Validation of IOTA-ADNEX Model in Discriminating Characteristics of Adnexal Masses: A Comparison with Subjective Assessment
Abstract
1. Introduction
2. Materials and Methods
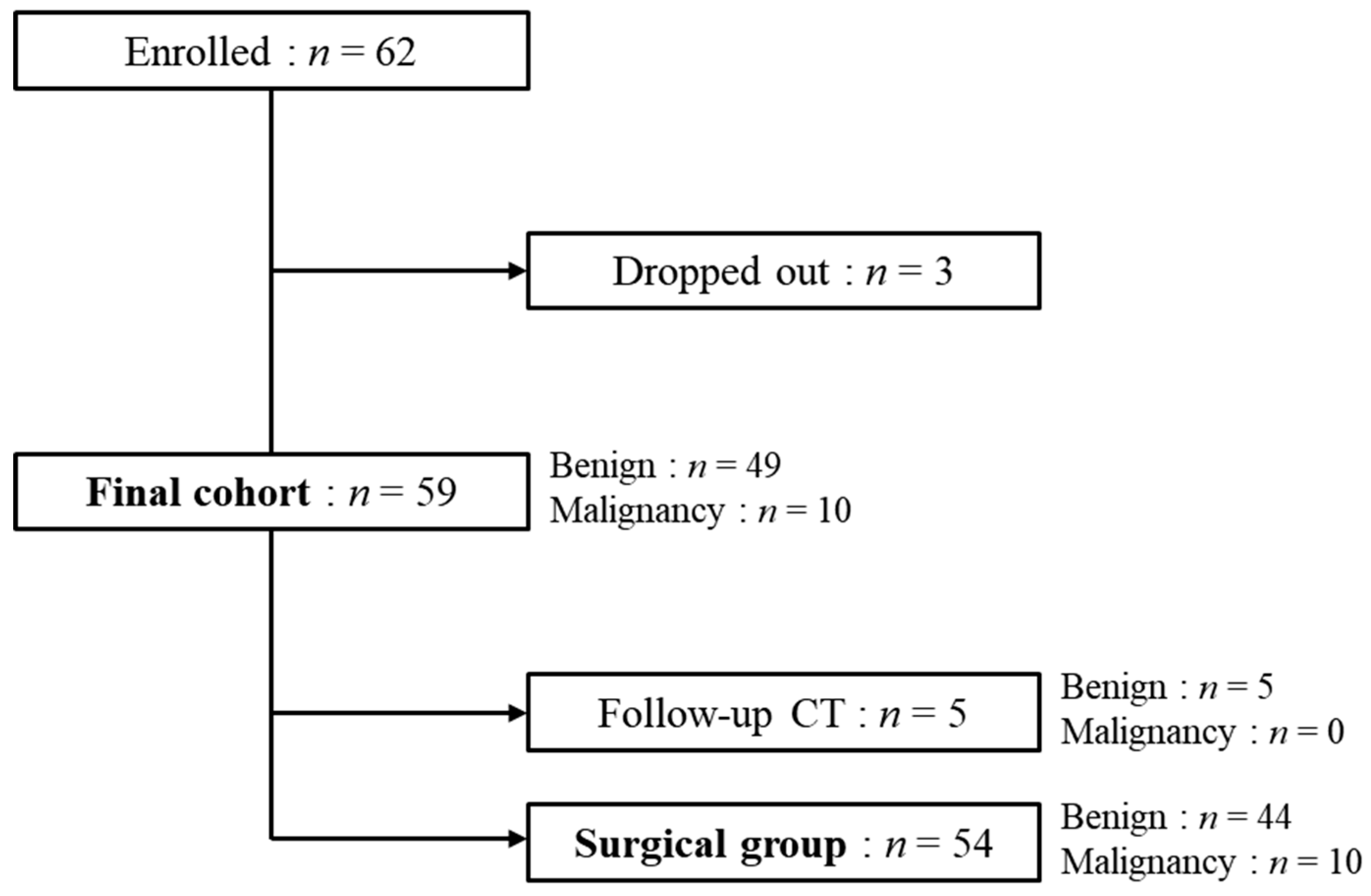
2.1. Patient Selection and Study Design
2.2. Ultrasound Examination
2.3. Statistical Analysis
3. Results
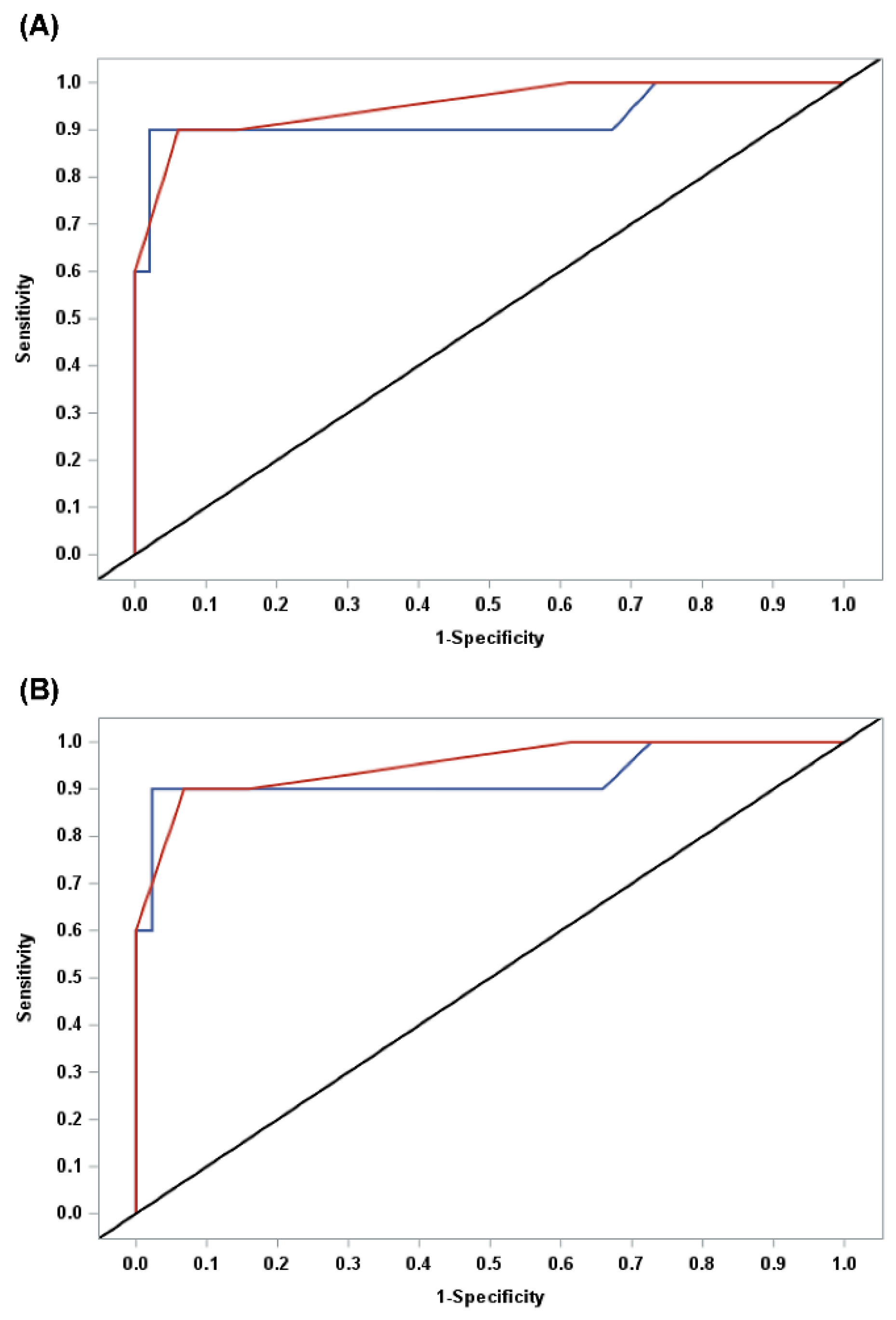
3.1. Diagnostic Performance of IOTA-ADNEX Models
3.2. ADNEX Model vs. Subjective Assessment
3.3. Optimal Cut-Off
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abramowicz, J.; Timmerman, D. Ovarian mass-differentiating benign from malignant: The value of the International Ovarian Tumor Analysis ultrasound rules. Am. J. Obstet. Gynecol. 2017, 217, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Rochon, J.; Pfisterer, J.; Hoskins, W.J. Variations in institutional infrastructure, physician specialization and experience, and outcome in ovarian cancer: A systematic review. Gynecol. Oncol. 2009, 112, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 22 February 2020).
