Effects of Trigger Point Dry Needling for the Management of Knee Pain Syndromes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Experimental Section
2.1. Systematic Literature Search
2.2. Selection Criteria
2.3. Screening, Selection Process and Data Extraction
2.4. Assessment of Methodological Quality and Risk of Bias
2.5. Level of Evidence
2.6. Data Synthesis and Analysis
3. Results
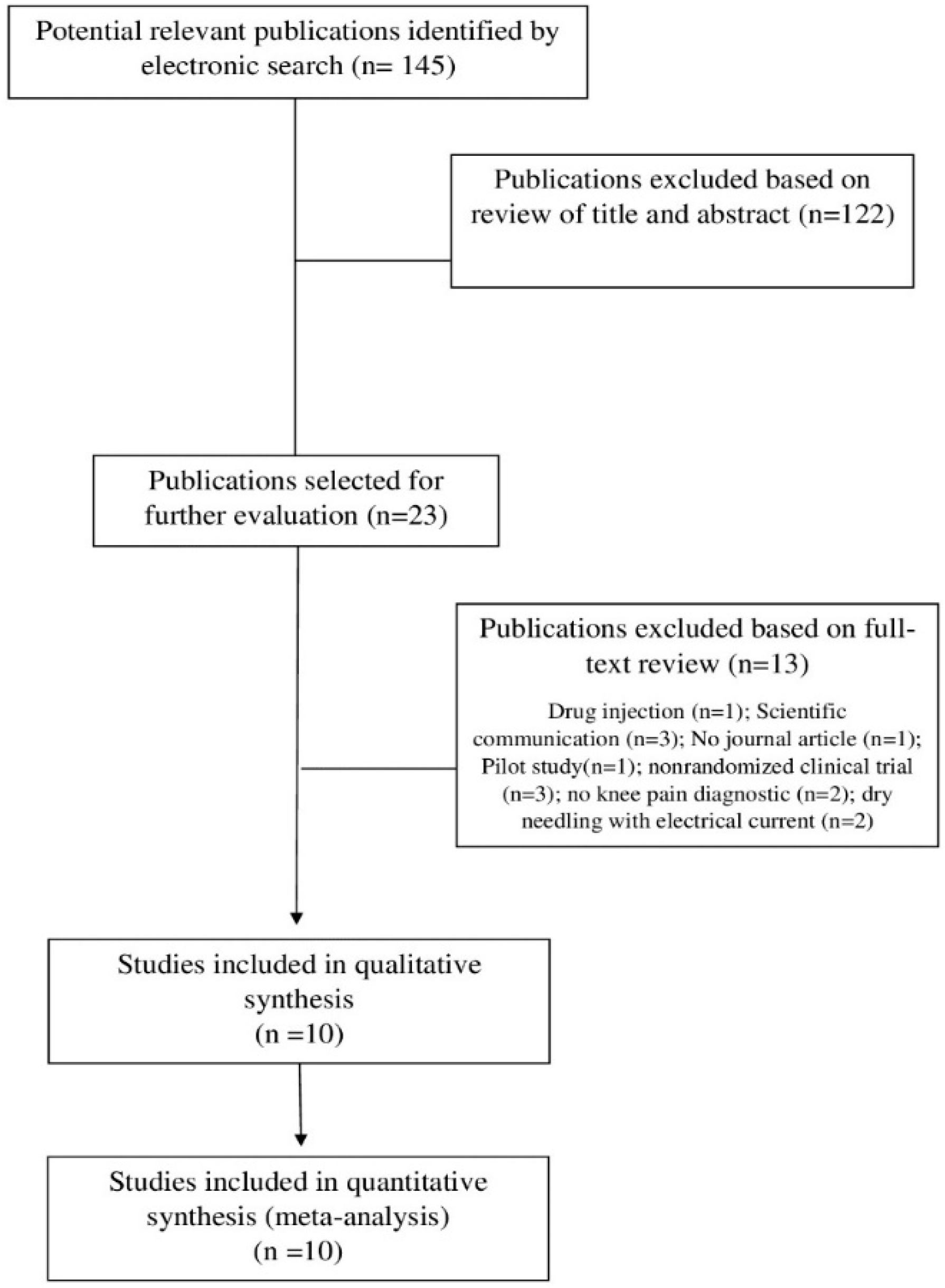
3.1. Study Selection
3.2. Study Characteristics
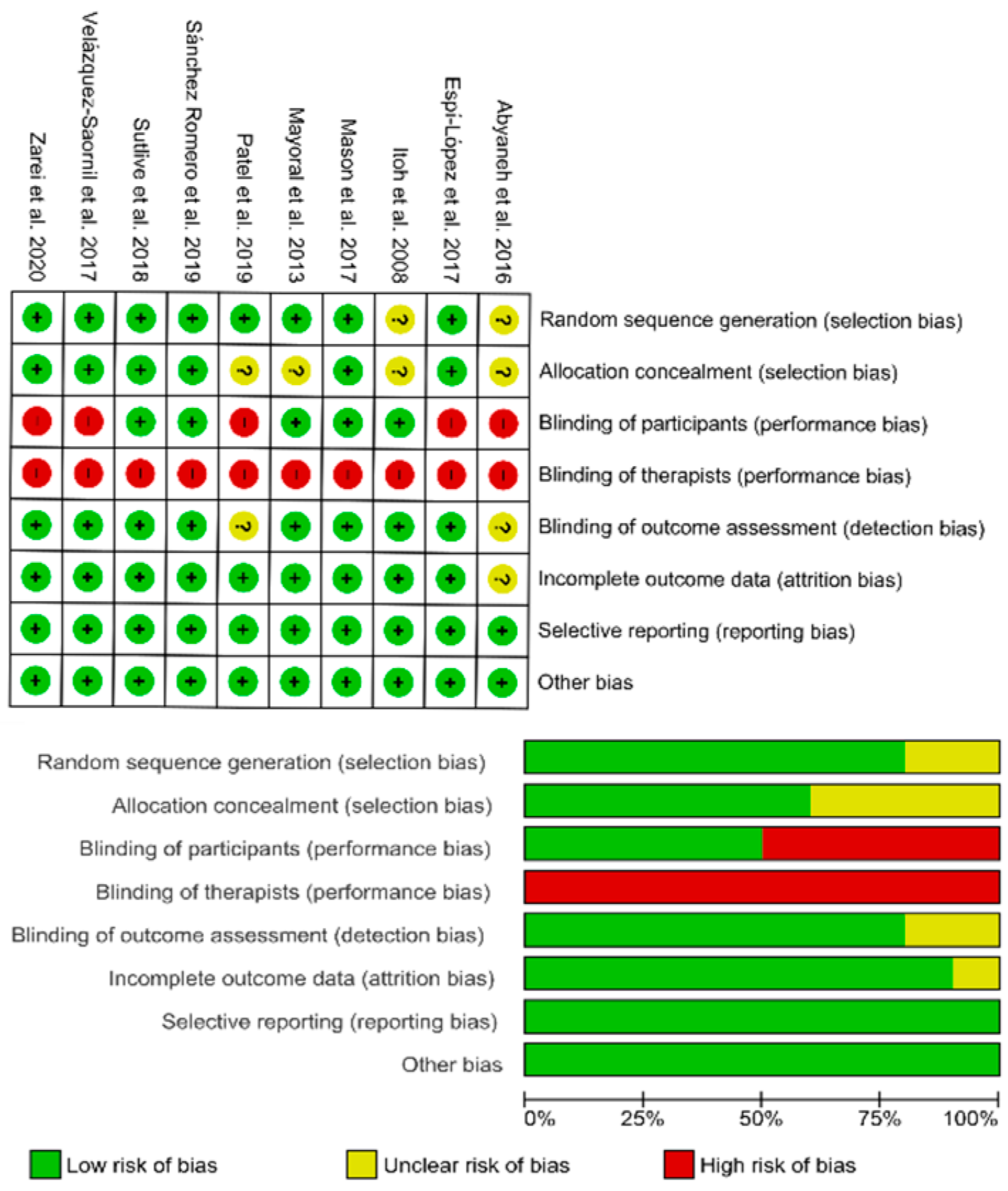
3.3. Methodological Quality
3.4. Risk of Bias
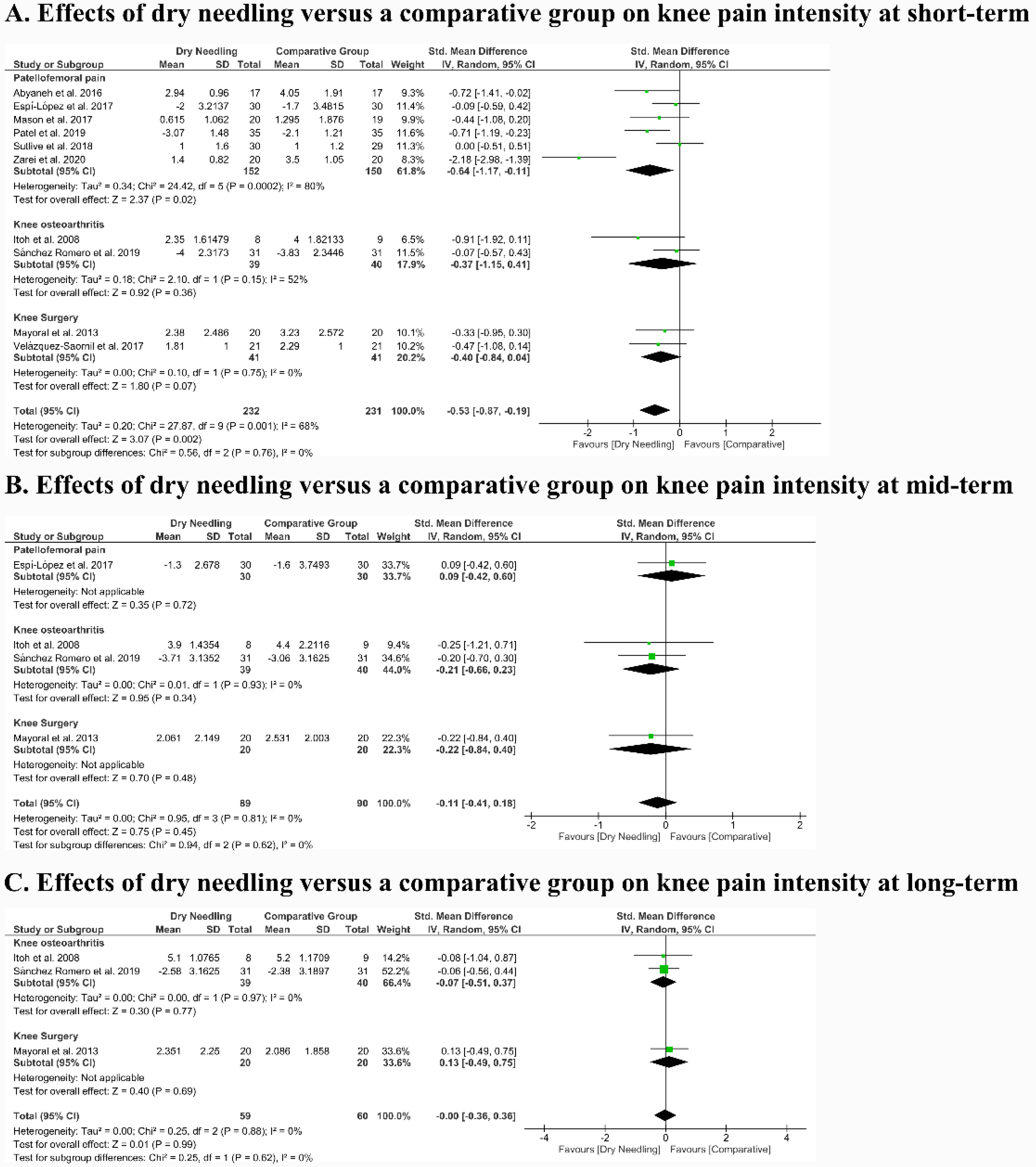
3.5. Effects of Dry Needling on Knee Pain Intensity
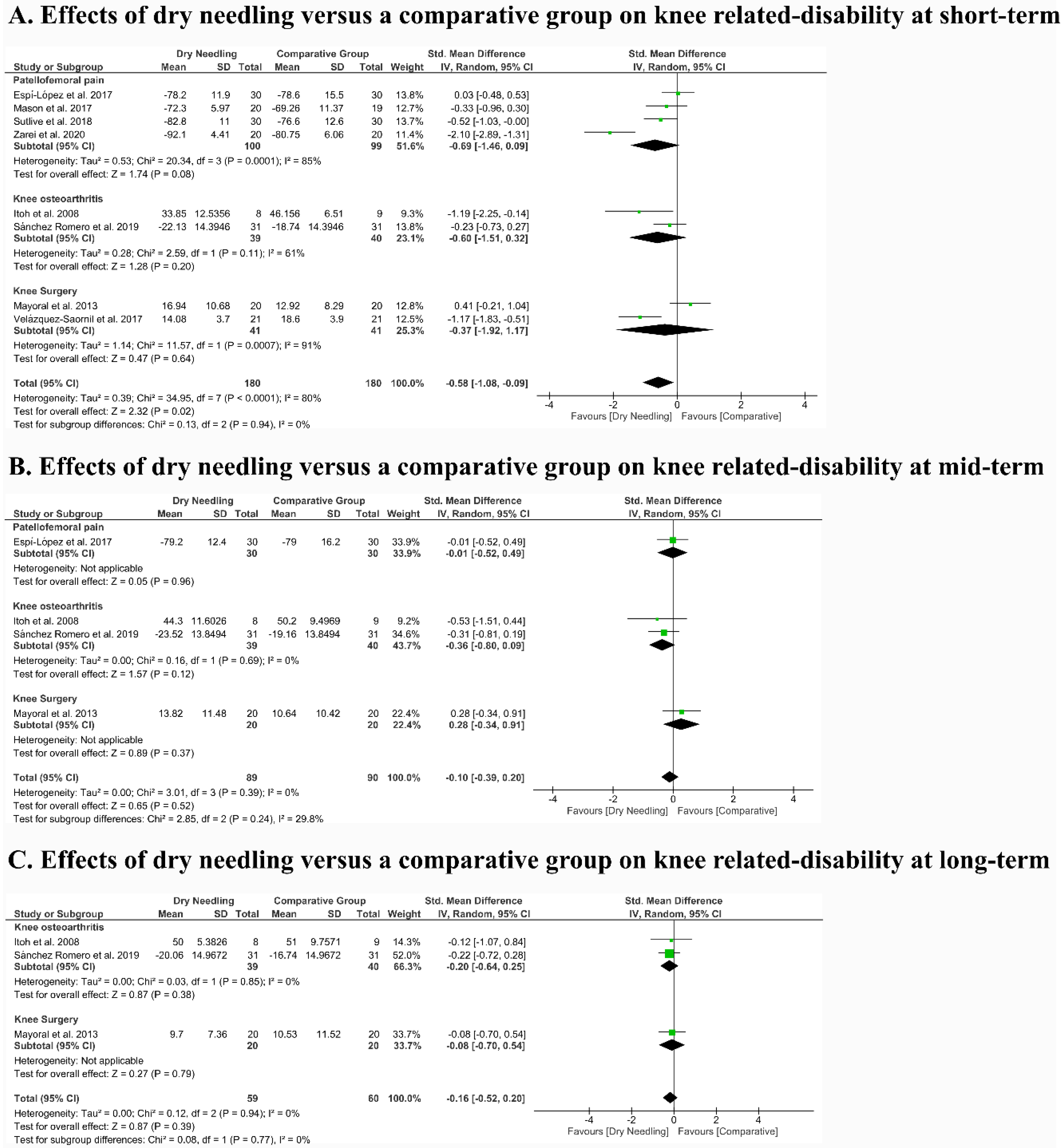
3.6. Effects of Dry Needling on Related Disability
3.7. Quality of Evidence (GRADE)
3.8. Adverse Events
4. Discussion
4.1. Effectiveness of Trigger Point Dry Needling
4.2. Safety of Trigger Point Dry Needling
4.3. Strengths and Limitations
4.4. Clinical and Research Implications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calmbach, W.L.; Hutchens, M. Evaluation of patients presenting with knee pain: Part I. History, physical examination, radiographs, and laboratory tests. Am. Fam. Physician 2003, 68, 907–912. [Google Scholar]
- Jordan, J.M.; Helmick, C.G.; Renner, J.B.; Luta, G.; Dragomir, A.D.; Woodard, J.; Fang, F.; Schwartz, T.A.; Nelson, A.E.; Abbate, L.M. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J. Rheumatol. 2009, 36, 809–815. [Google Scholar] [CrossRef]
- Smith, B.E.; Selfe, J.; Thacker, D.; Hendrick, P.; Bateman, M.; Moffatt, F.; Rathleff, M.S.; Smith, T.O.; Logan, P. Incidence and prevalence of patellofemoral pain: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0190892. [Google Scholar] [CrossRef]
- Boling, M.; Padua, D.; Marshall, S.; Guskiewicz, K.; Pyne, S.; Beutler, A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand. J. Med. Sci. Sports 2010, 20, 725–730. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Madaleno, F.O.; Santos, B.A.; Araújo, V.L.; Oliveira, V.C.; Resende, R.A. Prevalence of knee osteoarthritis in former athletes: A systematic review with meta-analysis. Brazilian J. Phys. Ther. 2018, 22, 437–451. [Google Scholar] [CrossRef]
