Effects of Bag Mask Ventilation and Advanced Airway Management on Adherence to Ventilation Recommendations and Chest Compression Fraction: A Prospective Randomized Simulator-Based Trial
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.3. Scenario
2.4. Data Analysis
2.5. Statistics
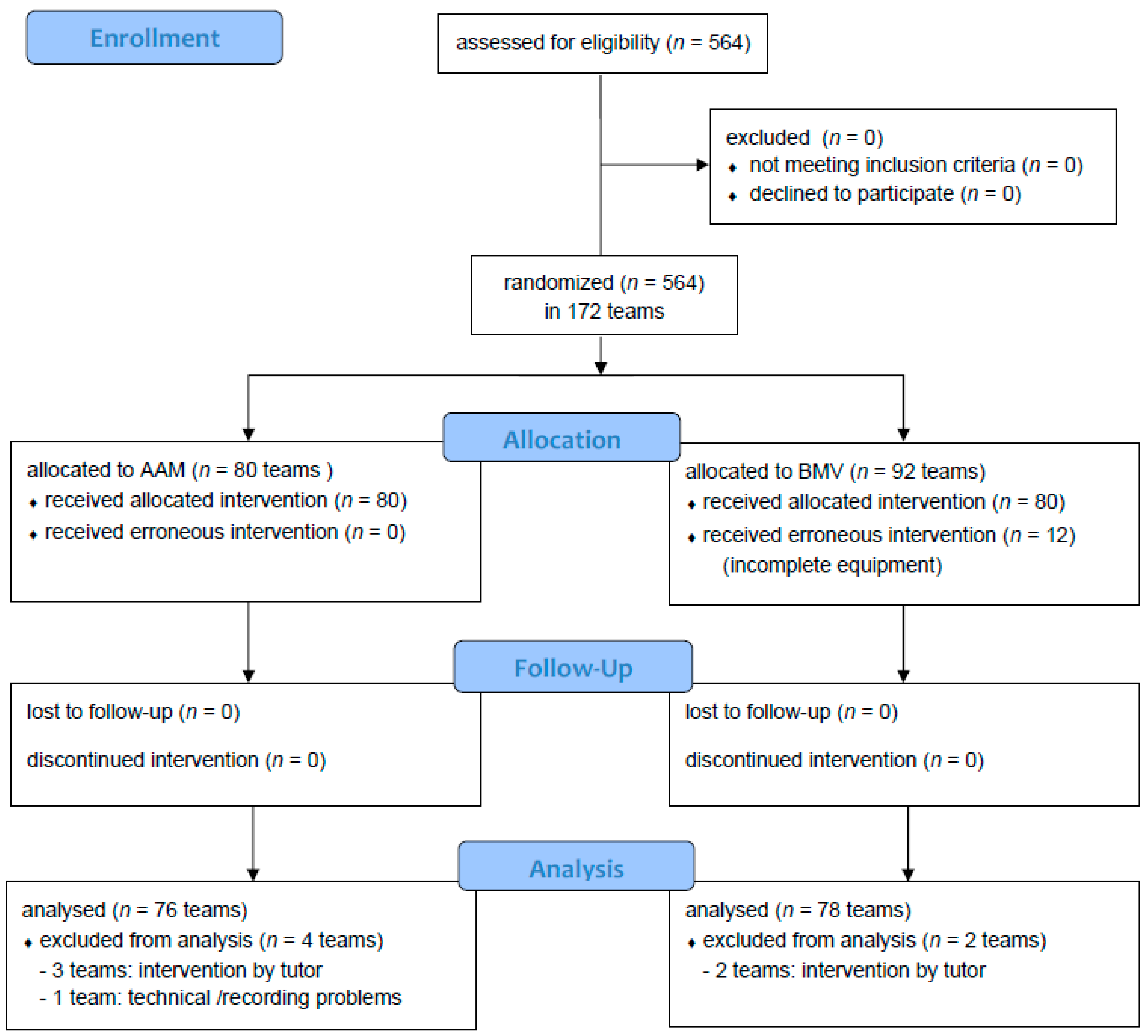
3. Results
3.1. Participants
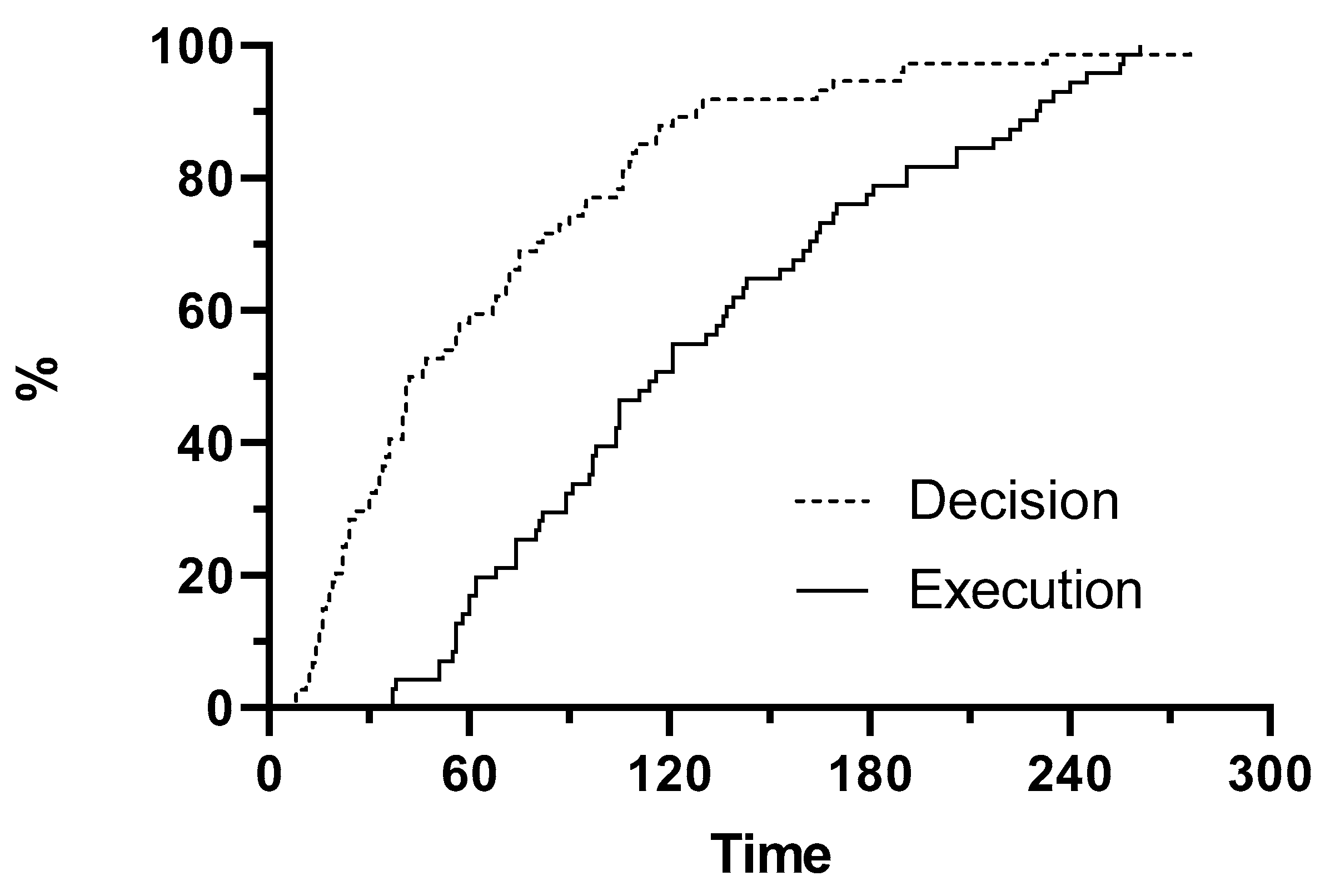
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soar, J.; Nolan, J.P.; Bottiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.P.; Soar, J. Airway techniques and ventilation strategies. Curr. Opin. Crit. Care 2008, 14, 279–286. [Google Scholar] [CrossRef]
- Lyon, R.M.; Ferris, J.D.; Young, D.M.; McKeown, D.W.; Oglesby, A.J.; Robertson, C. Field intubation of cardiac arrest patients: A dying art? Emerg. Med. J. 2010, 27, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.L.; Gerecht, R.B.; Steuerwald, M.T.; McMullan, J.T. Endotracheal intubation versus supraglottic airway placement in out-of-hospital cardiac arrest: A meta-analysis. Resuscitation 2015, 93, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, A.; Avis, S.R.; Nicholson, T.C.; Holmberg, M.J.; Moskowitz, A.; Coker, A.; Berg, K.M.; Parr, M.J.; Donnino, M.W.; Soar, J.; et al. Advanced airway management during adult cardiac arrest: A systematic review. Resuscitation 2019, 139, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jabre, P.; Penaloza, A.; Pinero, D.; Duchateau, F.X.; Borron, S.W.; Javaudin, F.; Richard, O.; de Longueville, D.; Bouilleau, G.; Devaud, M.L.; et al. Effect of Bag-Mask Ventilation vs Endotracheal Intubation During Cardiopulmonary Resuscitation on Neurological Outcome After Out-of-Hospital Cardiorespiratory Arrest: A Randomized Clinical Trial. JAMA 2018, 319, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Szydlo, D.; Stouffer, J.A.; Lin, S.; Carlson, J.N.; Vaillancourt, C.; Sears, G.; Verbeek, R.P.; Fowler, R.; Idris, A.H.; et al. Endotracheal intubation versus supraglottic airway insertion in out-of-hospital cardiac arrest. Resuscitation 2012, 83, 1061–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benger, J.R.; Kirby, K.; Black, S.; Brett, S.J.; Clout, M.; Lazaroo, M.J.; Nolan, J.P.; Reeves, B.C.; Robinson, M.; Scott, L.J.; et al. Effect of a Strategy of a Supraglottic Airway Device vs Tracheal Intubation During Out-of-Hospital Cardiac Arrest on Functional Outcome: The AIRWAYS-2 Randomized Clinical Trial. JAMA 2018, 320, 779–791. [Google Scholar] [CrossRef]