- Geomini, P.; Kruitwagen, R.F.; Bremer, G.L.; Cnossen, J.; Mol, B.W. The Accuracy of Risk Scores in Predicting Ovarian Malignancy. Obstet. Gynecol. 2009, 113, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast Jr, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2008, 112, 40–46. [Google Scholar] [CrossRef]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ferrazzi, E.; Ameye, L.; Konstantinovic, M.L.; Van Calster, B.; Collins, W.P.; Vergote, I.; Van Huffel, S.; et al. Logistic Regression Model to Distinguish Between the Benign and Malignant Adnexal Mass Before Surgery: A Multicenter Study by the International Ovarian Tumor Analysis Group. J. Clin. Oncol. 2005, 23, 8794–8801. [Google Scholar] [CrossRef]
- Timmerman, D.; Van Calster, B.; Testa, A.C.; Guerriero, S.; Fischerova, D.; Lissoni, A.A.; Van Holsbeke, C.; Fruscio, R.; Czekierdowski, A.; Jurkovic, D.; et al. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: A temporal and external validation study by the IOTA group. Ultrasound Obstet. Gynecol. 2010, 36, 226–234. [Google Scholar] [CrossRef]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ 2010, 341, c6839. [Google Scholar] [CrossRef]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Van Calster, B.; Bourne, T.; Ajossa, S.; Testa, A.C.; Guerriero, S.; Fruscio, R.; Lissoni, A.; Czekierdowski, A.; Savelli, L.; et al. External Validation of Diagnostic Models to Estimate the Risk of Malignancy in Adnexal Masses. Clin. Cancer Res. 2011, 18, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef] [PubMed]
- Araujo, K.G.; Jales, R.M.; Pereira, P.N.; Yoshida, A.; Andrade, L.D.A.; Sarian, L.O.; Derchain, S. Performance of the IOTA ADNEX model in preoperative discrimination of adnexal masses in a gynecological oncology center. Ultrasound Obstet. Gynecol. 2017, 49, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Van Calster, B.; Timmerman, D.; Nikman, S. Subjective ultrasound assessment, the ADNEX model and ultrasound-guided tru-cut biopsy to differentiate disseminated primary ovarian cancer from metastatic non-ovarian cancer. Ultrasound Obstet. Gynecol. 2016, 47, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Meys, E.M.J.; Jeelof, L.S.; Achten, n.M.J.; Slangen, B.F.M.; Lambrechts, S.; Kruitwagen, R.F.P.M.; Van Gorp, T. Estimating risk of malignancy in adnexal masses: External validation of the ADNEX model and comparison with other frequently used ultrasound methods. Ultrasound Obstet. Gynecol. 2017, 49, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Sayasneh, A.; Ferrara, L.; De Cock, B.; Saso, S.; Al-Memar, M.; Johnson, S.; Kaijser, J.; Carvalho, J.; Husicka, R.; Smith, A.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model: A multicentre external validation study. Br. J. Cancer 2016, 115, 542–548. [Google Scholar] [CrossRef]
- Szubert, S.; Wójtowicz, A.; Moszyński, R.; Żywica, P.; Dyczkowski, K.; Stachowiak, A.; Sajdak, S.; Szpurek, D.; Alcazar, J.L. External validation of the IOTA ADNEX model performed by two independent gynecologic centers. Gynecol. Oncol. 2016, 142, 490–495. [Google Scholar] [CrossRef]
- Wynants, L.; Timmerman, D.; Verbakel, J.Y.; Testa, A.; Savelli, L.; Fischerova, D.; Franchi, D.; Van Holsbeke, C.; Epstein, E.; Froyman, W.; et al. Clinical Utility of Risk Models to Refer Patients with Adnexal Masses to Specialized Oncology Care: Multicenter External Validation Using Decision Curve Analysis. Clin. Cancer Res. 2017, 23, 5082–5090. [Google Scholar] [CrossRef]