- Osthoff, A.-K.R.; Juhl, C.B.; Knittle, K.; Dagfinrud, H.; Hurkmans, E.; Braun, J.; Schoones, J.; Vlieland, T.P.M.V.; Niedermann, K. Effects of exercise and physical activity promotion: Meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis. RMD Open 2018, 4, e000713. [Google Scholar] [CrossRef]
- Anwer, S.; Alghadir, A.; Zafar, H.; Brismée, J.-M. Effects of orthopaedic manual therapy in knee osteoarthritis: A systematic review and meta-analysis. Physiotherapy 2018, 104, 264–276. [Google Scholar] [CrossRef]
- Neal, B.S.; Lack, S.D.; Lankhorst, N.E.; Raye, A.; Morrissey, D.; van Middelkoop, M. Risk factors for patellofemoral pain: A systematic review and meta-analysis. Br. J. Sport Med. 2019, 53, 270–281. [Google Scholar] [CrossRef]
- Øiestad, B.E.; Juhl, C.B.; Eitzen, I.; Thorlund, J.B. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 171–177. [Google Scholar] [CrossRef]
- Dor, A.; Kalichman, L. A myofascial component of pain in knee osteoarthritis. J. Bodyw. Mov. Ther. 2017, 21, 642–647. [Google Scholar] [CrossRef]
- Simons, D.G.; Travell, J.G.; Simon, L. Myofascial Pain and Dysfunction. The Trigger Point Manual, 3rd ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Ge, H.-Y.; Arendt-Nielsen, L.; Madeleine, P. Accelerated muscle fatigability of latent myofascial trigger points in humans. Pain Med. 2012, 13, 957–964. [Google Scholar] [CrossRef]
- Ibarra, J.M.; Ge, H.-Y.; Wang, C.; Vizcaíno, V.M.; Graven-Nielsen, T.; Arendt-Nielsen, L. Latent myofascial trigger points are associated with an increased antagonistic muscle activity during agonist muscle contraction. J. Pain 2011, 12, 1282–1288. [Google Scholar] [CrossRef]
- Roach, S.; Sorenson, E.; Headley, B.; San Juan, J.G. Prevalence of myofascial trigger points in the hip in patellofemoral pain. Arch. Phys. Med. Rehabil. 2013, 94, 522–526. [Google Scholar] [CrossRef]