- Andersen, L.W.; Raymond, T.T.; Berg, R.A.; Nadkarni, V.M.; Grossestreuer, A.V.; Kurth, T.; Donnino, M.W.; for the American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Association Between Tracheal Intubation During Pediatric In-Hospital Cardiac Arrest and Survival. JAMA 2016, 316, 1786–1797. [Google Scholar] [CrossRef]
- Andersen, L.W.; Granfeldt, A.; Callaway, C.W.; Bradley, S.M.; Soar, J.; Nolan, J.P.; Kurth, T.; Donnino, M.W.; for the American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Association Between Tracheal Intubation During Adult In-Hospital Cardiac Arrest and Survival. JAMA 2017, 317, 494–506. [Google Scholar] [CrossRef]
- Hanif, M.A.; Kaji, A.H.; Niemann, J.T. Advanced airway management does not improve outcome of out-of-hospital cardiac arrest. Acad. Emerg. Med. 2010, 17, 926–931. [Google Scholar] [CrossRef]
- Hasegawa, K.; Hiraide, A.; Chang, Y.; Brown, D.F. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA 2013, 309, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanghavi, P.; Jena, A.B.; Newhouse, J.P.; Zaslavsky, A.M. Outcomes After Out-of-Hospital Cardiac Arrest Treated by Basic vs Advanced Life Support. JAMA Intern. Med. 2015, 175, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Grossestreuer, A.V.; Donnino, M.W. “Resuscitation time bias”—A unique challenge for observational cardiac arrest research. Resuscitation 2018, 125, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, J.; Chilwan, M.; Field, R.; Davies, R.; Gao, F.; Perkins, G.D. The impact of airway management on quality of cardiopulmonary resuscitation: An observational study in patients during cardiac arrest. Resuscitation 2014, 85, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.H.; Bartels, U.; Schultens, A.; Steffen, T.; Torney, A.; Bahr, J.; Graf, B.M. Influence of airway management strategy on “no-flow-time” during an “Advanced life support course” for intensive care nurses - A single rescuer resuscitation manikin study. BMC Emerg. Med. 2008, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinverni, S.; Bartiaux, M.; Cavallotto, F.; De Longueville, D.; Mols, P.; Gorlicki, J.; Adnet, F. Does endotracheal intubation increases chest compression fraction in out of hospital cardiac arrest: A substudy of the CAAM trial. Resuscitation 2019, 137, 35–40. [Google Scholar] [CrossRef]
