Endocrinopathies Associated with Immune Checkpoint Inhibitor Cancer Treatment: A Review
Abstract
1. Introduction
2. Hypopituitarism
3. Adrenal Insufficiency
4. Thyroid Dysfunction
5. Type 1 Diabetes Mellitus
6. Treatment
6.1. Hypopituitarism
6.2. Adrenal Insufficiency
6.3. Thyroid Dysfunction
6.4. Type 1 Diabetes Mellitus
7. Adrenal Insufficiency in Tumor-Bearing Patients
8. Adrenal Crisis
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Salomon, B.; Bluestone, J.A.C. Omplexities of Cd28/B7: Ctla-4 C Ostimulatory P Athways In A Utoimmunity And T Ransplantation. Annu. Rev. Immunol. 2001, 19, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Teft, W.A.; Kirchhof, M.G.; Madrenas, J. A molecular perspective of ctla-4 function. Annu. Rev. Immunol. 2006, 24, 65–97. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Bulliard, Y.; Jolicoeur, R.; Windman, M.; Rue, S.M.; Ettenberg, S.; Knee, D.A.; Wilson, N.S.; Dranoff, G.; Brogdon, J.L. Activating fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 2013, 210, 1685–1693. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory t cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 2018, 40, 17–65. [Google Scholar] [CrossRef]
- Davies, M.; Duffield, E.A. Safety of checkpoint inhibitors for cancer treatment: Strategies for patient monitoring and management of immune-mediated adverse events. ImmunoTargets Ther. 2017, 6, 51–71. [Google Scholar] [CrossRef]
- Postow, M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. B 2015, 76–83. [Google Scholar] [CrossRef] [PubMed]
- De Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Win, M.A.; Thein, K.Z.; Qdaisat, A.; Yeung, S.C.J. Acute symptomatic hypocalcemia from immune checkpoint therapy-induced hypoparathyroidism. Am. J. Emerg. Med. 2017, 35, 1039.e5–1039.e7. [Google Scholar] [CrossRef]
- Umeguchi, H.; Takenoshita, H.; Inoue, H.; Kurihara, Y.; Sakaguchi, C.; Yano, S.; Hasuzawa, N.; Sakamoto, S.; Sakamoto, R.; Ashida, K. Autoimmune-Related Primary Hypoparathyroidism Possibly Induced by the Administration of Pembrolizumab: A Case Report. J. Oncol. Pract. 2018, 14, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.; Sanchez, G.O.; Herzig, P.; Läubli, H. Inflammation-induced hypoparathyroidism triggered by combination immune checkpoint blockade for melanoma. J. Immunother. Cancer 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Piranavan, P.; Li, Y.; Brown, E.; Kemp, E.H.; Trivedi, N. Immune Checkpoint Inhibitor-Induced Hypoparathyroidism Associated with Calcium-Sensing Receptor-Activating Autoantibodies. J. Clin. Endocrinol. Metab. 2018, 104, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015, 13. [Google Scholar] [CrossRef]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy-immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens a systematic review and meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- González-Rodríguez, E.; Rodríguez-Abreu, D. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Cukier, P.; Santini, F.C.; Scaranti, M.; Hoff, A.O. Endocrine side effects of cancer immunotherapy. Endocr. Relat. Cancer 2017, 24, T331–T347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tella, S.H.; Rivero, J.D.e.l.; Kommalapati, A.; Ebenuwa, I.; Gulley, J.; Strauss, J.; Brownell, I. Anti-PD-L1 treatment induced central diabetes insipidus. J. Clin. Endocrinol. Metab. 2018, 103, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.; Reynolds, K.; Zubiri, L.; Lawrence, D.; Cohen, J.V.; Sullivan, R.J.; Nachtigall, L.; Tritos, N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 2019, 181, 211–219. [Google Scholar] [CrossRef]
- Kanie, K.; Iguchi, G.; Bando, H.; Fujita, Y.; Odake, Y.; Yoshida, K.; Matsumoto, R.; Fukuoka, H.; Ogawa, W.; Takahashi, Y. Two Cases of Atezolizumab-Induced Hypophysitis. J. Endocr. Soc. 2018, 2, 91–95. [Google Scholar] [CrossRef]
- Cho, K.Y.; Miyoshi, H.; Nakamura, A.; Kurita, T.; Atsumi, T. Hyponatremia can be a powerful predictor of the development of isolated ACTH deficiency associated with nivolumab treatment. Endocr. J. 2017, 64, 235–236. [Google Scholar] [CrossRef]
- Okano, Y.; Satoh, T.; Horiguchi, K.; Toyoda, M.; Osaki, A.; Matsumoto, S.; Tomaru, T.; Nakajima, Y.; Ishii, S.; Ozawa, A.; et al. Nivolumab-induced hypophysitis in a patient with advanced malignant melanoma. Endocr. J. 2016, 63, 905–912. [Google Scholar] [CrossRef]
- Albarel, F.; Gaudy, C.; Castinetti, F.; Carré, T.; Morange, I.; Conte-Devolx, B.; Grob, J.J.; Brue, T. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur. J. Endocrinol. 2015, 172, 195–204. [Google Scholar] [CrossRef]
- Ryder, M.; Callahan, M.; Postow, M.A.; Wolchok, J.; Fagin, J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 2014, 21, 371–381. [Google Scholar] [CrossRef]
- Trainer, H.; Hulse, P.; Higham, C.E.; Trainer, P.; Lorigan, P. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinol. Diabetes Metab. Case Rep. 2016, 2016. [Google Scholar] [CrossRef]
- Paepegaey, A.-C.; Lheure, C.; Ratour, C.; Lethielleux, G.; Clerc, J.; Bertherat, J.; Kramkimel, N.; Groussin, L. Polyendocrinopathy Resulting From Pembrolizumab in a Patient With a Malignant Melanoma. J. Endocr. Soc. 2017, 1, 646–649. [Google Scholar] [CrossRef]
- Min, L.; Ibrahim, N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013, 1, e15. [Google Scholar] [CrossRef]
- Inaba, H.; Ariyasu, H.; Okuhira, H.; Yamamoto, Y.; Akamatsu, H.; Katsuda, M.; Jinnin, M.; Hara, I.; Akamizu, T. Endocrine dysfunctions during treatment of immune-checkpoint inhibitors. Trends Immunother. 2018, 2. [Google Scholar] [CrossRef]
- Gan, E.H.; Mitchell, A.L.; Plummer, R.; Pearce, S.; Perros, P. Tremelimumab-Induced Graves Hyperthyroidism. Eur. Thyroid J. 2017, 6, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, S.; Fujiwara, Y.; Iwama, S.; Ohashi, K.; Kuchiba, A.; Arima, H.; Yamazaki, N.; Kitano, S.; Yamamoto, N.; Ohe, Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018, 109, 3583–3590. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Tsunekawa, T.; Onoue, T.; Takagi, H.; Hagiwara, D.; Ito, Y.; Morishita, Y.; et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J. Endocr. Soc. 2018, 2, 241–251. [Google Scholar] [CrossRef]