- Sánchez-Romero, E.A.; Pecos-Martín, D.; Calvo-Lobo, C.; García-Jiménez, D.; Ochoa-Sáez, V.; Burgos-Caballero, V.; Fernández-Carnero, J. Clinical features and myofascial pain syndrome in older adults with knee osteoarthritis by sex and age distribution: A cross-sectional study. Knee 2019, 26, 165–173. [Google Scholar] [CrossRef]
- Dommerholt, J.; Fernandez-de-las Peñas, C. Trigger Point Dry Needling: An Evidence and Clinical-Based Approach, 2nd ed.; Elsevier, Churchill Livingstone: London, UK, 2019. [Google Scholar]
- APTA. Description of Dry Needling in Clinical Practice: An Educational Resource Paper; APTA Public Policy, Pract Prof Aff Unit: Alexandria, VA, USA, 2013. [Google Scholar]
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The Effectiveness of Trigger Point Dry Needling for Musculoskeletal Conditions by Physical Therapists: A Systematic Review and Meta-analysis. J. Orthop. Sport Phys. Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef]
- Morihisa, R.; Eskew, J.; McNamara, A.; Young, J. Dry needling in subjects with muscular trigger points in the lower quarter: A systematic review. Int. J. Sports Phys. Ther. 2016, 11, 1. [Google Scholar] [PubMed]
- Collins, N.J.; Barton, C.J.; van Middelkoop, M.; Callaghan, M.J.; Rathleff, M.S.; Vicenzino, B.T.; Davis, I.S.; Powers, C.M.; Macri, E.M.; Hart, H.F.; et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: Recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br. J. Sports Med. 2018, 52, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Bossuyt, P.; Chang, S.; Muti, P.; Jaeschke, R.; Guyatt, G.H. GRADE: Assessing the quality of evidence for diagnostic recommendations. BMJ Evid. Based Med. 2008, 13, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Austin, T.M.; Richter, R.R.; Sebelski, C.A. Introduction to the GRADE approach for guideline development: Considerations for physical therapist practice. Phys. Ther. 2014, 94, 1652–1659. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G (Eds.) Chapter 9: Analyzing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.2.0 (Updated June 2017); Available online: www.training.cochrane.org/handbook (accessed on 1 May 2020).
