D.B.; Brown, J.M.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Y.; Zhao, L.; Zhao, F.; Feng, J.; Li, S.; Chen, H.; Sun, J.; Zhu, B.; Geng, R.; et al. Gut microbiota in patients after surgical treatment for colorectal cancer. Environ. Microbiol. 2019, 21, 772–783. [Google Scholar] [CrossRef]
- Lederer, A.K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef]
- Ku, H.J.; Lee, J.H. Development of a novel long-range 16S rRNA universal primer set for metagenomic analysis of gastrointestinal microbiota in newborn infants. J. Microbiol. Biotechnol. 2014, 24, 812–822. [Google Scholar] [CrossRef]
- de Vos, W.M. Microbe profile: Akkermansia muciniphila: A conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology 2017, 163, 646–648. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Ose, R.; Hirano, K.; Maeno, S.; Nakagawa, J.; Salminen, S.; Tochio, T.; Endo, A. The ability of human intestinal anaerobes to metabolize different oligosaccharides: Novel means for microbiota modulation? Anaerobe 2018, 51, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Sherafat, S.; Alebouyeh, M.; Moghim, S.; Ahmadi Amoli, H.; Ghasemian-Safaei, H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol. Hepatol. Bed Bench 2018, 11, 101–109. [Google Scholar] [PubMed]
- Juul, T.; Ahlberg, M.; Biondo, S.; Emmertsen, K.J.; Espin, E.; Jimenez, L.M.; Matzel, K.E.; Palmer, G.; Sauermann, A.; Trenti, L.; et al. International validation of the low anterior resection syndrome score. Ann. Surg. 2014, 259, 728–734. [Google Scholar] [CrossRef]
- Kochan, P.; Chmielarczyk, A.; Szymaniak, L.; Brykczynski, M.; Galant, K.; Zych, A.; Pakosz, K.; Giedrys-Kalemba, S.; Lenouvel, E.; Heczko, P.B. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient--is the time right to revise probiotic safety guidelines? Clin. Microbiol. Infect. 2011, 17, 1589–1592. [Google Scholar] [CrossRef]
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.J.; Ziring, D.A. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J. Clin. Gastroenterol. 2013, 47, 437–439. [Google Scholar] [CrossRef]
- Komatsu, S.; Yokoyama, Y.; Nagino, M. Gut microbiota and bacterial translocation in digestive surgery: The impact of probiotics. Langenbecks Arch. Surg. 2017, 402, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.; van Santvoort, H.C.; Renooij, W.; de Smet, M.B.; Boermeester, M.A.; Fischer, K.; Timmerman, H.M.; Ali, U.A.; Cirkel, G.A.; Bollen, T.L.; et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann. Surg. 2009, 250, 712–719. [Google Scholar] [CrossRef]
- Heath, M.L.; Nghiem, P. Merkel cell carcinoma: If no breslow, then what? J. Surg. Oncol. 2007, 95, 614–615. [Google Scholar] [CrossRef]