- Alhusseini, M.; Samantray, J. Hypothyroidism in Cancer Patients on Immune Checkpoint Inhibitors with anti-PD1 Agents: Insights on Underlying Mechanisms. Exp. Clin. Endocrinol. Diabetes 2017, 125, 267–269. [Google Scholar] [CrossRef]
- Orlov, S.; Salari, F.; Kashat, L.; Walfish, P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J. Clin. Endocrinol. Metab. 2015, 100, 1738–1741. [Google Scholar] [CrossRef]
- De Filette, J.; Jansen, Y.; Schreuer, M.; Everaert, H.; Velkeniers, B.; Neyns, B.; Bravenboer, B. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J. Clin. Endocrinol. Metab. 2016, 101, 4431–4439. [Google Scholar] [CrossRef]
- Delivanis, D.A.; Gustafson, M.P.; Bornschlegl, S.; Merten, M.M.; Kottschade, L.; Withers, S.; Dietz, A.B.; Ryder, M. Pembrolizumab-induced thyroiditis: Comprehensive clinical review and insights into underlying involved mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef]
- Yamauchi, I.; Sakane, Y.; Fukuda, Y.; Fujii, T.; Taura, D.; Hirata, M.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid 2017, 27, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Shiba, M.; Inaba, H.; Ariyasu, H.; Kawai, S.; Inagaki, Y.; Matsuno, S.; Iwakura, H.; Yamamoto, Y.; Nishi, M.; Akamizu, T. Fulminant type 1 diabetes mellitus accompanied by positive conversion of anti-insulin antibody after the administration of anti-CTLA-4 antibody following the discontinuation of anti-PD-1 antibody. Intern. Med. 2018, 57, 2029–2034. [Google Scholar] [CrossRef]
- Baden, M.Y.; Imagawa, A.; Abiru, N.; Awata, T.; Ikegami, H.; Uchigata, Y.; Oikawa, Y.; Osawa, H.; Kajio, H.; Kawasaki, E.; et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol. Int. 2019, 10, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, A.; Hanafusa, T.; Awata, T.; Ikegami, H.; Uchigata, Y.; Osawa, H.; Kawasaki, E.; Kawabata, Y.; Kobayashi, T.; Shimada, A.; et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J. Diabetes Investig. 2012, 3, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Hodi, F.S.; Giobbie-Hurder, A.; Ott, P.A.; Luke, J.J.; Donahue, H.; Davis, M.; Carroll, R.S.; Kaiser, U.B. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: A retrospective cohort study. Clin. Cancer Res. 2015, 21, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Tajima, T.; Katabami, T.; Iwasaki, Y.; Tanahashi, Y.; Sugawara, A.; Hasegawa, T.; Mune, T.; Oki, Y.; Nakagawa, Y.; et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: A Japan endocrine society clinical practice guideline. Endocr. J. 2016, 63, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-Y.; Lo, C.-Y. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin. Endocrinol. (Oxf). 2002, 56, 95–101. [Google Scholar] [CrossRef]
- Barker, N.W. The pathologic anatomy in twenty-eight cases of Addison’s disease. Arch. Pathol. 1929, 8, 432–450. [Google Scholar]
