Abstract
Animal studies and the scarce clinical trials available that have been conducted suggest that bioactive surfaces on dental implants could improve the osseointegration of such implants. The purpose of this systematic review was to compare the effectiveness of osseointegration of titanium (Ti) dental implants using bioactive surfaces with that of Ti implants using conventional surfaces such as sandblasted large-grit acid-etched (SLA) or similar surfaces. Applying the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement, the MEDLINE, PubMed Central and Web of Science databases were searched for scientific articles in April 2020. The keywords used were “dental implants”, “bioactive surfaces”, “biofunctionalized surfaces”, and “osseointegration”, according to the question: “Do bioactive dental implant surfaces have greater osseointegration capacity compared with conventional implant surfaces?” Risk of bias was assessed using the Cochrane Collaboration tool. 128 studies were identified, of which only 30 met the inclusion criteria: 3 clinical trials and 27 animal studies. The average STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) and ARRIVE (Animal Research: Reporting of In Vivo Experiments) scores were 15.13 ± 2.08 and 17.7±1.4, respectively. Implant stability quotient (ISQ) was reported in 3 studies; removal torque test (RTT)—in 1 study; intraoral periapical X-ray and microcomputed tomography radiological evaluation (RE)—in 4 studies; shear force (SF)—in 1 study; bone-to-implant contact (BIC)—in 12 studies; and BIC and bone area (BA) jointly—in 5 studies. All animal studies reported better bone-to-implant contact surface for bioactive surfaces as compared to control implants with a statistical significance of p < 0.05. Regarding the bioactive surfaces investigated, the best results were yielded by the one where mechanical and chemical treatment methods of the Ti surfaces were combined. Hydroxyapatite (HA) and calcium–phosphate (Ca–Ph) were the most frequently used bioactive surfaces. According to the results of this systematic review, certain bioactive surfaces have a positive effect on osseointegration, although certain coating biomolecules seem to influence early peri-implant bone formation. Further and more in-depth research in this field is required to reduce the time needed for osseointegration of dental implants.
1. Introduction
The concept of osseointegration was introduced by Brånemark et al. in 1969 [1] to be later defined by Albrektsson et al. as “a direct structural and functional connection between living bone and the surface of a load-bearing titanium (Ti) implant” [2]. Machined dental implant surfaces were the starting point [3] and, since then, different modifications to Ti surfaces have been tested in an attempt to improve the biological conditions and properties of osseointegration. Ti is a low bioactivity biomaterial, which is why different surface treatments have been developed aimed at improving osseointegration capacity [4].
Implant surface modification is one of the most novel and productive research fields acquiring relevance in the search for a system that meets the ideal functional and biological goals [5,6]. Surface topography plays a crucial role in osseointegration and it is known that cell response can be modulated by adapting implant surface texture. Rough micro-, submicro- and nanoscale topographies can be very effective in promoting osseointegration [7,8,9]. Some authors have reported that sandblasted and acid-etched (SLA) implants achieve osseointegration even in the absence of primary stability [8].
The main systems that have been developed and are used to achieve adequate implant surface roughness are sandblasting, acid etching, anodizing, and titanium plasma spraying [10]. Other strategies that have been proposed for the improvement of titanium surface osseointegration include coatings with hydroxyapatite, bioactive glasses, bisphosphonates, or collagen [11,12,13,14].
The bio-functionalization of a certain material consists of a modification of the physicochemical properties of its surface, which would allow an improvement in the biological response of an organism when it comes into contact with it.
Currently, most research is focused on antibacterial and antiadhesive surfaces which include both materials that are able to reduce bacterial adhesion on implant surfaces and active antibacterial materials with a defined antimicrobial activity [15,16].
Bioactive surfaces are those capable of achieving a faster and better quality of osseointegration with the aim of solving such problems as poor bone quality or reducing waiting times for prosthetic loading [17]. Figure 1 provides a graph demonstrating the increase in publications regarding this topic in the last twenty years (elaborated with the data from the US National Library of Medicine).
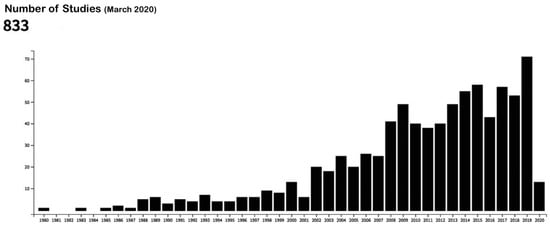
Figure 1.
Publications in the US National Library of Medicine database with the following keywords: “bioactive surfaces” and “dental implants.” Source: US National Library of Medicine [18].
Systematic reviews are an essential tool to synthesize the scientific information available, enhancing the validity of the findings of individual studies while at the same time detecting areas of uncertainty that require further research. Keeping this in mind, the purpose of this work was to conduct a systematic review of the literature comparing the effectiveness of osseointegration of bioactive dental implant surfaces with that of implants without such surface morphology.
2. Methods
We performed study selection according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guidelines for reporting systematic reviews and meta-analyses [19].
2.1. Protocol
The search strategy was conducted using the population, intervention, comparison, and outcome (PICO) framework based on the following question: “Do bioactive dental implant surfaces have greater osseointegration capacity compared with conventional implant surfaces?”