- Lange, M.M.; den Dulk, M.; Bossema, E.R.; Maas, C.P.; Peeters, K.C.; Rutten, H.J.; Klein Kranenbarg, E.; Marijnen, C.A.; van de Velde, C.J. Cooperative Clinical Investigators of the Dutch Total Mesorectal Excision Trial. Risk factors for faecal incontinence after rectal cancer treatment. Br. J. Surg. 2007, 94, 1278–1284. [Google Scholar] [CrossRef]
- Levack, M.M.; Savitt, L.R.; Berger, D.L.; Shellito, P.C.; Hodin, R.A.; Rattner, D.W.; Goldberg, S.M.; Bordeianou, L. Sigmoidectomy syndrome? Patients’ perspectives on the functional outcomes following surgery for diverticulitis. Dis. Colon Rectum 2012, 55, 10–17. [Google Scholar] [CrossRef]
- Elfeki, H.; Larsen, H.M.; Emmertsen, K.J.; Christensen, P.; Youssef, M.; Khafagy, W.; Omar, W.; Laurberg, S. Bowel dysfunction after sigmoid resection for cancer and its impact on quality of life. Br. J. Surg. 2019, 106, 142–151. [Google Scholar] [CrossRef]
- van Heinsbergen, M.; Janssen-Heijnen, M.L.; Leijtens, J.W.; Slooter, G.D.; Konsten, J.L. Bowel dysfunction after sigmoid resection underestimated: Multicentre study on quality of life after surgery for carcinoma of the rectum and sigmoid. Eur. J. Surg. Oncol. 2018, 44, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113, 4435–4440. [Google Scholar]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed]
- Kosiewicz, M.M.; Zirnheld, A.L.; Alard, P. Gut microbiota, immunity, and disease: A complex relationship. Front. Microbiol. 2011, 2, 180. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 2018, 9, 2872. [Google Scholar] [CrossRef]
- Willmannm, M.; Vehreschild, M.J.G.T.; Biehl, L.M.; Vogel, W.; Dörfel, D.; Hamprecht, A.; Seifert, H.; Autenrieth, I.B.; Peter, S. Distinct impact of antibiotics on the gut microbiome and resistome: A longitudinal multicenter cohort study. BMC Biol. 2019, 17, 76. [Google Scholar]
- McSorley, S.T.; Steele, C.W.; McMahon, A.J. Meta-analysis of oral antibiotics, in combination with preoperative intravenous antibiotics and mechanical bowel preparation the day before surgery, compared with intravenous antibiotics and mechanical bowel preparation alone to reduce surgical-site infections in elective colorectal surgery. BJS Open. 2018, 2, 185–194. [Google Scholar] [PubMed]
- Vo, E.; Nader N Massarweh, N.N.; Chai, C.Y.; Cao, H.S.T.; Zamani, N.; Abraham, S.; Adigun, S.; Awad, S.S. Association of the Addition of Oral Antibiotics to Mechanical Bowel Preparation for Left Colon and Rectal Cancer Resections With Reduction of Surgical Site Infections. JAMA Surg. 2018, 153, 114–121. [Google Scholar] [CrossRef] [PubMed]

| Placebo (n = 31) | Probiotics (n = 29) | p-Value | ||
|---|---|---|---|---|
| Age, years, mean (SD) | 61.03 (7.02) | 60.10 (10.37) | 0.69 * | |
| Sex | Male | 13 (41.94) | 19 (65.52) | 0.07 † |
| Female | 18 (58.06) | 10 (34.48) | ||
| BMI, kg/m2 | 24.04 (3.53) | 24.42 (2.90) | 0.65 * | |
| Exercise | No | 15 (48.39) | 20 (68.97) | 0.31 ‡ |
| Times/week | <3 | 5 (16.13) | 3 (10.34) | |
| ≥3 | 11 (35.48) | 6 (20.69) | ||