- Dragoo, J.L.; Wasterlain, A.S.; Braun, H.J.; Nead, K.T. Platelet-rich plasma as a treatment for patellar tendinopathy: A double-blind, randomized controlled trial. Am. J. Sports Med. 2014, 42, 610–618. [Google Scholar] [CrossRef]
- De-la-Llave-Rincón, A.I.; Loa-Barbero, B.; Palacios-Ceña, M.; Salom-Moreno, J.; Ortega-Santiago, R.; Ambite-Quesada, S.; Arias-Buría, J.L.; Fernandez-De-Las-Peñas, C. Manual therapy combined with dry needling for the management of patients with patellofemoral pain syndrome. Man. Ther. 2016, 25, e82. [Google Scholar] [CrossRef]
- Dragoo, J.; Wasterlain, A. Double-blind randomized controlled trial of platelet-rich-plasma versus dry needling for treatment of patellar tendinosis. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, e118–e119. [Google Scholar] [CrossRef]
- Wasterlain, A.S.; Braun, H.J.; Dragoo, J.L. Platelet-rich plasma as a treatment for patellar tendinopathy: A double-blind randomized controlled trial. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, e31–e32. [Google Scholar] [CrossRef]
- Sanchez-Romero, E.A.; Pecos-Martin, D.; Calvo-Lobo, C.; Ochoa-Saez, V.; Burgos-Caballero, V.; Fernandez-Carnero, J. Effects of dry needling in an exercise program for older adults with knee osteoarthritis: A pilot clinical trial. Medicine 2018, 97, e11255. [Google Scholar] [CrossRef]
- James, S.L.J.; Ali, K.; Pocock, C.; Robertson, C.; Walter, J.; Bell, J.; Connell, D.; Bradshaw, C. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br. J. Sports Med. 2007, 41, 518–521. [Google Scholar] [CrossRef]
- Nunez-Cortes, R.; Cruz-Montecinos, C.; Vasquez-Rosel, A.; Paredes-Molina, O.; Cuesta-Vargas, A. Dry Needling Combined with Physical Therapy in Patients with Chronic Postsurgical Pain Following Total Knee Arthroplasty: A Case Series. J. Orthop. Sports Phys. Ther. 2017, 47, 209–216. [Google Scholar] [CrossRef]
- Ortega-Cebrian, S.; Luchini, N.; Whiteley, R. Dry needling: Effects on activation and passive mechanical properties of the quadriceps, pain and range during late stage rehabilitation of ACL reconstructed patients. Phys. Ther. Sport 2016, 21, 57–62. [Google Scholar] [CrossRef]
- Da Graca-Tarragó, M.; Deitos, A.; Brietzke, A.P.; Torres, I.L.S.; Stefani, L.C.; Fregni, F.; Caumo, W. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. 2016, 17, 877–891. [Google Scholar] [CrossRef][Green Version]
- Dunning, J.; Butts, R.; Young, I.; Mourad, F.; Galante, V.; Bliton, P.; Tanner, M.; Fernandez-de-las-Penas, C. Periosteal Electrical Dry Needling as an Adjunct to Exercise and Manual Therapy for Knee Osteoarthritis: A Multi-Center Randomized Clinical Trial. Clin. J. Pain 2018, 34, 1149–1158. [Google Scholar] [CrossRef]
- Abyaneh, H.M.; Mosallanezhad, Z.; Mohammadalizade, H.; Bakhshi, E.; Vahedi, G.; Nourbakhsh, M.R. Physiotherapy with and without superficial dry needling affects pain and muscle strength in patients with patellofemoral pain syndrome. Iran. Rehabil. J. 2016, 14, 23–30. [Google Scholar]
- Espí-López, G.V.; Serra-Añó, P.; Vicent-Ferrando, J.; Sánchez-Moreno-Giner, M.; Arias-Buría, J.L.; Cleland, J.; Fernández-de-las-Peñas, C. Effectiveness of Inclusion of Dry Needling in a Multimodal Therapy Program for Patellofemoral Pain: A Randomized Parallel-Group Trial. J. Orthop. Sport Phys. Ther. 2017, 47, 392–401. [Google Scholar] [CrossRef]
- Itoh, K.; Hirota, S.; Katsumi, Y.; Ochi, H.; Kitakoji, H. Trigger point acupuncture for treatment of knee osteoarthritis—A preliminary RCT for a pragmatic trial. Acupunct. Med. 2008, 26, 17–26. [Google Scholar] [CrossRef]
- Mason, J.S.; Crowell, M.; Dolbeer, J.; Morris, J.; Terry, A.; Koppenhaver, S.; Goss, D.L. The effectiveness of dry needling and stretching vs. stretching alone on hamstring flexibility in patients with knee pain: A randomized controlled trial. Int. J. Sports Phys. Ther. 2016, 11, 672–683. [Google Scholar]
- Mayoral, O.; Salvat, I.; Martin, M.T.; Martin, S.; Santiago, J.; Cotarelo, J.; Rodriguez, C. Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: A randomized, double-blinded, placebo-controlled trial. Evid. Based Complement. Alternat. Med. 2013, 2013, 694941. [Google Scholar] [CrossRef]
- Sanchez Romero, E.A.; Fernandez-Carnero, J.; Calvo-Lobo, C.; Ochoa Saez, V.; Burgos Caballero, V.; Pecos-Martin, D. Is a Combination of Exercise and Dry Needling Effective for Knee OA? Pain Med. 2020, 21, 349–363. [Google Scholar] [CrossRef]
- Sutlive, T.G.; Golden, A.; King, K.; Morris, W.B.; Morrison, J.E.; Moore, J.H.; Koppenhaver, S. Short-term effects of trigger point dry needling on pain and disability in subjects with patellofemoral pain syndrome. Int. J. Sports Phys. Ther. 2018, 13, 462–473. [Google Scholar] [CrossRef]
- Velazquez-Saornil, J.; Ruiz-Ruiz, B.; Rodriguez-Sanz, D.; Romero-Morales, C.; Lopez-Lopez, D.; Calvo-Lobo, C. Efficacy of quadriceps vastus medialis dry needling in a rehabilitation protocol after surgical reconstruction of complete anterior cruciate ligament rupture. Medicine 2017, 96, e6726. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Bervis, S.; Piroozi, S.; Motealleh, A. Added Value of Gluteus Medius and Quadratus Lumborum Dry Needling in Improving Knee Pain and Function in Female Athletes with Patellofemoral Pain Syndrome: A Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2020, 101, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.; Srivastava, A.; Shyam, A.; Sancheti, P. Immediate Effect of Dry Needling Vs Ultrasound on Releasing Trigger Points in Quadriceps in Patients with Patello-Femoral Pain Syndrome on Pain. Int. J. Physiother. Res. 2019, 7, 3287–3294. [Google Scholar] [CrossRef]
- Rogan, S.; Haehni, M.; Luijckx, E.; Dealer, J.; Reuteler, S.; Taeymans, J. Effects of Hip Abductor Muscles Exercises on Pain and Function in Patients with Patellofemoral Pain: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2019, 33, 3174–3187. [Google Scholar] [CrossRef]