To answer this question, a sample population group of patients undergoing treatment with Ti dental implants with bioactive surfaces was selected. Controls were patients who were treated with conventional implant surfaces. The outcomes reviewed in the literature were the BIC (bone-to-implant contact), BA (bone area), RTT (removal torque test), RE (radiological evaluation), SF (shear force), or ISQ (implant stability quotient) values reported in the different selected studies.
2.2. Search Method for the Identification of Studies
A search in the MEDLINE, PubMed Central, and Web of Science electronic databases was conducted in April 2020 to identify relevant scientific articles. The search terms used were “Ti dental implants”, “bioactive surfaces”, “biofunctionalized surfaces”, and “osseointegration.”
2.3. Inclusion and Exclusion Criteria
Inclusion criteria:
- (a)
- Studies published in English.
- (b)
- Studies with Ti implants.
- (c)
- Human studies with bioactive titanium implant placement procedures.
- (d)
- Animal studies with bioactive titanium implant placement procedures.
- (e)
- Studies evaluating BIC (bone-to-implant contact), BA (bone area), ISQ (implant stability quotient), RTT (removal torque test), RE (intraoral periapical X-ray and microcomputed tomography radiological evaluation), or SF (shear force).
Exclusion criteria:
- (a)
- Studies using conventional Ti implants (SLA or similar surfaces).
- (b)
- In vitro studies.
- (c)
- Narrative reviews and systematic reviews.
- (d)
- Case studies.
- (e)
- Irrelevant (didactics, Delphi surveys…) and duplicate studies and those that did not meet the established inclusion criteria.
2.4. Data Extraction and Analysis
Studies that made no reference to the research question were removed and the titles and abstracts of the articles selected were obtained and entered in an Excel spreadsheet. Two reviewers (N.L.-V. and A.L.-V.) selected the titles and abstracts independently. Discrepancies in terms of study inclusion were discussed between the two mentioned reviewers until consensus was reached. Subsequently, full texts of the selected studies were obtained for their review and inclusion.
2.5. Risk of Bias (RoB) of Included Articles
The Cochrane Collaboration, London, UK, tool was used to assess methodology of the scientific evidence in all the selected studies as previously described [20].
2.6. Quality of the Reports of the Included Studies
This was assessed according to the modified STROBE statement (STrengthening the Reporting of OBservational studies in Epidemiology) (Table 1) [21] and ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) (Table 2) [22], which include a total of 22 items. Each item was assessed by reviewers N.L.-V. and A.L.-V. who attributed scores of 0 (not reported) or 1 (reported) carrying out a complete count of all the studies included.

Table 1.
Checklist of the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) criteria reported in the human studies.

Table 2.
Checklist of the ARRIVE (Animal Research: Reporting of In Vivo Experiments) criteria reported in the included studies.
3. Results
3.1. Characteristics of the Studies
By April 2020, a total of 128 studies had been gathered and subsequently assessed by the reviewers. From these, 92 studies were removed due to their being in vitro trials, duplicates, systematic reviews, or irrelevant, leaving a total of 30 studies: 27 were carried out on animals [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] and 3 on humans [23,24,25] (Figure 2 “Flowchart”). Table 3 and Table 4 provide a general description of the details of each study. Table S1 (PRISMA Checklist), describes section/items and pages.
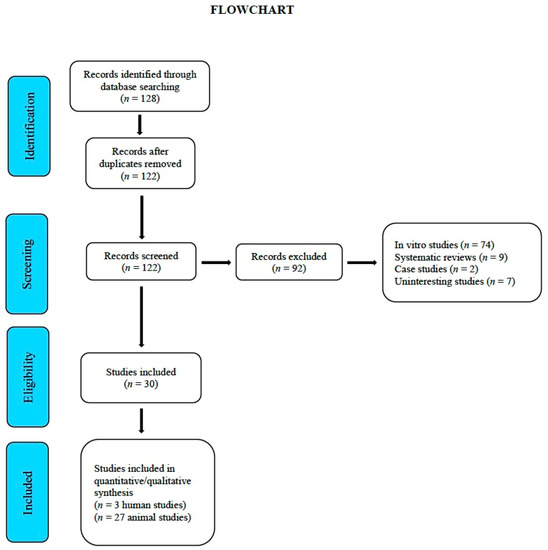
Figure 2.
Flowchart.

Table 3.
Animal studies.

Table 4.
Human studies.
3.2. ISQ, BIC, BA, RTT, RE, and SF Information
Of the clinical studies, only Gursoytrak and Ataoglu reported ISQ, finding no significant difference between Ti surfaces and bioactive (alkali-modified) surfaces after twelve weeks [23]; Malchiodi and colleagues [24] reported the BIC value by means of radiological analysis of the bone-implant interface in three biopsies in CaP-coated Ti implants; Mistry and colleagues [25] compared two modified Ti surfaces, one with HA and the other with bioactive glass, reporting bone-implant contact values obtained from CT scans (no significant differences).
In animal studies, ISQ was reported by 2 studies [26,27], both of which showed an increase when HA and alumina bioactive surfaces were used. BIC was reported in 11 studies [28,29,30,32,33,35,36,37,38,39,40,42,43,44,45,46,49,50,51]; BIC and BA together—5 studies [28,32,35,36,39]; RTT was reported in a study using rat tibiae where machined Ti surfaces and HA coated surfaces were compared [48]; RE (microcomputed tomography) is reported in 2 studies [31,34], SF—in one study [47].