| Alcohol | No | 16 (51.61) | 14 (48.28) | 0.56 ‡ |
| Bottles/week | Former use | 2 (6.45) | 6 (20.69) | |
| <1 | 4 (12.90) | 3 (10.34) | ||
| <4 | 3 (9.68) | 3 (10.34) | ||
| ≥4 | 6 (19.35) | 3 (10.34) | ||
| Smoking | None | 16 (51.61) | 14 (48.28) | 0.74 † |
| Ex-smoker | 7 (22.58) | 9 (31.03) | ||
| Smoker | 8 (25.81) | 6 (20.69) | ||
| Comorbidities | 16 (57.1) | 21 (71.4) | 0.227 † | |
| Pathological stage | Stage I | 6 (19.4) | 12 (41.4) | 0.148 |
| Stage II | 8 (25.8) | 6 (20.7) | ||
| Stage III | 15 (48.4) | 11 (42.3) | ||
| Stage IV | 2 (6.5) | 0 (0) | ||
| No. of harvested LNs, mean (SD) | 24.6 (12.6) | 22.6 (7.8) | 0.469 * | |
| Lymphatic invasion | Negative | 19 (61.3) | 22 (75.9) | 0.23 † |
| Positive | 12 (38.7) | 7 (24.1) | ||
| Vascular invasion | Negative | 22 (71.0) | 25 (86.2) | 0.15 † |
| Positive | 9 (29.0) | 4 (13.8) | ||
| Perineural invasion | Negative | 19 (61.3) | 24 (82.8) | 0.65 † |
| Positive | 12 (38.7) | 5 (17.2) |
| Visit 2 vs. 3 | Genus | Placebo-Visit 2 | Placebo-Visit 3 | Ratio | Probiotics-Visit 2 | Probiotics-Visit 3 | Ratio |
| Set I bacteria | Bifidobacterium | 4.388% | 2.810% | −36.0% | 3.638% | 3.225% | −11.3% |
| Akkermansia | 0.859% | 0.229% | −73.3% | 1.178% | 1.338% | 13.5% | |
| Parabacteroides | 1.914% | 0.561% | −70.7% | 0.397% | 0.507% | 27.7% | |
| Veillonella | 0.448% | 0.176% | −60.8% | 0.226% | 1.192% | 428.3% | |
| Lactobacillus | 2.231% | 1.084% | −51.4% | 0.669% | 0.526% | −21.3% | |
| Erysipelatoclostridium | 0.419% | 0.085% | −79.7% | 0.077% | 0.072% | −6.0% | |
| Set II bacteria | Prevotella | 7.368% | 9.650% | 31.0% | 10.286% | 15.469% | 50.4% |
| Alloprevotella | 0.687% | 0.822% | 19.6% | 1.053% | 1.368% | 29.9% | |
| Fusobacterium | 0.174% | 0.215% | 23.3% | 1.176% | 0.041% | −96.6% | |
| Porphyromonas | 0.713% | 1.050% | 47.3% | 0.465% | 0.012% | −97.4% | |
| Visit 3 vs. 6 | Genus | Placebo-Visit 3 | Placebo-Visit 6 | Ratio | Probiotics-Visit 3 | Probiotics-Visit 6 | Ratio |
| Set I bacteria | Bifidobacterium | 2.810% | 6.873% | * 144.6% | 3.225% | 9.484% | ** 194.1% |
| Akkermansia | 0.229% | 2.224% | 870.1% | 1.338% | 4.164% | 211.2% | |
| Parabacteroides | 0.561% | 3.715% | ** 562.6% | 0.507% | 5.836% | * 1051.3% | |
| Veillonella | 0.176% | 2.445% | * 1291.6% | 1.192% | 2.483% | 108.2% | |
| Lactobacillus | 1.084% | 1.195% | 10.2% | 0.526% | 0.875% | 66.2% | |
| Erysipelatoclostridium | 0.085% | 0.541% | * 535.7% | 0.072% | 0.356% | * 393.4% | |
| Set II bacteria | Prevotella | 9.650% | 2.214% | * −77.1% | 15.469% | 2.292% | *** −85.2% |
| Alloprevotella | 0.822% | 0.097% | −88.2% | 1.368% | 0.004% | ** −99.7% | |
| Fusobacterium | 0.215% | 0.917% | 326.3% | 0.041% | 0.227% | 459.2% | |
| Porphyromonas | 1.050% | 0.287% | −72.7% | 0.012% | 0.001% | * −91.0% | |