- Giles, L.S.; Webster, K.E.; McClelland, J.A.; Cook, J. Does quadriceps atrophy exist in individuals with patellofemoral pain? A systematic literature review with meta-analysis. J. Orthop. Sport. Phys. Ther. 2013, 43, 766–776. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic muscle inhibition after ACL reconstruction: A scoping review of the efficacy of interventions. Br. J. Sport Med. 2019, 53, 289–298. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J.; Lewis, G.N.; Dalbeth, N. Quadriceps arthrogenic muscle inhibition: The effects of experimental knee joint effusion on motor cortex excitability. Arthritis Res. Ther. 2014, 16, 502. [Google Scholar] [CrossRef]
- Pedroso, M.G.; de Almeida, A.C.; Aily, J.B.; de Noronha, M.; Mattiello, S.M. Fatty infiltration in the thigh muscles in knee osteoarthritis: A systematic review and meta-analysis. Rheumatol. Int. 2019, 39, 627–635. [Google Scholar] [CrossRef]
- Hislop, A.C.; Collins, N.J.; Tucker, K.; Deasy, M.; Semciw, A.I. Does adding hip exercises to quadriceps exercises result in superior outcomes in pain, function and quality of life for people with knee osteoarthritis? A systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 263–271. [Google Scholar] [CrossRef] [PubMed]
- French, H.P.; Smart, K.M.; Doyle, F. Prevalence of neuropathic pain in knee or hip osteoarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2017, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boyce, D.; Wempe, H.; Campbell, C.; Fuehne, S.; Zylstra, E.; Smith, G.; Wingard, C.; Jones, R. Adverse events aossciated with therapeutic dry needling. Int. J. Sports Phys. Ther. 2020, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Glenzer, S.; Johnson, A.; Nimityongskul, P. Deep Infection Following Dry Needling in a Young Athlete. J. Bone Jt. Surg. 2018, 8, e73. [Google Scholar] [CrossRef]
- Moody, P.W.; Fehring, T.K.; Springer, B.D. Periarticular needle-based therapies can cause periprosthetic knee infections. Arthroplast. Today 2020, 6, 241–245. [Google Scholar] [CrossRef]
- Braithwaite, F.A.; Walters, J.L.; Li, L.S.K.; Moseley, G.L.; Williams, M.T.; McEvoy, M.P. Blinding Strategies in Dry Needling Trials: Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1461–1480. [Google Scholar] [CrossRef]
- Krey, D.; Borchers, J.; McCamey, K. Tendon needling for treatment of tendinopathy: A systematic review. Phys. Sportsmed. 2015, 43, 80–86. [Google Scholar] [CrossRef]
- Mendonça, L.D.M.; Leite, H.R.; Zwerver, J.; Henschke, N.; Branco, G.; Oliveira, V.C. How strong is the evidence that conservative treatment reduces pain and improves function in individuals with patellar tendinopathy? A systematic review of randomised controlled trials including GRADE recommendations. Br. J. Sports Med. 2020, 54, 87–93. [Google Scholar] [CrossRef]
| PubMed Search Formula |
| #1 "Patellofemoral Pain Syndrome"(MeSH Terms) OR "Chondromalacia Patellae"(MeSH Terms) OR "Osteoarthritis, Knee"(MeSH Terms) OR "Knee Osteoarthritis" OR "Arthroplasty, Replacement, Knee"(MeSH Terms) OR "Knee Arthroplasty" OR "Knee Prosthesis"(MeSH Terms) OR "Knee Injuries"(MeSH Terms) OR "Anterior Cruciate Ligament Injuries"(MeSH Terms) OR "ACL Injury" OR "Medial Collateral Ligament Knee Injury" OR "Knee Joint Injury" OR "Knee Dislocation"(MeSH Terms) OR "Meniscectomy"(MeSH Terms) OR "Meniscus Injury" OR "Tibial Meniscus Injuries"(MeSH Terms) OR "Meniscus Tear" OR "Bucket Handle Tear" OR "Flap Tear" OR "Patellar Tendinopathy" OR "Patellar Tendonitis" OR "Patellar Tendinosis" OR "Jumper Knee" #2 "Dry Needling"(Mesh) OR "Intramuscular Stimulation" (Title/Abstract) OR "Muscular Needling" (Title/Abstract) #3 #1 AND #2 |
| Results: 37 |
| CINAHL/Medline Search Formula (EBSCO)/WOS Search Formula |
| ("Patellofemoral Pain Syndrome" OR "Chondromalacia Patellae" OR "Knee Osteoarthritis" OR "Knee Arthritis" OR "Knee Arthroplasty" OR "Knee Prosthesis" OR "Knee Injury" OR "Anterior Cruciate Ligament Injury" OR "ACL Injury" OR "Medial Collateral Ligament Knee Injury" OR "Knee Dislocation" OR "Meniscectomy" OR "Meniscus Injury" OR "Tibial Meniscus Injuries" OR "Meniscus Tear" OR "Bucket Handle Tear" OR "Flap Tear" OR "Patellar Tendinopathy" OR "Patellar Tendonitis" OR "Patellar Tendinosis" OR "Jumper Knee") AND ("Dry Needling" OR "Muscular Needling" OR "Intramuscular stimulation") NOT "Acupuncture" |
| Results: 38 |
| PEDro Search Formula |
| Abstract & Title: Knee Pain, Patellofemoral Pain, Knee Osteoarthritis Therapy: Dry Needling Body part: Lower Leg or Knee Method: Clinical trial When Searching: AND |
| Results: 11 |
| SCOPUS Search Formula |
| TITLE-ABS-KEY ("Patellofemoral Pain Syndrome" OR "Chondromalacia Patellae" OR "Knee Osteoarthritis" OR "Knee Arthritis" OR "Knee Arthroplasty" OR "Knee Prosthesis" OR "Knee Injury" OR "Anterior Cruciate Ligament Injury" OR "ACL Injury" OR "Medial Collateral Ligament Knee Injury" OR "Knee Joint Injury" OR "Meniscectomy" OR "Knee Dislocation" OR "Meniscus Injury" OR "Tibial Meniscus Injuries" OR "Meniscus Tear" OR "Bucket Handle Tear" OR "Flap Tear" OR "Patellar Tendinopathy" OR "Patellar Tendonitis" OR "Patellar Tendinosis" OR "Jumper Knee") AND TITLE-ABS-KEY ("Dry Needling" OR "Muscular needling" OR "Intramuscular stimulation") |
| Results: 52 |
| Cochrane Library Search Formula |
| #1 "Patellofemoral Pain Syndrome"(MeSH Terms) #2 "Chondromalacia Patellae" #3 "Osteoarthritis, Knee"(MeSH Terms) #4 "Arthroplasty, Replacement, Knee"(MeSH Terms) #5 "Knee Arthroplasty" #6 "Knee Prosthesis"(MeSH Terms) #7 "Knee Injuries"(MeSH Terms) #8 "Anterior Cruciate Ligament Injuries"(MeSH Terms) #9 "ACL Injury" #10 "Medial Collateral Ligament Knee Injury" #11 "Knee Joint Injury" #12 "Knee Dislocation"(MeSH Terms) #13 "Meniscectomy"(MeSH Terms) #14 "Meniscus Injury" #15 "Tibial Meniscus Injuries"(MeSH Terms) #16 "Meniscus Tear" #17 "Bucket Handle Tear" #18 "Flap Tear" #19 "Patellar Tendinopathy" #20 "Patellar Tendonitis" #21 "Patellar Tendinosis" #22 "Jumper Knee" #23 "Dry Needling"(Mesh) #24 "Intramuscular stimulation" #25 "Muscular needling" #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #27 #23 OR #24 OR #25 #28 #26 AND #27 |
| Results: 42 Trials: 39 |
| Study | Design | Group | Sample Size | Male/Female (%) | Age (years) | Pain Duration |
|---|---|---|---|---|---|---|
| Patellofemoral Pain Syndrome | ||||||