3.3. Synthesis of Included Studies
Thirteen studies used rabbits as animal models [23,27,30,31,32,37,38,39,43,45,48,49], 5—pigs [28,34,41,44,51], 4—dogs [29,46,47,52], 3—rats [33,35,42], 2—goat and non-human primates [36,50]. Sixteen modifications of the Ti surface were used, the most used bioactive surface being HA [27,29,36,44,46,49]. In humans, the worst results were reported by Malchiodi and colleagues who studied a bioactive surface of CaP. The study of Germanier and colleagues [51] who compared SLA surfaces (sandblasted large-grit acid-etched) with surfaces modified by a bioactive peptide found significant differences in the extent of BIC during the early stages of bone regeneration (RGD (Arg–Gly–Asp)-SLA 61.68 ± 4.21, SLA 43.62 ± 10.79), reporting the highest statistical significance (p < 0.001) in terms of peri-implant bone growth among all the included studies. All animal studies [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] reported an increase in peri-implant bone formation when using the surfaces studied, as compared to control surfaces (p > 0.05), 6 of them reporting the best results (p < 0.01) in the formation of peri-implant bone in the surfaces studied [30,31,33,34,36,45].
3.4. Risk of Bias (RoB Cochrane Collaboration’s Tool)
In the studies considered is shown in Figure 3 and Figure 4. Among the clinical trials, only one [23] met the blinding of participants and personnel and the blinding of outcome assessment criteria. The random sequence generation and allocation concealment domains were not met in any of the clinical trials considered.
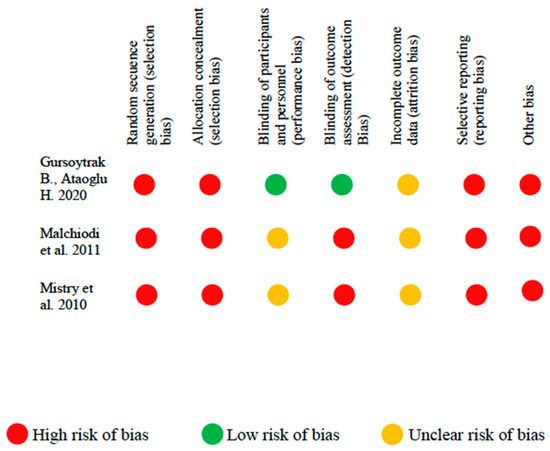
Figure 3.
Risk of bias in clinical studies.
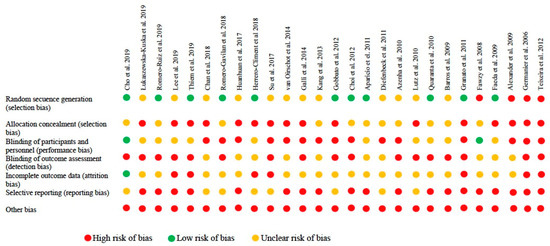
Figure 4.
Risk of bias in animal studies.
Among the animal studies, 46% met the random sequence generation domain and only 2 [26,48] met the blinding of participants and personnel criteria.
The average STROBE and ARRIVE scores were 15.13 ± 2.08 and 17.7 ± 1.4, respectively. According to the ARRIVE checklist, the study by Mistry et al. obtained the highest scores [25]; item 11 (accommodation and handling of animals) was only reported in 5 studies [26,27,28,29,30]; items 19, 20, and 21 (3Rs reported, adverse events, and study limitations) were not reported in any of the included studies. The maximum scores were achieved in the studies by Cho et al., 2019, Łukaszewska-Kuska et al., 2019, Romero-Ruiz et al., 2019, and Thiem et al., 2019 [26,27,28,29]. As regards the STROBE statement checklist, items 9 (bias), 14 (descriptive data), 19 (limitations), 20 (interpretation), and 21 (generalizability) were not reported in any of the studies (Table 3 and Table 4).
4. Discussion
The main purpose of implant surface modifications is to modulate the host tissue and favor osseointegration. This review assessed the capacity of improvement of osseointegration of bioactive surface modifications as compared to the conventional titanium surface (SLA or similar surfaces). Although a large part of research in implant dentistry is currently focused on the study of bioactive surfaces, only three studies based on human trials were found: one compared the ISQ of two types of implants, with and without a bioactive surface [23]; another studied a series of calcium phosphate (CaP)-coated Ti implants [24]; and the third study analyzed the effectiveness of hydroxyapatite and bioactive glass-coated Ti implants [25]. One of the reasons for the scarcity of this type of studies could be their ethical implications, since, for obvious ethical reasons, it is not possible to obtain gold standard histological samples (biopsies) to analyze bone formation around implants; however, one of the included clinical trials [24] studied through biopsy a series of samples obtained from patients. Nevertheless, even though bone-to-implant contact can be assessed using techniques such as electron microscopy [53,54], researchers have established histomorphometric analysis as the most widely used method in most studies [55,56].
The rest of the selected studies [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are based on animal research using different animal models and a variety of Ti surface coatings, with HA and Ca–Ph being the most common [27,28,29,36,46,52]. These six studies provided remarkable results as regards osseointegration, with conclusive findings such as activation of osteoblastic activity and healing time through the increase of bone-to-implant interaction during the first 2 months after placement [29,49].
