Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient Evaluation Measures
- Medical and gynecological examination: after obtaining a detailed medical, obstetric, and gynecological history, a vulvovaginal examination was performed. This included vaginal pH measurement, saline and 10% potassium hydroxide microscopy, yeast cultures and sexually transmitted infections (STIs) screening. In addition, localized vestibular atrophy, characterized by vestibular (but not vaginal) mucosal thinning, dryness and erythema was assessed. This particular vestibular atrophy is not referred to as “vaginal atrophy”, is often ignored and thus, its description is usually absent from clinical assessment. Patients were asked to provide dyspareunia history (primary/secondary), duration of dyspareunia, and current or prior use of systemic hormonal contraception (HC): oral contraceptives, transdermal patch, or vaginal ring.
- Pain evoked during vaginal intercourse: Self-report of pain intensity ratings experienced during sexual intercourse were assessed using a 0–10 Visual Analogue Scale (VAS), with 0 representing no pain and 10 being the worst possible pain.
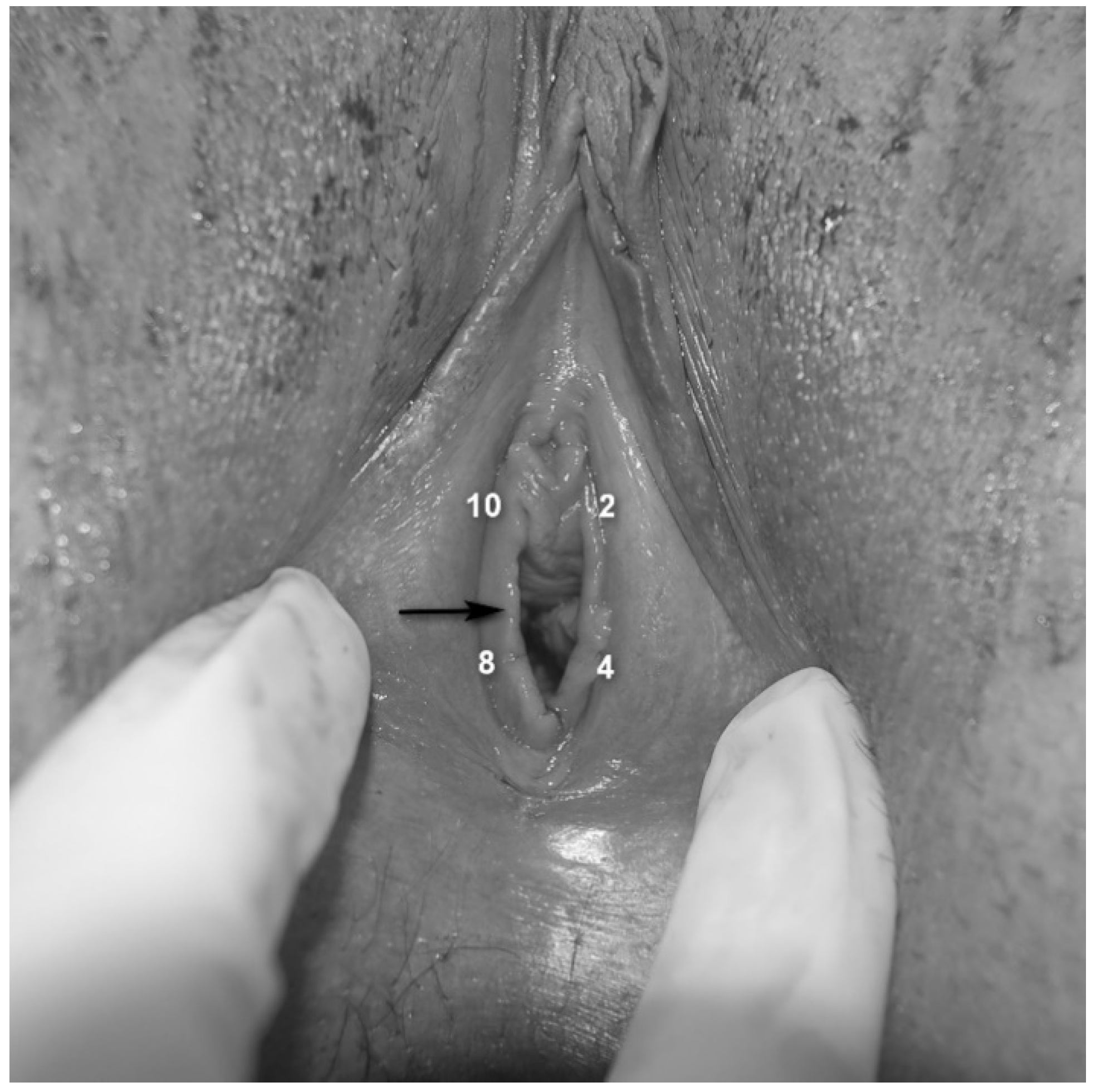
- Assessment of vestibular pain [25]: Vestibular tenderness was assessed by the Q-tip test, using a moistened cotton-tip applicator and touching the vestibule in four defined points (2, 4, 8 and 10 o’clock—Figure 1), with an interval of 5 s between each stimulus. The Q-tip test was performed twice, first to localize vestibular tenderness at each point (yes/no) and secondly, to quantify pain intensity using a Numeric Pain Scale (NPS) ranging from 0 to 10 at each point, with 0 corresponding to no pain and 10 being the worst possible pain.