- Wang, H.E.; Simeone, S.J.; Weaver, M.D.; Callaway, C.W. Interruptions in cardiopulmonary resuscitation from paramedic endotracheal intubation. Ann. Emerg. Med. 2009, 54, 645–652. [Google Scholar] [CrossRef]
- Abo, B.N.; Hostler, D.; Wang, H.E. Does the type of out-of-hospital airway interfere with other cardiopulmonary resuscitation tasks? Resuscitation 2007, 72, 234–239. [Google Scholar] [CrossRef]
- Aufderheide, T.P.; Sigurdsson, G.; Pirrallo, R.G.; Yannopoulos, D.; McKnite, S.; von, B.C.; Sparks, C.W.; Conrad, C.J.; Provo, T.A.; Lurie, K.G. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation 2004, 109, 1960–1965. [Google Scholar] [CrossRef] [Green Version]
- Abella, B.S.; Alvarado, J.P.; Myklebust, H.; Edelson, D.P.; Barry, A.; O’Hearn, N.; Vanden Hoek, T.L.; Becker, L.B. Quality of Cardiopulmonary Resuscitation During In-Hospital Cardiac Arrest. JAMA: J. Am. Med. Assoc. 2005, 293, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsch, S.C.; Muller, C.; Marquardt, K.; Conrad, G.; Tschan, F.; Hunziker, P.R. Human factors affect the quality of cardiopulmonary resuscitation in simulated cardiac arrests. Resuscitation 2004, 60, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Marsch, S.C.; Tschan, F.; Semmer, N.; Spychiger, M.; Breuer, M.; Hunziker, P.R. Performance of first responders in simulated cardiac arrests. Crit. Care Med. 2005, 33, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Tschan, F.; Vetterli, M.; Semmer, N.K.; Hunziker, S.; Marsch, S.C. Activities during interruptions in cardiopulmonary resuscitation: A simulator study. Resuscitation 2011, 82, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Wik, L.; Kramer-Johansen, J.; Myklebust, H.; Sorebo, H.; Svensson, L.; Fellows, B.; Steen, P.A. Quality of Cardiopulmonary Resuscitation During Out-of-Hospital Cardiac Arrest. JAMA: J. Am. Med. Assoc. 2005, 293, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Sall, F.S.; De, L.A.; Pazart, L.; Pugin, A.; Capellier, G.; Khoury, A. To intubate or not: Ventilation is the question. A manikin-based observational study. BMJ Open Respir Res. 2018, 5, e000261. [Google Scholar] [CrossRef]
- Cheng, A.; Kessler, D.; Mackinnon, R.; Chang, T.P.; Nadkarni, V.M.; Hunt, E.A.; Duval-Arnould, J.; Lin, Y.; Cook, D.A.; Pusic, M.; et al. Reporting Guidelines for Health Care Simulation Research: Extensions to the CONSORT and STROBE Statements. Simul. Healthc. 2016, 11, 25. [Google Scholar] [CrossRef]