Hydroxyapatite (HA) is a non-inflammatory, non-toxic, and non-immunogenic material with osteoconductive and bioactive properties [57]. HA coating has been proposed for implant surface modification to promote bone healing and osseointegration, which would allow early functional loading. Nevertheless, there are currently no standard manufacturing guidelines for HA deposition on implant surfaces [58]. In the past, multiple failures with HA-coated implants were reported [59]; however, it seems that such failures could be due to poor quality coatings and product crystallization [60].
A systematic review conducted by Qadir and colleagues [57] affirms that the topography and chemical properties of amorphous HA coating surfaces influence cell behavior and ion-substituted HA coatings significantly increase cell adhesion, but can have a cytotoxic effect that slows the growth of the cells that are attached to the coating’s surface areas, however, some authors question the effectiveness of hydrophilic surfaces and HA-coated surfaces in terms of osteoblastic activation [29].
The healing times reported in the different studies included varied widely from 1 to 13 weeks [31,39]; in a study using goats, van Oirschot and colleagues [36] found that at 4 weeks, HA-coated Ti had an osseointegrating effect (BIC and BA values) superior to shot blasting/acid etching (grit-blasted/acid-etched implants); Faeda and colleagues [49] studied Ti surfaces modified through laser ablation and subsequently coated with HA measuring implant extraction force using RTT. The average removal torque was higher in HA-coated implants obtaining significant values (p = 0.05) at 4, 8, and 12 weeks and comparing them with control implants (only laser ablation implants and machined surface implants). It is also worth noting that Mistry and colleagues [25] found similar results between HA-coated implants and implants covered with bioactive glass in their clinical trial.
Another modification of the Ti surface coating used in the different studies included in this systematic review was calcium–phosphate (Ca–Ph) [23,39,42,45]. Bioactive Ca and Ph-based ceramics have received considerable attention over the years, leading to highly osteoconductive coatings [45,61]. In a study in rat tibiae, Diefenbeck and colleagues [42], using Ca–Ph-coated Ti implants, found a high rate of early osseointegration compared to conventional surface Ti implants. It should be noted that the best results in terms of BIC at 3, 4, and 8 weeks, were obtained with surfaces modified with Ca–Ph [45] despite the fact that certain authors exclusively attribute osteoconductive properties to it [62].
The highest statistical significance was found in the study by Germanier and colleagues [51] who compared conventional surfaces of Ti (SLA) and surfaces modified by a double peptide, RDG (Arg–Asp–Gly) and RGD (Arg–Gly–Asp). At 2 weeks, RGD-coated implants yielded significantly higher percentages of bone-to-implant contact than controls (p < 0.001). This RGD peptide could have osteogenic properties that correlated with effects that would alter cell binding and dissemination, generating a more differentiated cell morphology [63].
A large part of the included studies compared the surfaces studied with SLA or similar surfaces [26,27,28,29,30,33,34,41,46,51]. Bioactive and biofunctional concepts were unclear in the included studies, and only 9 of them [26,31,33,38,40,43,44,47,51] clearly specified biofunctionalization of the Ti surface.
New methods of surface preparation are currently under constant investigation. The successful osseointegration of dental implants depends on the amount of bone that is in direct contact with the implant surface. Destruction of the bone-implant-contact area (BIC) could lead to implant failure. Early osseointegration is influenced by the roughness and coatings of the Ti surfaces [64,65], however, infection is frequently the cause of failure of dental implants [66]. Implant infections are generally associated with Gram-negative periodontal pathogens (Porphyromonas gingivalis, Prevotella intermedia/Prevotella nigrescens, and Actinobacillus actinomycetemcomitans); various antimicrobial and antibiotic peptides are proposed in order to solve these drawbacks [67]. In contact with air, Ti undergoes an oxidation process that is of major importance in the osseointegration process; however, the oxide on the Ti surface includes a large number of impurities, which would hinder the osseointegration process [68].
Plasma biology is a new interdisciplinary research area that is currently being used to functionalize surfaces and improve their biocompatibility [69]. The relationship between plasma treatment of Ti surfaces and differentiation of bone tissue has been reported in several studies [70].
The objective of current technologies [71] is to generate thin plasmas using small and easy-to-use devices. Ujino and colleagues [72] showed that osteogenic adhesion and differentiation increased in the cells grown on plasma-treated Ti discs as compared to those raised on untreated discs. Their device uses piezoelectric mechanical resonance to amplify electrical energy and generate high voltage. In this way, it ionizes the surrounding atmospheric air and produces plasma. Conventional plasma devices require vacuum and processing is limited and expensive. In contrast, the Piezobrush® PZ2 device (Relyon Plazma GmbH, Regensburg, Germany) used by Ujino and colleagues is compact and suitable for use in dental practices.
Because of its decontaminating properties, argon plasma (Ar), which is widely used as a coagulant in digestive surgery [73], has been proposed by certain authors [52,74,75,76,77] to improve early integration of Ti implants due to its decontaminating properties.
At low temperature, the Ar-oxygen plasma could be highly effective in cleaning surfaces, eliminating chemical residues, contaminants, and impurities in Ti, and producing an activating effect on the surface of the implant, which would improve cell proliferation and adhesion and, as a consequence, mineralization [74]; however, the devices used are expensive and work at high temperatures or low pressures, making them difficult to use in a regular dental office. Teixeira and colleagues [52] proposed its use in a dental office immediately before implantation through the use of non-thermal plasmas applied by means of manageable devices (KinPenTM® device, INP, Greifswald, Germany) which allow the modification of the Ti surface at room temperature. Similarly, in a study in Beagle dogs, Giro and colleagues [78] reported significant effects in implants treated with low-temperature Ar plasma. Therefore, the low-temperature Ar plasma could be used directly in a dental office both for surface disinfection and for direct application, in root canal disinfection, or in other surgical techniques that are conducted immediately prior to implantation [79].