- Pain rating in response to deep muscle palpation: patients were requested to report pain intensity using a 0–10 NPS, in response to pressure applied bilaterally to the puborectalis muscles with the examiner’s index finger.
- Pelvic floor muscle hypertonicity: the physician’s impression of hypertonicity (mild, moderate and severe) of the pelvic floor musculature was measured by applying pressure with the examiner’s index finger bilaterally to the puborectalis muscles.
- Assessment of rigid/constricting hymenal ring: This was done by placing 2 fingers at the introitus and stretching the hymenal ring (Figure 1) laterally [26,27], avoiding pressing or stretching of the underlying muscles. If insertion of two fingers was impossible due to obliterating hymenal tissue (but not contraction of the muscles or vaginismus), or if this hymenal-ring stretching provoked pain similar to the pain experienced by the patient with penetration and the physician identified a thick/rigid hymen, the patient was reported to have a “constricting hymen”.
- Umbilical hypersensitivity: Umbilical tenderness was assessed by a dry cotton-tip applicator by touching it gently and asking the patient to report hypersensitivity (yes or no). Given the common endodermal embryological origin of the vestibular mucosa and the umbilicus, hypersensitivity to touch in this location was considered to be a possible representative of congenital vestibular neuroproliferation [28].
- Level of desire and vaginal lubrication were assessed by calculation of the relevant domains in the Female Sexual Function Index, which was completed by the participants.
2.3. Allocation into the Anterior and Posterior Vestibular Tenderness Groups
2.4. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Characteristics of Circumferential Vs. Posterior-Only Vestibular Tenderness Hypersensitivity
3.3. Characteristics of Vestibular-Hypersensitivity in the Circumferential Vestibular Tenderness and the Posterior-Only Vestibular Tenderness Groups
3.4. Construction of the Four Vestibular Tenderness Subgroups
3.4.1. The Distinctive Characteristics of the Four Subgroups
3.4.2. Four Group Comparisons of Experimental Provoked Pain Measures
3.5. Prediction of Augmented Pain Hypersensitivity at the Anterior Vestibule
3.6. Prediction of Augmented Pain Hypersensitivity at the Posterior Vestibule
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual for Mental Disorders, 5th ed.; American Psychiatric Press: Philadelphia, PA, USA, 2013. [Google Scholar]
- Harlow, B.L.; Wise, L.A.; Stewart, E.G. Prevalence and predictors of chronic lower genital tract discomfort. Am. J. Obstet. Gynecol. 2001, 185, 545–550. [Google Scholar] [CrossRef]
- Laumann, E.O.; Paik, A.; Rosen, RC. Sexual dysfunction in the United States: Prevalence and predictors. J. Am. Med. Assoc. 1999, 281, 537–544. [Google Scholar] [CrossRef]
- Danielsson, I.; Sjöberg, I.; Stenlund, H.; Wikman, M. Prevalence and incidence of prolonged and severe dyspareunia in women: Results from a population study. Scand. J. Pub. Health 2003, 31, 113–118. [Google Scholar] [CrossRef]
- Bornstein, J.; Goldstein, A.T.; Stockdale, C.K.; Bergeron, S.; Pukall, C.; Zolnoun, D.; Coady, D. 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and Vulvodynia. Obstet. Gynecol. 2016, 127, 745–751. [Google Scholar] [CrossRef]
- Pukall, C.F.; Binik, Y.M.; Khalifé, S.; Amsel, R.; Abbott, F.V. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain 2002, 96, 163–175. [Google Scholar] [CrossRef]