| Abyaneh et al. 2016 | RCT | G1 G2 | 17 17 | NR NR | 37.88 ± 9.53 33.58 ± 8 | 1.88 ± 1.16 years 2.11 ± 1.16 years |
| Espí-López et al 2017 | RCT | G1 G2 | 30 30 | 15/15 16/14 | 29.7 ± 9.5 29.2 ± 10.5 | 9.5 ± 5.8 years 8.5 ± 6.3 years |
| Mason et al. 2017 | RCT | G1 G2 | 20 19 | 20 17/2 | 20.3 ± 1.08 20.16 ± 2.12 | 17.75 ± 26.10 weeks 14.3 ± 16.36 weeks |
| Sutlive et al. 2018 | RCT | G1 G2 | 30 30 | 56.7% male 66.7% male | 30.3 ± 5.5 31.1 ± 5.1 | 27.4 ± 29.7 months 53.0 ± 66.8 months |
| Patel et al. 2019 | RCT | G1 G2 | 35 35 | NR NR | 26 ± 5 33.3 ± 3 | >3 months >3 months |
| Zarei et al. 2020 | RCT | G1 G2 | 20 20 | 0/20 0/20 | 22.25 ± 3.25 25.65 ± 8.49 | >3 months >3 months |
| Knee Osteoarthritis | ||||||
| Itoh et al. 2008 | RCT | G1 G2 G3 | 8 9 7 | NR NR NR | 74.2 ± 8.1 73.3 ± 6.5 70.5 ± 8.1 | 7.5 ± 6.0 years 6.1 ± 6.8 years 5.6 ± 5.1 years |
| Sánchez-Romero et al. 2019 | RCT | G1 G2 | 31 31 | 21/10 23/8 | 72.97 ± 6.29 71.65 ± 5.00 | 62.88 ± 40.75 months 68.55 ± 30.31 months |
| Post-Surgery Knee Pain | ||||||
| Mayoral et al. 2013 | RCT | G1 G2 | 20 20 | 11/29 | 71.65 ± 6.06 72.90 ± 7.85 | NR NR |
| Velázquez-Saornil et al. 2017 | RCT | G1 G2 | 22 22 | 16/6 12/10 | 31.4 ± 8.3 34.4 ± 8.6 | 15.6 ± 1.5 days 15.5 ± 2.0 days |
| Study | Group | TrP Criteria | Needle Approach | No. Punctures | Targeted Muscles | Gauge (mm) | Depth (mm) | Time | Frequency Incisions (Hz) | No. Incisions | LTR | Therapist |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patellofemoral Pain Syndrome | ||||||||||||
| Abyaneh et al. 2016 | G1: Superficial Dry Needling (DN) | NO | At about 8 cm above the lateral femoral condyle of the knee joint line in vastus lateralis muscle At 8 cm above the medial femoral condyle of the knee joint line in vastus medialis muscle At 8 cm above the vase of the patella in the rectus femoris muscles. | 3 | Vastus lateralis Vastus medialis Rectus femoris | 50 length | 10 | 6 | NR | NR | No | Physical therapist |
| Espí-López et al. 2017 | G1: DN plus manual and exercise therapy | YES | Fast-in and fast-out technique at the active TrP | NR | Vastus lateralis Vastus medialis | 0.32 × 40 | 15–20 vastus medialis 30–35 vastus lateralis | Until no more local twitch responses were elicited | 1 | NR | Yes | Physical therapist |
| Mason et al. 2017 | G1: TrP DN | YES | Fast-in fast-out (Hong’s technique) at latent TrP | NR | Hamstrings’ muscles | NR | NR | NR | NR | NR | Yes | Physical therapist |
| G2: TrP Sham DN | NO | At three points over the lateral hamstrings and three points over the medial hamstrings without the intention of locating any TrPs. The simulation was performed with a small nail | NA | Hamstrings’ muscles | NA | NA | NR | NA | NA | NA | Physical therapist | |
| Sutlivee et al. 2018 | G1: TrP DN | YES | Fast-in fast-out (Hong’s technique) at two TrP of each of three targeted quadriceps or the most painful location | 6 | Vastus medialis, rectus femoris and vastus lateralis | 0.25 × 40 | NR | 5–10 s | NR | NR | Yes | Physical therapist |
| G2: TrP Sham DN | YES | Simulation at the TrP without puncture | ipsilateral to the symptomatic knee | NA | NA | 5–10 s | NA | NA | NA | Physical therapist | ||
| Patel et al. 2019 | G1: TrP DN | YES | NR | NR | All trigger points of quadriceps muscle of the symptomatic knee | NR | NR | 10 min | NR | NR | NR | Physical therapist |
| Zarei et al. 2020 | G1: TrP DN | YES | Fast-in fast-out technique | NR | Gluteus medius Quadratus Lumborum | 0.30 × 10 0.30 × 50 | NR | NR | NR | NR | Yes | Physical therapist |
| Knee Osteoarthritis | ||||||||||||
| Itoh et al. 2008 | G1: DN | YES | At the trigger points of the lumbar and lower extremity, using the “sparrow pecking” technique | 3.3 | Quadriceps, ilipsoas, sartorius, adductors, popliteus, gluteus minimus | 0.2 × 50 | 10–30 mm | 10 min | NR | NR | Yes | Acupuncturist |
| G2: Sham DN | YES | At trigger points with steel needles, but the tips had been cut off to prevent the needle penetrating the skin. The acupuncturist inserted the needle and then used the sparrow pecking technique, then removed the needles | 3.1 | Quadriceps, ilipsoas, sartorius, adductors, popliteus, gluteus minimus | 0.2 × 50 | Not penetrating the skin | 10 min | NR | NR | No | Acupuncturist | |
| Sánchez-Romero et al. 2019 | G1: DN plus exercise therapy | YES | At TrP, fast-in fast-out technique | NR | In all muscles with TrP of the symptomatic knees | 0.30 × 40 0.30 × 60 0.30 × 75 | According to the muscle selected and the subject | NR | NR | 15 | Yes | Physical Therapist |
| G2: Sham DN plus exercise therapy | YES | Simulation | NR | In all muscles with TrP of the symptomatic knees | Sham Needle | NA | NA | NR | NA | NA | Physical Therapist | |
| Post-Surgery Knee Pain | ||||||||||||
| Mayoral et al. 2013 | G1: DN | YES | At TrP using Hong’s-fast-in fast-out technique | NR | Tensor fasciae latae, hip adductors, hamstrings, quadriceps gastrocnemius, popliteus | 0.30 × 50 | NR | NR | NR | 20 | Yes | Physical Therapist |
| G2: Sham DN | NO | Simulated TrP DN | NA | NA | NA | NA | NA | NA | NA | NA | Physical Therapist | |
| Velázquez-Saornil et al. 2017 | G1: DN | YES | On the most active TrP of the vastus medialis of the affected knee, fast-in fast-out technique | 1 | Vastus Medialis | 0.25 × 25 | Varied according to the subject | 1–2 min until LTR exhaustion, patient tolerance limit or 20 incisions | NR | 20 incisions | Yes | Physical Therapy |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patellofemoral Pain Syndrome | |||||||||||
| Abyaneh et al. 2016 | Y | N | Y | N | N | N | Y | Y | Y | N | 5/10 |
| Espí-López et al. 2017 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Mason et al. 2017 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 9/10 |
| Sutlive et al. 2018 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 9/10 |
| Patel et al. 2019 | Y | N | Y | N | N | N | Y | Y | Y | Y | 6/10 |
| Zarei et al. 2019 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Knee Osteoarthritis | |||||||||||
| Itoh et al. 2008 | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Sánchez-Romero et al. 2019 | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 |