| Visit 6 vs. 7 | Genus | Placebo-Visit 6 | Placebo-Visit 7 | Ratio | Probiotics-Visit 6 | Probiotics-Visit 7 | Ratio |
| Set I bacteria | Bifidobacterium | 6.873% | 7.339% | 6.8% | 9.484% | 9.961% | 5.0% |
| Akkermansia | 2.224% | 7.041% | 216.7% | 4.164% | 9.256% | 122.3% | |
| Parabacteroides | 3.715% | 4.203% | 13.1% | 5.836% | 3.847% | −34.1% | |
| Veillonella | 2.445% | 0.660% | −73.0% | 2.483% | 2.786% | 12.2% | |
| Lactobacillus | 1.195% | 0.917% | −23.3% | 0.875% | 1.239% | 41.6% | |
| Erysipelatoclostridium | 0.541% | 0.666% | 23.2% | 0.356% | 0.487% | 37.1% | |
| Set II bacteria | Prevotella | 2.214% | 2.562% | 15.7% | 2.292% | 3.287% | 43.4% |
| Alloprevotella | 0.097% | 0.091% | −5.9% | 0.004% | 0.004% | −7.9% | |
| Fusobacterium | 0.917% | 0.620% | −32.4% | 0.227% | 0.237% | 4.4% | |
| Porphyromonas | 0.287% | 0.302% | 5.3% | 0.001% | 0.000% | −79.3% | |
| Visit 2 vs. 7 | Genus | Placebo-Visit 2 | Placebo-Visit 7 | Ratio | Probiotics-Visit 2 | Probiotics-Visit 7 | Ratio |
| Set I bacteria | Bifidobacterium | 4.388% | 7.339% | 67.2% | 3.638% | 9.961% | * 173.8% |
| Akkermansia | 0.859% | 7.041% | 720.1% | 1.178% | 9.256% | * 685.4% | |
| Parabacteroides | 1.914% | 4.203% | 119.6% | 0.397% | 3.847% | * 869.3% | |
| Veillonella | 0.448% | 0.660% | 47.4% | 0.226% | 2.786% | * 1134.4% | |
| Lactobacillus | 2.231% | 0.917% | −58.9% | 0.669% | 1.239% | 85.1% | |
| Erysipelatoclostridium | 0.419% | 0.666% | 58.9% | 0.077% | 0.487% | 535.9% | |
| Set II bacteria | Prevotella | 7.368% | 2.562% | −65.2% | 10.286% | 3.287% | * −68.0% |
| Alloprevotella | 0.687% | 0.091% | −86.7% | 1.053% | 0.004% | * −99.7% | |
| Fusobacterium | 0.174% | 0.620% | 255.5% | 1.176% | 0.237% | −79.9% | |
| Porphyromonas | 0.713% | 0.302% | −57.6% | 0.465% | 0.000% | −100.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.J.; Lee, J.-H.; Kye, B.-H.; Oh, H.-K.; Cho, Y.B.; Kim, Y.-T.; Kim, J.Y.; Sung, N.Y.; Kang, S.-B.; Seo, J.-M.; et al. Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 2181. https://doi.org/10.3390/jcm9072181
Park IJ, Lee J-H, Kye B-H, Oh H-K, Cho YB, Kim Y-T, Kim JY, Sung NY, Kang S-B, Seo J-M, et al. Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Clinical Medicine. 2020; 9(7):2181. https://doi.org/10.3390/jcm9072181
Chicago/Turabian StylePark, In Ja, Ju-Hoon Lee, Bong-Hyeon Kye, Heung-Kwon Oh, Yong Beom Cho, You-Tae Kim, Joo Yun Kim, Na Young Sung, Sung-Bum Kang, Jeong-Meen Seo, and et al. 2020. "Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial" Journal of Clinical Medicine 9, no. 7: 2181. https://doi.org/10.3390/jcm9072181
APA StylePark, I. J., Lee, J.-H., Kye, B.-H., Oh, H.-K., Cho, Y. B., Kim, Y.-T., Kim, J. Y., Sung, N. Y., Kang, S.-B., Seo, J.-M., Sim, J.-H., Lee, J.-L., & Lee, I. K. (2020). Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Clinical Medicine, 9(7), 2181. https://doi.org/10.3390/jcm9072181