- Bohm-Starke, N.; Hilliges, M.; Falconer, C.; Rylander, E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol. Obstet. Investig. 1998, 46, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Reissing, E.D.; Brown, C.; Lord, M.J.; Binik, Y.M.; Khalifé, S. Pelvic floor muscle functioning in women with vulvar vestibulitis syndrome. J. Psychosom. Obstet. Gynaecol. 2005, 26, 107–113. [Google Scholar] [CrossRef]
- Gentilcore-Saulnier, E.; McLean, L.; Goldfinger, C.; Pukall, C.F.; Chamberlain, S. Pelvic floor muscle assessment outcomes in women with and without provoked vestibulodynia and the impact of a physical therapy program. J. Sex. Med. 2010, 7 2 Pt 2, 1003–1022. [Google Scholar] [CrossRef]
- Wesselmann, U.; Bonham, A.; Foster, D. Vulvodynia: Current state of the biological science. Pain 2014, 155, 1696–1701. [Google Scholar] [CrossRef]
- Pukall, C.F.; Goldstein, A.T.; Bergeron, S.; Foster, D.; Stein, A.; Kellogg-Spadt, S.; Bachmann, G. Vulvodynia: Definition, prevalence, impact, and pathophysiological factors. J. Sex. Med. 2016, 13, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Lev-Sagie, A.; Witkin, S.S. Recent advances in understanding provoked vestibulodynia. F1000Research 2016, 5, 2581. [Google Scholar] [CrossRef] [PubMed]
- Granot, M.; Lavee, Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J. Sex Marital Ther. 2005, 31, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.G. Vulvar vestibulitis syndrome. J. Rep. Med. 1987, 32, 110–114. [Google Scholar]
- Stockdale, C.K.; Lawson, H.W. 2013 Vulvodynia guideline update. J. Low. Genit. Tract Dis. 2014, 18, 93–100. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Rubin, R.; Goldstein, A.T. Current uses of surgery in the treatment of genital pain. Curr. Sex Health Rep. 2014, 3, 252–258. [Google Scholar] [CrossRef]
- Goldstein, A. Moving beyond the diagnosis of vestibulodynia” A holiday wish list. J. Sex. Med. 2009, 6, 3227–3229. [Google Scholar] [CrossRef]
- Donders, G.; Bellen, G. Characteristics of the pain observed in the focal vulvodynia syndrome (VVS). Med. Hypoth. 2012, 78, 11–14. [Google Scholar] [CrossRef]
- Pukall, C.F.; Mitchell, L.S.; Goldstein, A.T. Non-medical, medical, and surgical approaches for the treatment of provoked vestibulodynia. Curr. Sex Health Rep. 2016, 8, 240–248. [Google Scholar] [CrossRef]
- Tommola, P.; Unkila-Kallio, L.; Paetau, A.; Meri, S.; Kalso, E.; Paavonen, J. Immune activation enhances epithelial nerve growth in provoked vestibulodynia. Am. J. Obstet. Gynecol. 2016, 215, 768-e1. [Google Scholar] [CrossRef]
- Halperin, R.; Zehavi, S.; Vaknin, Z.; Ben-Ami, I.; Pansky, M.; Schneider, D. The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gyn. Obstet. Investig. 2005, 59, 75–79. [Google Scholar] [CrossRef]
- Goldstein, A.; Burrows, L.; Goldstein, I. Can oral contraceptives cause vestibulodynia? J. Sex. Med. 2010, 7 Pt 1, 1585–1587. [Google Scholar] [CrossRef]
- Malykhina, A.P. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience 2007, 149, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Engel-Yeger, B.; Ohel, G.; Aronson, D.; Granot, M. The association between supra-physiological levels of estradiol and response patterns to experimental pain. Eur. J. Pain. 2010, 14, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.T.; Pukall, C.F.; Brown, C.; Bergeron, S.; Stein, A.; Kellogg-Spadt, S. Vulvodynia: Assessment and Treatment. J. Sex. Med. 2016, 13, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Brashear, D.B.; Munsick, R.A. Hymenal Dyspareunia. J. Sex Edu. Ther. 1991, 17, 27–31. [Google Scholar] [CrossRef]
- Grillo, L.; Grillo, D. Mangement of dyspareunia secondary to hymenal remnants. Obstet. Gynecol. 1980, 56, 510–514. [Google Scholar] [PubMed]