On the other hand, the experimental rodent models (rats and rabbits) used in many of the studies included, as well as the choice of implant location (tibia and femur), are not considered adequate models for the extrapolation of results to humans, among other reasons, because they lack cortical bone remodeling and because they stop growing much later than other mammals [80]. Additionally, the bones of rabbits, which are the most commonly used species in the studies included in this systematic review, are the most dissimilar in structure to human bone [81]. While none of the species meets all the requirements of an ideal model, understanding the differences in bone architecture and remodeling among the different experimental animal species could help researchers to select an adequate species for a specific research question.
Nonetheless, this systematic review is not free from limitations as regards number, quality, and methodology of the studies included, both animal and human. First, only three clinical trials were found [23,24,25], which is insufficient to confirm the results they describe as significant in humans. Second, the ecological fallacy in the interpretation of results due to the heterogeneity in the characteristics of the studies (methodological diversity) and also to the population samples used in each of them (clinical diversity) [82]. And, third, the concepts of bioactivation and biofunctionalization of surfaces are not always clear in the different studies included in this systematic review, leading to great heterogeneity of results.
This heterogeneity of results could be due to the surface coating process and the different methods used to evaluate the bone–implant surface contact: mechanical, histomorphometric, and radiological (ISQ, RTT, RE, SF, BIC, and BA). Although Yang and colleagues [83] found greater osseointegration and bone apposition using an electrochemical process in the modification of surfaces, the limited information provided by most of the studies makes it difficult to determine the best methods of surface modification. Finally, another important aspect regarding the limitations of our study was the publication bias, and therefore we are aware that our conclusions can only be applied to the sample of included studies.
Therefore, to determine the effect of bioactive and biofunctionalized surfaces on implant osseointegration, it is necessary to reduce the risk for bias of the studies, eliminate confounding factors, and establish a clear definition of adequate parameters, all of this aimed at obtaining the results that might be useful in a wide range of clinical applications so that scientific evidence may support the practice of clinical dentistry.
The purpose of this review was to assess the impact of bioactive surface modification on implant osseointegration. However, it proved difficult to conclude that such modifications might be beneficial in terms of osseointegration, mainly because the risk of bias was high in most of the studies included and their analysis was complicated and problematic, hampering the interpretation of results.
5. Conclusions
The effect of bioactive modifications of dental implant surfaces is not always beneficial for osseointegration, although certain biomolecules used for coating seem to influence early peri-implant bone formation. All the materials proposed in the different studies included in this systematic review to modify the implant surfaces of Ti and improve its osseointegration have different advantages and limitations in terms of mechanical properties, biocompatibility, and osseointegration potential. Therefore, long-term clinical trials are required to validate the success of implants using this type of biomolecular coating. On the other hand, it should be noted that the results obtained using animal models cannot always be extrapolated to human clinical reality.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/7/2047/s1, Table S1: PRISMA Checklist.
Author Contributions
Study concept and design, N.L.-V., S.H.-H., and A.L.-V.; data collection (literature search and study selection), N.L.-V., J.F.-F., and J.M.R.; data analysis and interpretation (literature), J.F.F., B.M.d.S.; drafting of the manuscript, N.L.-V.; A.L.-V.; critical revision of the manuscript for important intellectual content, A.L.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BIC | bone implant contact |
| BA | bone area |
| RTT | removal torque test |
| RE | radiological evaluation |
| SF | shear force |
| ISQ | implant stability quotient |
| HA | hydroxyapatite |
| SiO2 | silicon dioxide |
| Sr | strontium |
| SLA | sandblasted with long-grit corundum followed by acid etching with sulfuric and hydrochloric acid |
| Al2O3 | aluminum oxide |
| Ca | calcium |
| Ta | tantalum |
| Ti | titanium |
| P | phosphorus |
| Ar | argon |
| P2O5 | phosphorus oxide |
| CaO | calcium oxide |
| NaOH | sodium hydroxide |
| CaP | calcium phosphate |
References
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Sennerby, L. State of the art in oral implants. J. Clin. Periodontol. 1991, 18, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, A.; Omar, M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titaniun oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, 515–527. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef]
- Lu, X.; Xiong, S.; Chen, Y.; Zhao, F.; Hu, Y.; Guo, Y.; Wu, B.; Huang, P.; Yang, B. Effects of statherin on the biological properties of titanium metals subjected to different surface modification. Colloids Surf. B Biointerfaces 2020, 188, 110783. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Verardi, S.; Swoboda, J.; Rebaudi, F.; Rebaudi, A. Osteointegration of Tissue-Level Implants with Very Low Insertion Torque in Soft Bone. Implant. Dent. 2018, 27, 5–9. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; McLachlan, T.; Cai, Y.; Hyzy, S.L.; Schneider, J.M.; Schwartz, Z.; Sandhage, K.H.; Boyan, B. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials 2012, 33, 8986–8994. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Towse, R. The osseointegration properties of titanium implants with hydroxyapatite submicron-scale features in the rabbit tibia. Int. J. Periodontics Restor. Dent. 2014, 34, e18–e25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez-Sastre, S.; Gonzalo-Orden, J.M.; Altónaga, J.A.; Orden, M.A. Coating titanium implants with bioglass and with hydroxyapatite. A comparative study in sheep. Int. Orthop. 1998, 22, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Oh, T.-J.; Bae, T.-S.; Lee, M.-H.; Soh, Y.; Kim, B.-I.; Kim, H.S. Effect of Bisphosphonates on Anodized and Heat-Treated Titanium Surfaces: An Animal Experimental Study. J. Periodontol. 2011, 82, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Sverzut, A.T.; Crippa, G.E.; Morra, M.; De Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Effects of type I collagen coating on titanium osseointegration: Histomorphometric, cellular and molecular analyses. Biomed. Mater. 2012, 7, 35007. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed. Nanotechnol. Boil. Med. 2011, 7, 22–39. [Google Scholar] [CrossRef]
- Stanford, C. Surface modifications of dental implants. Aust. Dent. J. 2008, 53, S26–S33. [Google Scholar] [CrossRef]
- US National Library of Medicine. Available online: https://www.nlm.nih.gov (accessed on 3 April 2020).