- Kleinman, M.E.; Brennan, E.E.; Goldberger, Z.D.; Swor, R.A.; Terry, M.; Bobrow, B.J.; Gazmuri, R.J.; Travers, A.H.; Rea, T. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, 414–435. [Google Scholar] [CrossRef] [Green Version]
- Link, M.S.; Berkow, L.C.; Kudenchuk, P.J.; Halperin, H.R.; Hess, E.P.; Moitra, V.K.; Neumar, R.W.; O’Neil, B.J.; Paxton, J.H.; Silvers, S.M.; et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, 444–464. [Google Scholar] [CrossRef]
- Perkins, G.D.; Handley, A.J.; Koster, R.W.; Castren, M.; Smyth, M.A.; Olasveengen, T.; Monsieurs, K.G.; Raffay, V.; Grasner, J.T.; Wenzel, V.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015, 95, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Neumar, R.W.; Otto, C.W.; Link, M.S.; Kronick, S.L.; Shuster, M.; Callaway, C.W.; Kudenchuk, P.J.; Ornato, J.P.; McNally, B.; Silvers, S.M.; et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, 729–767. [Google Scholar] [CrossRef] [Green Version]
- Gough, C.J.R.; Nolan, J.P. To intubate or not to intubate? Curr. Opin. Crit. Care 2018, 24, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Nolan, J.P. Airway management in cardiopulmonary resuscitation. Curr. Opin. Crit. Care 2013, 19, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.R.; Schmicker, R.H.; Stephens, S.; Carlson, J.N.; Callaway, C.; Herren, H.; Idris, A.H.; Sopko, G.; Puyana, J.C.J.; Daya, M.R.; et al. Outcomes With the Use of Bag-Valve-Mask Ventilation During Out-of-hospital Cardiac Arrest in the Pragmatic Airway Resuscitation Trial. Acad. Emerg. Med. 2020, 27, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Schmicker, R.H.; Daya, M.R.; Stephens, S.W.; Idris, A.H.; Carlson, J.N.; Colella, M.R.; Herren, H.; Hansen, M.; Richmond, N.J.; et al. Effect of a Strategy of Initial Laryngeal Tube Insertion vs Endotracheal Intubation on 72-Hour Survival in Adults With Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2018, 320, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.L.; McMullan, J.T.; Wang, H.E.; Xie, C.; Xu, P.; Hart, K.W.; Stolz, U.; Lindsell, C.J. Timing of Advanced Airway Placement after Witnessed Out-of-Hospital Cardiac Arrest. Prehosp. Emerg. Care 2019, 23, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Izawa, J.; Iwami, T.; Gibo, K.; Okubo, M.; Kajino, K.; Kiyohara, K.; Nishiyama, C.; Nishiuchi, T.; Hayashi, Y.; Kiguchi, T.; et al. Timing of advanced airway management by emergency medical services personnel following out-of-hospital cardiac arrest: A population-based cohort study. Resuscitation 2018, 128, 16–23. [Google Scholar] [CrossRef]