| Post-Surgery Knee Pain | |||||||||||
| Mayoral et al. 2013 | Y | N | Y | Y | N | Y | Y | Y | Y | Y | 8/10 |
| Velázquez-Saornil et al. 2017 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 |
| Study | Intervention(s) | Sample Size | Intervention Duration (Sessions/Weeks) | Comparison and Outcome Measure | Between-Groups Differences (95%CI) (SMD) |
|---|---|---|---|---|---|
| Patellofemoral Pain Syndrome | |||||
| Abyaneh et al. 2016 | G1: Superficial dry needling plus routine physical therapy G2: Routine physical therapy | N = 17 N = 17 | DN: 5 ss 1 every two days for 10 days Routine Physical Therapy: 5 × 2 weeks | Pain (VAS) G1 vs. G2 | 0wk: −1.11 (−2.13, −0.09) (−0.72) |
| Espí-López et al. 2017 | G1: Manual therapy and exercise plus dry needling G2: Manual therapy and exercise | N = 30 N = 30 | 1 × 3 weeks 1 × 3 weeks | Pain (NPRS) G1 vs. G2 G1 vs. G2 Pain (KOOS) G1 vs. G2 G1 vs. G2 Symptoms (KOOS) G1 vs. G2 G1 vs. G2 Function in daily living (KOOS) G1 vs. G2 G1 vs. G2 Function in sport and recreation (KOOS) G1 vs. G2 G1 vs. G2 Quality of life (KOOS) G1 vs. G2 G1 vs. G2 Disability (IKDC) G1 vs. G2 G1 vs. G2 Pain (IKDC pain subscale) G1 vs. G2 G1 vs. G2 Function (IKDC function subscale) G1 vs. G2 G1 vs. G2 | 15d: −0.3 (−0.9, 0.3) (−0.09) 3mo: 0.3 (−0.2, 0.8) (0.09) 15d: −2.9 (−5.8, 0.0) (−0.) 3mo: −2.1 (−4.6, 0.4) (−0.13) 15d: −0.7 (−2.4, 1.0) (−0.06) 3mo: −0.8 (−1.9, 0.3) (−0.06) 15d: −0.9 (−1.8, 0.0) (−0.08) 3mo: −2.8 (−5.7, 0.1) (−0.21) 15d: 0.2 (−1.0, 1.4) (0.01) 3mo: −3.2 (−6.4, 0.0) (−0.16) 15d: 1.2 (−1.0, 3.4) (0.14) 3mo: 3.5 (−0.5, 7.5) (0.21) 15d: 2.9 (0.0, 5.8) (0.19) 3mo: 2.3 (−0.1, 4.7) (0.17) 15d: 1.9 (−2.0, 5.8) (0.18) 3mo: 0.2 (−0.1, 0.5) (0.02) 15d: −2.3 (−6.0, 1.4) (−0.24) 3mo: 1.5 (0.0, 3.0) (0.36) |
| Mason et al. 2017 | G1: Dry needling and Stretching G2: Sham Dry Needling and Stretching | N = 20 N = 19 | 2 × 1 week 2 × 1 week | Deep squat pain (VAS) G1 vs. G2 Step down pain (VAS) G1 vs. G2 Disability (LEFS) G1 vs. G2 Active Knee Extension G1 vs. G2 Active Straight Leg Raise G1 vs. G2 Deep squat range of motion G1 vs. G2 | 7d: −6.00 (−17.80, 5.80) (−0.31) 7d: −6.80 (−16.63, 3.03) (−0.44) 7d: 3.04 (−2.70, 8.78) (0.33) 7d: 0.31 (−6.23, 6.85) (0.03) 7d: 0.04 (−5.12, 5.20) (0.00) 7d: 3.38 (−10.50, 17.26) (0.15) |
| Sutlive et al. 2018 | G1: DN and isometric and stretching quadriceps home-exercises G2: Sham DN and isometric and stretching quadriceps home-exercises | N = 30 N = 30 | 1 session 1 session | Pain squat (NPRS) G1 vs. G2 Pain upstairs (NPRS) G1 vs. G2 Pain down stairs (NPRS) G1 vs. G2 Disability function (LEFS) G1 vs. G2 Disability (Kujala) G1 vs. G2 | 72hr: 0.60 (−0.40, 1.60) (0.30) 72hr: 0.00 (−0.72, 0.72) (0.00) 72hr: 0.40 (−0.24, 1.04) (0.31) 72hr: 3.50 (−2.90, 9.90) (0.28) 72hr: 6.20(0.21, 12.19) (0.52) |
| Patel et al. 2019 | G1: Dry needling G2: Ultrasound | N = 35 N = 35 | DN: 1 session Ultrasound: 1 session | Pain (NPRS) G1 vs. G2 Sensitivity (PPT) G1 vs. G2 | 0wk: −0.97 (−1.60, −0.34) (−0.71) 0wk: 5.28 (2.57, 7.99) (0.90) |
| Zarei et al. 2019 | G1: Dry needling plus exercise program G2: Exercise program | N = 20 N = 20 | DN: 1 × 4 weeks Exercise program: 5 × 4 weeks | Pain (NPRS) G1 vs. G2 G1 vs. G2 Disability (Kujala) G1 vs. G2 G1 vs. G2 Pain sensitivity (PPT gluteus medium) G1 vs. G2 G1 vs. G2 Pain sensitivity (PPT quadratus lumborum) G1 vs. G2 G1 vs. G2 Step-down test G1 vs. G2 G1 vs. G2 SEBT anterior G1 vs. G2 G1 vs. G2 SEBT posterolateral G1 vs. G2 G1 vs. G2 SEBT posteromedial G1 vs. G2 G1 vs. G2 | 0wk: −2.00 (−2.63, −1.37) (−1.94) 2wk: −2.10 (−2.68, −1.52) (−2.18) 0wk: 8.00 (4.51, 11.49) (1.39) 2wk: 11.35 (8.07, 14.63) (2.10) 0wk: 2.97 (2.53, 3.41) (4.09) 2wk: 3.45 (3.08, 3.82) (5.68) 0wk: 2.75 (2.29, 3.21) (3.64) 2wk: 2.88 (2.53, 3.23) (4.93) 0wk: 6.45 (4.03, 8.87) (1.62) 2wk: 7.15 (5.18, 9.12) (2.20) 0wk: 0.08 (0.00, 0.16) (0.62) 2wk: 0.09 (0.02, 0.16) (0.73) 0wk: 0.05 (−0.02, 0.12) (0.43) 2wk: 0.08 (0.01, 0.15) (0.71) 0wk: 0.08 (0.02, 0.14) (0.78) 2wk: 0.08 (0.02, 0.14) (0.78) |
| Knee Osteoarthritis | |||||
| Itoh et al. 2008 | G1: Trigger Point Dry needling G2: AcupunctureG3: Sham Dry Needling | N = 8 N = 9 N = 7 | 1 × 5 weeks 1 × 5 weeks 1 × 5 weeks | Pain (VAS) G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G3 G1 vs. G3 G1 vs. G3 G2 vs. G3 G2 vs. G3 G2 vs. G3 Disability (WOMAC) G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G3 G1 vs. G3 G1 vs. G3 G2 vs. G3 G2 vs. G3 G2 vs. G3 | 5wk: −1.65 (−3.28, −0.02) (−0.91) 10wk: −0.50 (−2.25, 1.25) (−0.25) 20wk: −0.10 (−1.17, 0.97) (−0.08) 5wk: −3.10 (−4.48, −1.72) (−2.09) 10wk: −1.25 (−3.14, 0.64) (−0.65) 20wk: −0.90 (−2.61, 0.81) (−0.52) 5wk: −1.45 (−2.88, −0.02) (−0.89) 10wk: −0.75 (−2.40, 0.90) (−0.39) 20wk: −0.80 (−2.51, 0.91) (−0.47) 5wk: −12.31 (−21.98, −2.63) (−1.19) 10wk: −5.90 (−16.06, 4.26) (−0.53) 20wk: −1.00 (−8.39, 6.39) (−0.12) 5wk: −18.45 (−28.92, −7.98) (−1.63) 10wk: −9.70 (−20.61, 1.21) (−0.84) 20wk: −4.00 (−14.87, 6.87) (−0.37) 5wk: −6.14 (−13.37, 1.09) (−0.81) 10wk: −3.80 (−13.43, 5.83) (−0.37) 20wk: −3.00 (−15.04, 9.04) (−0.24) |
| Sánchez-Romero et al. 2019 | G1: Trigger Point Dry needling plus therapeutic exercise G2: Sham Dry Needling plus therapeutic exercise | N = 31 N = 31 | 1 × 6 weeks Therapeutic exercise: 24 sessions for 12 weeks 1 × 6 weeks Therapeutic exercise: 24 sessions for 12 weeks | Pain (NPRS) G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 Disability (WOMAC) G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 Quality of life (EQ-5D) G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 Barthel Index G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 Time Up and Go Test G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 G1 vs. G2 Medication consumption G1 vs. G2 | 0wk: −0.17 (−1.33, 0.99) (−0.07) 3mo: −0.65 (−2.22, 0.92) (−0.20) 6mo: −0.80 (−2.45, 0.85) (−0.24) 9mo: −0.32 (−1.67, −1.0.3) (−0.12) 12mo: −0.20 (−1.02, 0.26) (−0.06) 0wk: −3.39 (−10.56, 3.78) (−0.23) 3mo: −4.36 (−11.25, 2.53) (−0.31) 6mo: −4.23 (−12.07, 3.61) (−0.27) 9mo: −4.06 (−11.55, 3.43) (−0.27) 12mo: −3.32 (−10.77, 4.13) (−0.22) 0wk: −0.77 (−2.05, 0.51) (−0.30) 3mo: −0.60 (−2.02, 0.82) (−0.21) 6mo: −0.70 (−1.97, 0.57) (−0.27) 9mo: −0.73 (−1.90, 0.44) (−0.31) 12mo: −0.50 (−1.95, 0.95) (−0.17) 0wk: 0.58 (−1.74, 2.90) (0.12) 3mo: 0.96 (−2.05, 3.97) (0.16) 6mo: 0.09 (−2.20, 2.38) (0.02) 9mo: 0.07 (−1.76, 1.90) (0.02) 12mo: −0.06 (2.27,.15) (−0.01) 0wk: −0.22 (−1.42, 0.98) (−0.09) 3mo: −0.23 (−1.61, 1.15) (−0.08) 6mo: −0.16 (−152, 1.20) (−0.06) 9mo: −0.58 (−1.83, 0.67) (−0.23) 12mo: −0.45 (−1.77, 0.87) (−0.17) 12mo: −1.62 (−2.79, −0.45) (−0.68) |