- Hutton, B.; Catalá-López, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Sterne, J.A.C. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2006; Chapter 8; Available online: www.cochrane-handbook.org (accessed on 30 March 2020).
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Stadlinger, B.; Pourmand, P.; Locher, M.C.; Schulz, M.C. Systematic review of animal models for the study of implant integration, assessing the influence of material, surface and design. J. Clin. Periodontol. 2012, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gursoytrak, B.; Ataoglu, H. Use of resonance frequency analysis to evaluate the effects of surface properties on the stability of different implants. Clin. Oral Implant. Res. 2019, 31, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, L.; Ghensi, P.; Cucchi, A.; Trisi, P.; Szmukler-Moncler, S.; Corrocher, G.; Gerosa, R. Early bone formation around immediately loaded FBR-coated implants after 8, 10 and 12 weeks: A human histologic evaluation of three retrieved implants. Minerva Stomatol. 2011, 60, 205–216. [Google Scholar] [PubMed]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-B.; Jung, S.Y.; Park, C.Y.; Kang, H.K.; Yeo, I.L.; Min, B.-M. A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces. Materials 2019, 12, 3400. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hedzelek, W.; Dorocka-Bobkowska, B. Effects of a hydroxyapatite coating on the stability of endosseous implants in rabbit tibiae. Dent. Med. Probl. 2019, 56, 123–129. [Google Scholar] [CrossRef]
- Romero-Ruiz, M.M.; Gil, F.; Ríos-Santos, J.V.; Lázaro-Calvo, P.; Ríos-Carrasco, B.; Herrero-Climent, M. Influence of a Novel Surface of Bioactive Implants on Osseointegration: A Comparative and Histomorfometric Correlation and Implant Stability Study in Minipigs. Int. J. Mol. Sci. 2019, 20, 2307. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.-M.; Ben Amara, H.; Lee, Y.-M.; Lim, Y.-J.; Kim, H.-Y.; Koo, K.-T. Bone healing dynamics associated with 3 implants with different surfaces: Histologic and histomorphometric analyses in dogs. J. Periodontal Implant. Sci. 2019, 49, 25–38. [Google Scholar] [CrossRef]
- Thiem, D.; Adam, M.; Ganz, C.; Gerber, T.; Kämmerer, P. The Implant Surface and Its Role in Affecting the Dynamic Processes of Bone Remodeling by Means of Distance Osteogenesis: A Comparative in vivo Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 133–140. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Lew, W.-Z.; Lu, E.; Loretz, T.; Lu, L.; Lin, C.-T.; Feng, S.-W. An evaluation of the biocompatibility and osseointegration of novel glass fiber reinforced composite implants: In vitro and in vivo studies. Dent. Mater. 2018, 34, 470–485. [Google Scholar] [CrossRef]
- Romero-Gavilan, F.; Araújo-Gomes, N.; Sanchez-Perez, A.M.; García-Arnáez, I.; Elortza, F.; Azkargorta, M.; De Llano, J.J.M.; Carda, C.; Gurruchaga, M.; Suay, J.; et al. Bioactive potential of silica coatings and its effect on the adhesion of proteins to titanium implants. Colloids Surf. B Biointerfaces 2017, 162, 316–325. [Google Scholar] [CrossRef]
- Huanhuan, J.; PengJie, H.; Sheng, X.; Binchen, W.; Li, S. The effect of strontium-loaded rough titanium surface on early osseointegration. J. Biomater. Appl. 2017, 32, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Climent, M.; Ruiz, M.M.R.; Calvo, P.L.; Ríos-Santos, J.V.; Pérez, R.; Gil Mur, F.J. Effectiveness of a new dental implant bioactive surface: Histological and histomorphometric comparative study in minipigs. Clin. Oral Investig. 2017, 22, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Komasa, S.; Li, P.; Nishizaki, M.; Chen, L.; Terada, C.; Yoshimine, S.; Nishizaki, H.; Okazaki, J. Synergistic effect of nanotopography and bioactive ions on peri-implant bone response. Int. J. Nanomed. 2017, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, B.A.J.A.; Meijer, G.J.; Bronkhorst, E.M.; Närhi, T.; Jansen, J.A.; Beucken, J.J.V.D. Comparison of different surface modifications for titanium implants installed into the goat iliac crest. Clin. Oral Implant. Res. 2014, 27, e57–e67. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Andersson, M.; Wennerberg, A.; Jimbo, R. Osteoconductive Potential of Mesoporous Titania Implant Surfaces Loaded with Magnesium: An Experimental Study in the Rabbit. Clin. Implant. Dent. Relat. Res. 2014, 17, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, O.B.; Min, S.-K.; Jung, S.Y.; Jang, D.H.; Kwon, T.-K.; Min, B.-M.; Yeo, I.L. The effect of the DLTIDDSYWYRI motif of the human laminin α2 chain on implant osseointegration. Biomaterials 2013, 34, 4027–4037. [Google Scholar] [CrossRef]