- Raschi, E.; Mazzarella, A.; Antonazzo, I.C.; Bendinelli, N.; Forcesi, E.; Tuccori, M.; Moretti, U.; Poluzzi, E.; De Ponti, F. Toxicities with Immune Checkpoint Inhibitors: Emerging Priorities From Disproportionality Analysis of the FDA Adverse Event Reporting System. Target Oncol. 2019, 14, 205–221. [Google Scholar] [CrossRef]
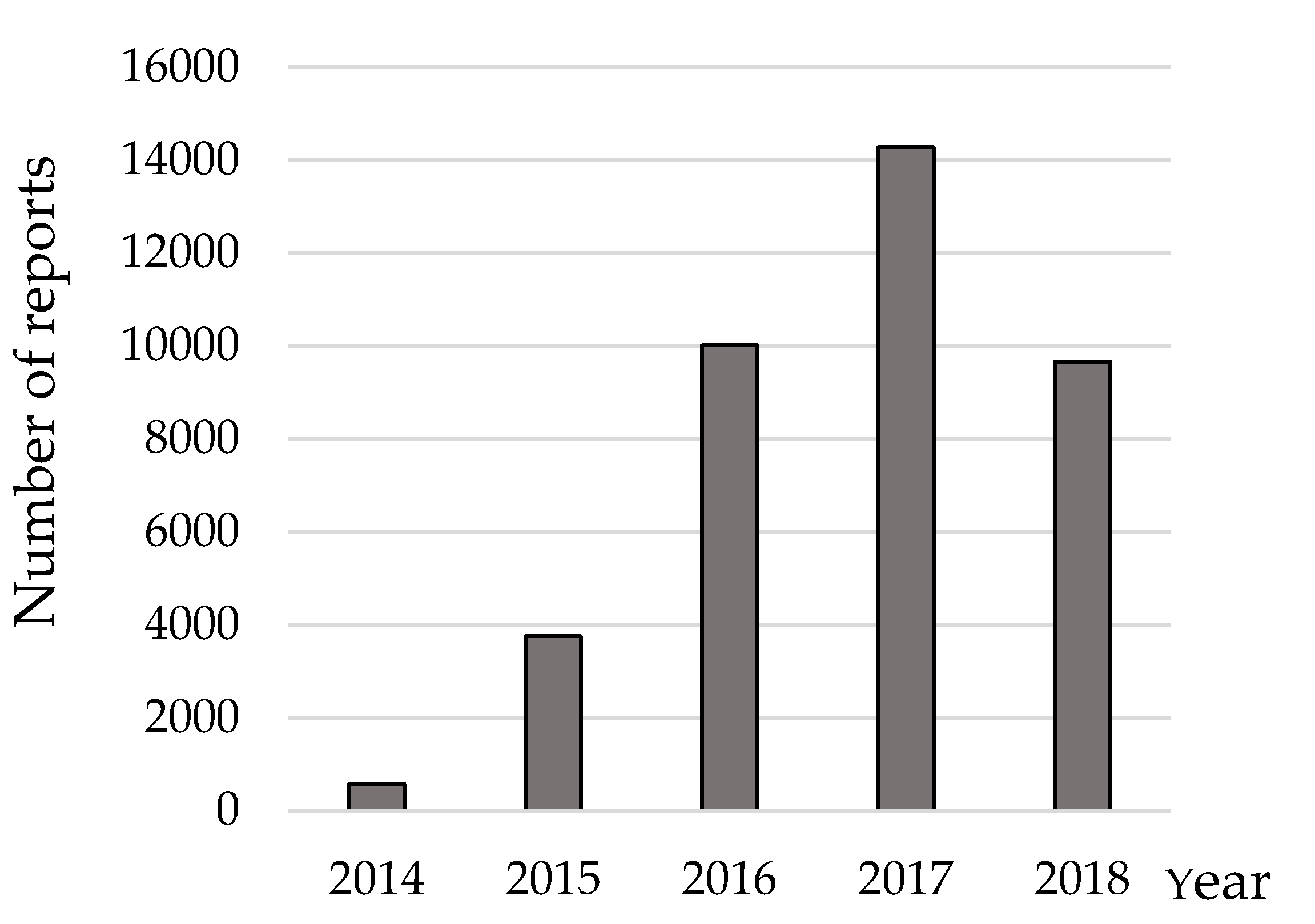
| Target | Drug | Indication | Major Endocrinopathies |
|---|---|---|---|
| Anti CTLA-4 antibody | Ipilimumab | Malignant melanoma | Hypopituitarism |
| Renal cell cancer | |||
| Anti-PD-1 antibody | Nivolumab | Malignant melanoma | Hypothyroidism Hyperthyroidism |
| Non-small cell lung cancer | |||
| Renal cell cancer | |||
| Hodgkin lymphoma | |||
| Head and neck cancer | |||
| Gastric cancer | |||
| Malignant mesothelioma | |||
| Colorectal cancer with high-frequency microsatellite instability (MSI-High) | |||
| Esophageal cancer | |||
| Pembrolizumab | Non-small cell lung cancer | Hypothyroidism Hyperthyroidism | |
| Hodgkin lymphoma | |||
| Urothelial cancer | |||
| Solid cancers with high-frequency microsatellite instability (MSI-High) | |||
| Renal cell cancer | |||
| Head and neck cancer | |||
| Anti PD-L1 antibody | Atezolizumab | Non-small cell lung cancer | Hypothyroidism |
| Small cell lung cancer | |||
| Breast cancer | |||
| Durvalumab | Non-small cell lung cancer | Hypothyroidism | |
| Hyperthyroidism | |||
| Avelumab | Merkel cell carcinoma renal cell cancer | Hypothyroidism |
| CTCAE Grade | Management | Treatment of Adverse Event |
|---|---|---|
| Grade 1 |
|
|
| Grade 2 |
|
|
| Grade 3 |
|
|
| Grade 4 |
|
|
| CTCAE Grade | Management | Treatment of Adverse Event |
|---|---|---|
| Grade 1 |
|
|
| Grade 2 |
|
|
| Grade 3 |
|
|
| Grade 4 |
|
|
| CTCAE Grade | Management | Treatment of Adverse Event |
|---|---|---|
| Grade 1 |
|
|
| Grade 2 |
|
|
| Grade 3 or 4 |
|
|
| CTCAE Grade | Management | Treatment of Adverse Event |
|---|---|---|
| Grade 1 |
|
|
| Grade 2 |
|
|
| Grade 3 or 4 |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okura, N.; Asano, M.; Uchino, J.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Fukui, M.; Takayama, K. Endocrinopathies Associated with Immune Checkpoint Inhibitor Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 2033. https://doi.org/10.3390/jcm9072033
Okura N, Asano M, Uchino J, Morimoto Y, Iwasaku M, Kaneko Y, Yamada T, Fukui M, Takayama K. Endocrinopathies Associated with Immune Checkpoint Inhibitor Cancer Treatment: A Review. Journal of Clinical Medicine. 2020; 9(7):2033. https://doi.org/10.3390/jcm9072033
Chicago/Turabian StyleOkura, Naoko, Mai Asano, Junji Uchino, Yoshie Morimoto, Masahiro Iwasaku, Yoshiko Kaneko, Tadaaki Yamada, Michiaki Fukui, and Koichi Takayama. 2020. "Endocrinopathies Associated with Immune Checkpoint Inhibitor Cancer Treatment: A Review" Journal of Clinical Medicine 9, no. 7: 2033. https://doi.org/10.3390/jcm9072033
APA StyleOkura, N., Asano, M., Uchino, J., Morimoto, Y., Iwasaku, M., Kaneko, Y., Yamada, T., Fukui, M., & Takayama, K. (2020). Endocrinopathies Associated with Immune Checkpoint Inhibitor Cancer Treatment: A Review. Journal of Clinical Medicine, 9(7), 2033. https://doi.org/10.3390/jcm9072033