| Post-Surgery Knee Pain | |||||
| Mayoral et al. 2013 | G1: Trigger Point Dry needling G2: Sham Dry Needling | N = 22 N = 22 | 1 session 1 session | Pain (VAS) G1 vs. G2 G1 vs. G2 G1 vs. G2 Pain (WOMAC) G1 vs. G2 G1 vs. G2 G1 vs. G2 Stiffness (WOMAC) G1 vs. G2 G1 vs. G2 G1 vs. G2 Disability (WOMAC) G1 vs. G2 G1 vs. G2 G1 vs. G2 ROM G1 vs. G2 Strength (Flexion) G1 vs. G2 Strength (Extension) G1 vs. G2 | 1mo: −0.85 (−2.42, 0.72) (−0.33) 3mo: −0.47 (−1.76, 0.82) (−0.22) 6mo: 0.27 (−1.01, 1.54) (0.13) 1mo: 0.93 (−1.21, 3.07) (0.26) 3mo: 1.24 (−0.54, 3.02) (0.42) 6mo: 0.11 (−1.67, 1.89) (0.04) 1mo: 0.09 (−0.81, 0.99) (0.06) 3mo: 0.07 (−1.01, 1.15) (0.04) 6mo: 0.09 (−1.17, 0.97) (−0.08) 1mo: 4.02 (−1.91, 9.95) (0.41) 3mo: 3.18 (−3.61, 9.97) (0.28) 6mo: −0.83 (−6.82, 5.16) (−0.08) 1mo: −3.01 (−13.87, 7.85) (−0.17) 1mo: −0.76 (−4.62, 3.10) (−0.12) 1mo: −1.10 (−5.27, 3.07) (−0.16) |
| Velázquez-Saornil et al. 2017 | G1: Rehabilitation plus TrP DN G2; Rehabilitation alone | N = 22 N = 22 | DN: 1 session Rehabilitation: 5 × 5 weeks | Pain (VAS) G1 vs. G2 Disability (WOMAC) G1 vs. G2 ROM G1 vs. G2 Balance (SEBT) G1 vs. G2 | 0wk: −0.48 (−1.08, 0.12) (−0.47) 0wk: −4.52 (−6.76, −2.28) (−1.17) 0wk: 2.86 (0.03, 5.69) (0.60) 0wk: 2.30 (−0.79, 5.39) (0.44) |
| Number of Studies | Risk of Bias | Inconsistency | Indirectness of Evidence | Imprecision | Publication Bias | Quality of Evidence | SMD (95% CI) |
|---|---|---|---|---|---|---|---|
| Effects of Dry Needling on Knee Pain at Short-term | |||||||
| Overall effect (n = 10) | No | Serious (I2 = 68%) | No | No | No | Moderate | −0.53 (−0.87, −0.19) * |
| Patellofemoral Pain (n = 6) | No | Serious (I2 = 80%) | No | No | No | Moderate | −0 64 (−1.17, −0.11) * |
| Knee Osteoarthritis (n = 2) | No | Serious (I2 = 52%) | No | Very serious | No | Very Low | −0.37 (−1.15, 0.41) |
| Post-Surgery Knee Pain (n = 2) | No | No (I2 = 0%) | No | Very serious | No | Low | −0.40 (−0.84, 0.04) |
| Effects of Dry Needling on Knee Pain at Mid-term | |||||||
| Overall effect (n = 4) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.11 (−0.41, 0.18) |
| Patellofemoral Pain (n = 1) | No | No | No | Very Serious | No | Low | 0.09 (−0.42, 0.60) |
| Knee Osteoarthritis (n = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.21 (−0.66, 0.23) |
| Post-Surgery Knee Pain (n = 1) | No | No | No | Very Serious | No | Low | −0.22 (−0.84, 0.40) |
| Effects of Dry Needling on Knee Pain at Long-term | |||||||
| Overall effect (n = 3) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.00 (−0.36, 0.36) |
| Knee Osteoarthritis (n = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.07 (−0.51, 0.37) |
| Post-Surgery Knee Pain (n = 1) | No | No | No | Very Serious | No | Low | 0.13 (−0.49, 0.75) |
| Effects of Dry Needling on RelatedDisability at Short-term | |||||||
| Overall effect (n = 8) | No | Serious (I2 = 80%) | No | No | No | Moderate | −0.58 (−1.08, −0.09) * |
| Patellofemoral Pain (n = 4) | No | Very Serious (I2 = 85%) | No | No | No | Low | −0 69 (−1.46, 0.09) |
| Knee Osteoarthritis (n = 2) | No | Serious (I2 = 61%) | No | Very serious | No | Very Low | −0.60 (−1.51, 0.32) |
| Post-Surgery Knee Pain (n = 2) | No | Very Serious (I2 = 91%) | No | Very serious | No | Very Low | −0.37 (−1.92, 1.17) |
| Effects of Dry Needling on RelatedDisability at Mid-term | |||||||
| Overall effect (n = 4) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.10 (−0.39, 020) |
| Patellofemoral Pain (n = 1) | No | No | No | Very Serious | No | Low | −0.01 (−0.52, 0.49) |
| Knee Osteoarthritis (n = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.36 (−0.80, 0.09) |
| Post-Surgery Knee Pain (n = 1) | No | No | No | Very Serious | No | Low | 0.28 (−0.34, 0.91) |
| Effects of Dry Needling on RelatedDisability at Long-term | |||||||
| Overall effect (n = 3) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.16 (−0.52, 0.20) |
| Knee Osteoarthritis (n = 2) | No | No (I2 = 0%) | No | Very Serious | No | Low | −0.20 (−0.64, 0.25) |
| Post-Surgery Knee Pain (n = 1) | No | No | No | Very Serious | No | Low | −0.08 (0.70, 0.54) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahou-El-Bachiri, Y.; Navarro-Santana, M.J.; Gómez-Chiguano, G.F.; Cleland, J.A.; López-de-Uralde-Villanueva, I.; Fernández-de-las-Peñas, C.; Ortega-Santiago, R.; Plaza-Manzano, G. Effects of Trigger Point Dry Needling for the Management of Knee Pain Syndromes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2044. https://doi.org/10.3390/jcm9072044
Rahou-El-Bachiri Y, Navarro-Santana MJ, Gómez-Chiguano GF, Cleland JA, López-de-Uralde-Villanueva I, Fernández-de-las-Peñas C, Ortega-Santiago R, Plaza-Manzano G. Effects of Trigger Point Dry Needling for the Management of Knee Pain Syndromes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(7):2044. https://doi.org/10.3390/jcm9072044
Chicago/Turabian StyleRahou-El-Bachiri, Youssef, Marcos J. Navarro-Santana, Guido F Gómez-Chiguano, Joshua A Cleland, Ibai López-de-Uralde-Villanueva, César Fernández-de-las-Peñas, Ricardo Ortega-Santiago, and Gustavo Plaza-Manzano. 2020. "Effects of Trigger Point Dry Needling for the Management of Knee Pain Syndromes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 7: 2044. https://doi.org/10.3390/jcm9072044
APA StyleRahou-El-Bachiri, Y., Navarro-Santana, M. J., Gómez-Chiguano, G. F., Cleland, J. A., López-de-Uralde-Villanueva, I., Fernández-de-las-Peñas, C., Ortega-Santiago, R., & Plaza-Manzano, G. (2020). Effects of Trigger Point Dry Needling for the Management of Knee Pain Syndromes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(7), 2044. https://doi.org/10.3390/jcm9072044