- Gobbato, L.; Arguello, E.; Sanz-Martín, I.; Hawley, C.E.; Griffin, T.J. Early Bone Healing Around 2 Different Experimental, HA Grit-Blasted, and Dual Acid-Etched Titanium Implant Surfaces. A Pilot Study in Rabbits. Implant. Dent. 2012, 21, 454–460. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, H.-J.; Jang, J.-U.; Yeo, I.L. Comparison Between Bioactive Fluoride Modified and Bioinert Anodically Oxidized Implant Surfaces in Early Bone Response Using Rabbit Tibia Model. Implant. Dent. 2012, 21, 124–128. [Google Scholar] [CrossRef]
- Aparicio, C.; Padrós, A.; Gil, F. In vivo evaluation of micro-rough and bioactive titanium dental implants using histometry and pull-out tests. J. Mech. Behav. Biomed. Mater. 2011, 4, 1672–1682. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Mückley, T.; Schrader, C.; Schmidt, J.; Zankovych, S.; Bossert, J.; Jandt, K.D.; Faucon, M.; Finger, U. The effect of plasma chemical oxidation of titanium alloy on bone-implant contact in rats. Biomaterials 2011, 32, 8041–8047. [Google Scholar] [CrossRef]
- Azenha, M.R.; Peitl, O.; Barros, V.M.D.R. Bone response to biosilicates with different crystal phases. Braz. Dent. J. 2010, 21, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Srour, S.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of titanium implants with a biomimetic active peptide (P-15) promotes early osseointegration. Clin. Oral Implant. Res. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Quaranta, A.; Iezzi, G.; Scarano, A.; Coelho, P.G.; Vozza, I.; Marincola, M.; Piattelli, A. A Histomorphometric Study of Nanothickness and Plasma-Sprayed Calcium–Phosphorous-Coated Implant Surfaces in Rabbit Bone. J. Periodontol. 2010, 81, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.R.M.; Júnior, A.B.N.; Papalexiou, V.; De Souza, S.L.S., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.M. Effect of biofunctionalized implant surface on osseointegration: A histomorphometric study in dogs. Braz. Dent. J. 2009, 20, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Granato, R.; Marin, C.; Gil, J.N.; Dodson, T.B.; Suzuki, M.; Coelho, P.G.; Chuang, S.-K. Thin Bioactive Ceramic-Coated Alumina-Blasted/Acid-Etched Implant Surface Enhances Biomechanical Fixation of Implants: An Experimental Study in Dogs. Clin. Implant. Dent. Relat. Res. 2011, 13, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.S.; Amer, M. An in vitro and in vivo evaluation of bioactive titanium implants following sodium removal treatment. Dent. Mater. 2009, 25, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Faeda, R.S.; Tavares, H.S.; Sartori, R.; Guastaldi, A.C.; Marcantonio, E. Biological Performance of Chemical Hydroxyapatite Coating Associated with Implant Surface Modification by Laser Beam: Biomechanical Study in Rabbit Tibias. J. Oral Maxillofac. Surg. 2009, 67, 1706–1715. [Google Scholar] [CrossRef]
- Alexander, F.; Christian, U.; Stefan, T.; Christoph, V.; Reinhard, G.; Georg, W. Long-term effects of magnetron-sputtered calcium phosphate coating on osseointegration of dental implants in non-human primates. Clin. Oral Implant. Res. 2009, 20, 183–188. [Google Scholar] [CrossRef]
- Germanier, Y.; Tosatti, S.; Broggini, N.; Textor, M.; Buser, D. Enhanced bone apposition around biofunctionalized sandblasted and acid-etched titanium implant surfaces. A histomorphometric study in miniature pigs. Clin. Oral Implant. Res. 2006, 17, 251–257. [Google Scholar] [CrossRef]
- Teixeira, H.S.; Marin, C.; Witek, L.; Freitas, A., Jr.; Silva, N.R.; Lilin, T.; Tovar, N.; Janal, M.N.; Coelho, P.G. Assessment of a chair-side argon-based non-thermal plasma treatment on the surface characteristics and integration of dental implants with textured surfaces. J. Mech. Behav. Biomed. Mater. 2012, 9, 45–49. [Google Scholar] [CrossRef]
- Linder, L.; Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Ivarsson, B.; Jonsson, U.; Lundström, I. Electron Microscopic Analysis of the Bone-Titanium Interface. Acta Orthop. Scand. 1983, 54, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Oyonarte, R.; Pilliar, R.M.; DePorter, D.; Woodside, D.G. Peri-implant bone response to orthodontic loading: Part 1. A histomorphometric study of the effects of implant surface design. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Hansson, H.; Albrektsson, T. Qualitative interfacial study between bone and tantalum, niobium or commercially pure titanium. Biomaterials 1990, 11, 277–280. [Google Scholar] [CrossRef]