- Burrows, L.J.; Klingman, D.; Pukall, C.F.; Goldstein, A.T. Umbilical hypersensitivity in women with primary vestibulodynia. J. Rep. Med. 2008, 53, 413–416. [Google Scholar]
- Andrews, J.C. Vulvodynia interventions-systematic review and evidence grading. Obstet. Gynecol. Surv. 2011, 66, 299–315. [Google Scholar] [CrossRef]
- Zolnoun, D.; Bair, E.; Essick, G.; Gracely, R.; Goyal, V.; Maixner, W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J. Pain 2012, 13, 910–920. [Google Scholar] [CrossRef]
- Witzeman, K.; Nguyen, R.H.; Eanes, A.; As-Sanie, S.; Zolnoun, D. Mucosal versus muscle pain sensitivity in provoked vestibulodynia. J. Pain Res. 2015, 8, 549–555. [Google Scholar] [CrossRef]
- Heddini, U.; Bohm-Starke, N.; Nilsson, K.W.; Johannesson, U. Provoked vestibulodynia--medical factors and comorbidity associated with treatment outcome. J. Sex. Med. 2012, 9, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.J.; Basha, M.; Goldstein, A.T. The effects of hormonal contraceptives on female sexuality: A review. J. Sex. Med. 2012, 9, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, U.; Blomgren, B.; Hilliges, M.; Rylander, E.; Bohm-Starke, N. The vulval vestibular mucosa-morphological effects of oral contraceptives and menstrual cycle. Brit. J. Derm. 2007, 157, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Brisson, J.; Fortier, M.; Morin, C.; Blanchette, C. Use of oral contraceptive pills and vulvar vestibulitis: A case-control study. Am. J. Epidem. 2002, 156, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bazin, S.; Bouchard, C.; Brisson, J.; Morin, C.; Meisels, A.; Fortier, M. Vulvar vestibulitis syndrome: An exploratory case-control study. Obstet. Gynecolo. 1994, 83, 47–50. [Google Scholar]
- Sjöberg, I.; Nylander Lundqvist, E.N. Vulvar vestibulitis in the north of Sweden. An epidemiologic case-control study. J. Rep. Med. 1997, 42, 166–168. [Google Scholar]
- Goldstein, A.T.; Belkin, Z.R.; Krapf, J.M.; Song, W.; Khera, M.; Jutrzonka, S.L.; Kim, N.N.; Burrows, L.J.; Goldstein, I. Polymorphisms of the androgen receptor gene and hormonal contraceptive induced provoked vestibulodynia. J. Sex. Med. 2014, 11, 2764–2771. [Google Scholar] [CrossRef]
- Greenstein, A.; Ben-Aroya, Z.; Fass, O.; Militscher, I.; Roslik, Y.; Chen, J.; Abramov, L. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J. Sex. Med. 2007, 4, 1679–1683. [Google Scholar] [CrossRef]
- Reed, B.D.; Harlow, S.D.; Sen, A.; Legocki, L.J.; Edwards, R.M.; Arato, N.; Haefner, H.K. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am. J. Obstet. Gynecol. 2012, 206, 170-e1. [Google Scholar] [CrossRef]
- Harlow, B.L.; Vitonis, A.F.; Stewart, E.G. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J. Rep. Med. 2008, 53, 102–110. [Google Scholar]
- Bohm-Starke, N.; Johannesson, U.; Hilliges, M.; Rylander, E.; Torebjörk, E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: A contributing factor in vulvar vestibulitis? J. Rep. Med. 2004, 49, 888–892. [Google Scholar]
- Burrows, L.J.; Goldstein, A.T. The Treatment of Vestibulodynia with Topical Estradiol and Testosterone. Sex Med. 2013, 1, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M. Modulation of pain by estrogens. Pain 2007, 132 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef]
- Leclair, C.M.; Goetsch, M.F.; Korcheva, V.B.; Anderson, R.; Peters, D.; Morgan, T.K. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstet. Gynecol. 2011, 117, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Bohm-Starke, N.; Hilliges, M.; Falconer, C.; Rylander, E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol. Obstet. Investig. 1999, 48, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Tympanidis, P.; Terenghi, G.; Dowd, P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br. J. Derm. 2003, 148, 1021–1027. [Google Scholar] [CrossRef]