- O’Neill, J.F.; Deakin, C.D. Do we hyperventilate cardiac arrest patients? Resuscitation 2007, 73, 82–85. [Google Scholar] [CrossRef]
- Gazmuri, R.J.; Ayoub, I.M.; Radhakrishnan, J.; Motl, J.; Upadhyaya, M.P. Clinically plausible hyperventilation does not exert adverse hemodynamic effects during CPR but markedly reduces end-tidal PCO(2). Resuscitation 2012, 83, 259–264. [Google Scholar] [CrossRef]
- Beesems, S.G.; Wijmans, L.; Tijssen Jan, G.P.; Koster, R.W. Duration of Ventilations During Cardiopulmonary Resuscitation by Lay Rescuers and First Responders. Circulation 2013, 127, 1585–1590. [Google Scholar] [CrossRef] [Green Version]
- Khoury, A.; De Luca, A.; Sall, F.S.; Pazart, L.; Capellier, G. Ventilation feedback device for manual ventilation in simulated respiratory arrest: A crossover manikin study. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, L.; Sellmann, T.; Wetzchewald, D.; Schwager, H.; Russo, S.; Marsch, S. Effects of Bag Mask Ventilation and Advanced Airway Management on Adherence to Ventilation Recommendations and Chest Compression Fraction: A Prospective Randomized Simulator-Based Trial. J. Clin. Med. 2020, 9, 2045. https://doi.org/10.3390/jcm9072045
Vogt L, Sellmann T, Wetzchewald D, Schwager H, Russo S, Marsch S. Effects of Bag Mask Ventilation and Advanced Airway Management on Adherence to Ventilation Recommendations and Chest Compression Fraction: A Prospective Randomized Simulator-Based Trial. Journal of Clinical Medicine. 2020; 9(7):2045. https://doi.org/10.3390/jcm9072045
Chicago/Turabian StyleVogt, Lea, Timur Sellmann, Dietmar Wetzchewald, Heidrun Schwager, Sebastian Russo, and Stephan Marsch. 2020. "Effects of Bag Mask Ventilation and Advanced Airway Management on Adherence to Ventilation Recommendations and Chest Compression Fraction: A Prospective Randomized Simulator-Based Trial" Journal of Clinical Medicine 9, no. 7: 2045. https://doi.org/10.3390/jcm9072045
APA StyleVogt, L., Sellmann, T., Wetzchewald, D., Schwager, H., Russo, S., & Marsch, S. (2020). Effects of Bag Mask Ventilation and Advanced Airway Management on Adherence to Ventilation Recommendations and Chest Compression Fraction: A Prospective Randomized Simulator-Based Trial. Journal of Clinical Medicine, 9(7), 2045. https://doi.org/10.3390/jcm9072045