- Park, Y.-S.; Yi, K.-Y.; Lee, I.-S.; Jung, Y.-C. Correlation between microtomography and histomorphometry for assessment of implant osseointegration. Clin. Oral Implant. Res. 2005, 16, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Boil. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D.C.N. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 2000, 28, 667–707. [Google Scholar] [CrossRef]
- Wheeler, S.L. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int. J. Oral Maxillofac. Implant. 1996, 11, 340–350. [Google Scholar] [CrossRef]
- Thierer, T.E.; Davliakos, J.P.; Keith, J.D.; Sanders, J.J.; Tarnow, D.P.; Rivers, J.A. Five-Year Prospective Clinical Evaluation of Highly Crystalline HA MP-1–Coated Dental Implants. J. Oral Implant. 2008, 34, 39–46. [Google Scholar] [CrossRef]
- Coelho, P.G.; Lemons, J.E. Physico/chemical characterization andin vivoevaluation of nanothickness bioceramic depositions on alumina-blasted/acid-etched Ti-6Al-4V implant surfaces. J. Biomed. Mater. Res. Part A 2009, 90, 351–361. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Liu, Y.; Jin, X.; Fan, C.; Ye, H.; Ou, M.; Lv, L.; Wu, G.; Zhou, Y. Bi-Functionalization of a Calcium Phosphate-Coated Titanium Surface with Slow-Release Simvastatin and Metronidazole to Provide Antibacterial Activities and Pro-Osteodifferentiation Capabilities. PLoS ONE 2014, 9, e97741. [Google Scholar] [CrossRef][Green Version]
- Tosatti, S.G.P.; Schwartz, Z.; Campbell, C.; Cochran, D.L.; Vandevondele, S.; Hubbell, J.A.; Denzer, A.; Simpson, J.; Wieland, M.; Lohmann, C.H.; et al. RGD-containing peptide GCRGYGRGDSPG reduces enhancement of osteoblast differentiation by poly(L-lysine)-graft-poly(ethylene glycol)-coated titanium surfaces. J. Biomed. Mater. Res. 2004, 68, 458–472. [Google Scholar] [CrossRef]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implant. 2005, 20, 569–577. [Google Scholar]
- Parvizi, J.; Ghanem, E.; Azzam, K.; Davis, E.; Jaberi, F.; Hozack, W. Periprosthetic infection: Are current treatment strategies adequate? Acta Orthop. Belg. 2008, 74, 793–800. [Google Scholar]
- Leonhardt, Å.; Renvert, S.; Dahlén, G. Microbial findings at failing implants. Clin. Oral Implant. Res. 1999, 10, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Parham, P.L., Jr.; Cobb, C.M.; French, A.A.; Love, J.W.; Drisko, C.L.; Killoy, W.J. Effects of an air-powder abrasive system on plasma-sprayed titanium implant surfaces: An in vitro evaluation. J. Oral Implant. 1989, 15, 78–86. [Google Scholar]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Johnson, M.J.; Go, D.B. Piezoelectric transformers for low-voltage generation of gas discharges and ionic winds in atmospheric air. J. Appl. Phys. 2015, 118, 243304. [Google Scholar] [CrossRef]
- Ujino, D.; Nishizaki, H.; Higuchi, S.; Komasa, S.; Okazaki, J. Effect of Plasma Treatment of Titanium Surface on Biocompatibility. Appl. Sci. 2019, 9, 2257. [Google Scholar] [CrossRef]
- Yankovic, F.; Castillo, C.; Saenz, R.; Navarrete, C. Endoscopic argon plasma coagulation in recurrent tracheoesophageal fistula. Clinical series and review of the literature. Gastroenterol. Hepatol. 2009, 32, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive Effects of Low-Temperature Argon-Oxygen Plasma on a Titanium Implant Surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Rakic, M.; Sculean, A.; Miron, R.; Muzzi, M.; Carossa, S.; Mussano, F. Effects of argon plasma treatment on the osteoconductivity of bone grafting materials. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, H.; Harder, S.; Schulz, L.Y.; Wiltfang, J.; Flörke, C.; Açil, Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J. Cranio-Maxillofac. Surg. 2019, 47, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.-Á.; Torres-Lagares, D. Superficial Characteristics of Titanium after Treatment of Chorreated Surface, Passive Acid, and Decontamination with Argon Plasma. J. Funct. Biomater. 2018, 9, 71. [Google Scholar] [CrossRef]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.F.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. Part A 2012, 101, 98–103. [Google Scholar] [CrossRef]
- Cha, S.; Park, Y.-S. Plasma in dentistry. Clin. Plasma Med. 2014, 2, 4–10. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Tangl, S.; Barnewitz, D.; Genzel, A.; Heimel, P.; Hruschka, V.; Redl, H.; Nau, T. A large animal model for standardized testing of bone regeneration strategies. BMC Veter Res. 2018, 14, 330. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 559–573. [Google Scholar] [CrossRef]
- Yang, G.L.; He, F.M.; Hu, J.A.; Wang, X.X.; Zhao, S.F. Effects of biomimetically and electrochemically deposited nanohydroxyapatite coatings on osseointegration of porous titanium implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 782–789. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).