- Bornstein, J.; Goldschmid, N.; Sabo, E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol. Obstet. Investig. 2004, 58, 171–178. [Google Scholar] [CrossRef]
- Weström, L.V.; Willén, R. Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome. Obstet. Gynecol. 1998, 91, 572–576. [Google Scholar] [CrossRef]
- Thibault-Gagnon, S.; Morin, M. Active and, passive components of pelvic floor muscle tone in women with provoked vestibulodynia: A perspective based on a review of the literature. J. Sex. Med. 2015, 12, 2178–2189. [Google Scholar] [CrossRef]
- Reissing, E.D.; Binik, Y.M.; Khalifé, S.; Cohen, D.; Amsel, R. Vaginal spasm, pain, and behavior: An empirical investigation of the diagnosis of vaginismus. Arch. Sex Behav. 2004, 33, 5–17. [Google Scholar] [CrossRef]



| Mean | Range | |
|---|---|---|
| Age | 26.2 ± 4.1 | 18–40 |
| Married/in a committed relationship | 82 (73.2%) | |
| Duration of Dyspareunia symptoms (years) | 4.1 ± 3.4 | 4 months–13 |
| Nullipara Para | 105 8 | |
| Education (years) | 14.23 ± 2.1 | 11–21 |
| Religiosity | Secular 84 (74.3%) Religious 14 (12.4%) Orthodox 15 (13.3%) |
| Circumferential Vestibular Sensitivity (n = 41) | Posterior-Only Vestibular Sensitivity (n = 72) | p Value | |
|---|---|---|---|
| Vestibular mucosal atrophy | 63.4% | 20.8% | <0.001 |
| Hormonal Contraceptive use | 14.1% | 13.6% | NS |
| Umbilical pain hypersensitivity | 46.3% | 18.1% | 0.001 |
| Rigid hymen | 0% | 51% | <0.001 |
| Pain intensity during intercourse | 8.2 ± 1.5 | 7.7 ± 1.8 | NS |
| Pain evoked by deep muscle palpation | 6.4 ± 2.3 | 6.3 ± 1.7 | NS |
| Primary PVD | 13.5% | 13.3% | NS |
| Unstandardized Coefficients | Coefficients Std. Error | Coefficients Beta | t | p | |
|---|---|---|---|---|---|
| Degree of muscle tonus | 0.361 | 0.436 | 0.081 | 0.828 | 0.410 |
| Pain during intercourse | 0.359 | 0.123 | 0.276 | 2.914 | 0.004 |
| Pain evoked by deep palpation | −0.044 | 0.114 | −0.041 | −0.386 | 0.701 |
| Umbilical sensitivity | 1.366 | 0.4192 | 0.283 | 3.262 | 0.002 |
| Vestibular atrophy | 1.140 | 0.391 | 0.251 | 2.917 | 0.004 |
| Unstandardized Coefficients | Coefficients Std. Error | Coefficients Beta | t | p | |
|---|---|---|---|---|---|
| Degree of muscle tonus | 0.203 | 0.361 | 0.053 | 0.368 | 0.714 |
| Pain during intercourse | 0.497 | 0.102 | .440 | 4.867 | 0.000 |
| Pain evoked by deep palpation | 0.162 | 0.094 | 0.173 | 1.719 | 0.089 |
| Umbilical sensitivity | 0.608 | 0.347 | 0.145 | 1.753 | 0.083 |
| Vestibular atrophy | 0.119 | 0.324 | 0.030 | 0.368 | 0.714 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lev-Sagie, A.; Wertman, O.; Lavee, Y.; Granot, M. Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse. J. Clin. Med. 2020, 9, 2023. https://doi.org/10.3390/jcm9072023
Lev-Sagie A, Wertman O, Lavee Y, Granot M. Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse. Journal of Clinical Medicine. 2020; 9(7):2023. https://doi.org/10.3390/jcm9072023
Chicago/Turabian StyleLev-Sagie, Ahinoam, Osnat Wertman, Yoav Lavee, and Michal Granot. 2020. "Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse" Journal of Clinical Medicine 9, no. 7: 2023. https://doi.org/10.3390/jcm9072023
APA StyleLev-Sagie, A., Wertman, O., Lavee, Y., & Granot, M. (2020). Vestibular Anatomic Localization of Pain Sensitivity in Women with Insertional Dyspareunia: A Different Approach to Address the Variability of Painful Intercourse. Journal of Clinical Medicine, 9(7), 2023. https://doi.org/10.3390/jcm9072023