- Timmerman, D.; Schwärzler, P.; Collins, W.P.; Claerhout, F.; Coenen, M.; Amant, F.; Vergote, I.; Bourne, T. Subjective assessment of adnexal masses with the use of ultrasonography: An analysis of interobserver variability and experience. Ultrasound Obstet. Gynecol. 1999, 13, 11–16. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Froyman, W.; Kaijser, J.; Wynants, L.; Landolfo, C.; Anthoulakis, C.; Vergote, I.; Bourne, T.; Timmerman, D. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis. ObGyn 2015, 7, 32–41. [Google Scholar]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Kaijser, J.; Sayasneh, A.; Van Hoorde, K.; Ghaem-Maghami, S.; Bourne, T.; Timmerman, D.; Van Calster, B. Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: A systematic review and meta-analysis. Hum. Reprod. Updat. 2013, 20, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Meys, E.; Kaijser, J.; Kruitwagen, R.F.; Slangen, B.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.Y.; Timmerman, D.; Van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef]
- Timmerman, D. The use of mathematical models to evaluate pelvic masses; can they beat an expert operator? Best Pr. Res. Clin. Obstet. Gynaecol. 2004, 18, 91–104. [Google Scholar] [CrossRef]
- Van Gorp, T.; Veldman, J.; Van Calster, B.; Cadron, I.; Leunen, K.; Amant, F.; Timmerman, D.; Vergote, I. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. Eur. J. Cancer 2012, 48, 1649–1656. [Google Scholar] [CrossRef]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef]
- Bottoni, P.; Scatena, R. The Role of CA 125 as Tumor Marker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 229–244. [Google Scholar] [CrossRef]
- Skates, S.J.; Xu, F.-J.; Yu, Y.-H.; Sjövall, K.; Einhorn, N.; Chang, Y.; Bast, R.C.; Knapp, R.C. Toward an optimal algorithm for ovarian cancer screening with longitudinal tumor markers. Cancer 1995, 76, 2004–2010. [Google Scholar] [CrossRef]
- Zhang, Z.; Bast, R.C.; Yu, Y.; Li, J.; Sokoll, L.J.; Rai, A.J.; Rosenzweig, J.M.; Cameron, B.; Wang, Y.Y.; Meng, X.-Y.; et al. Three Biomarkers Identified from Serum Proteomic Analysis for the Detection of Early Stage Ovarian Cancer. Cancer Res. 2004, 64, 5882–5890. [Google Scholar] [CrossRef]
- Abdel Wahab, C.; Rousset, P.; Bolze, P.A.; Thomassin-Naggara, I. Borderline Ovarian Tumours: CNGOF Guidelines for Clinical Practice—Imaging. Gynecol. Obstet. Fertil. Senol. 2020, 48, 260–276. [Google Scholar] [PubMed]
| Benign (n = 49) | Malignant (n = 10) | Total (n = 59) | p-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| age, yr (range) | 42 (20~68) | 59 (41~71) | 45 (20~71) | 0.001 |
| BMI, kg/m2 (range) | 22.2 (16.3~31.5) | 23.5 (19.2~29.0) | 22.4 (16.3~31.5) | 0.283 * |
| CA-125, U/mL (range) | 15.3 (2–74) | 181.3 (3–672) | 43.4 (2–672) | 0.005 |
| parity | 0.012 | |||
| No | 20 (40.8%) | 0 (0%) | 20 (33.9%) | |
| Yes | 29 (59.2%) | 10 (100%) | 39 (66.1%) | |
| Menopause | 0.054 | |||
| No | 37 (75.5%) | 4 (40%) | 41 (69.5%) | |
| Yes | 12 (24.5%) | 6 (60%) | 18 (30.5%) | |
| Family history of ovarian/breast cancer | >0.999 | |||
| No | 47 (95.9%) | 10 (100%) | 57 (96.6%) | |
| Yes | 2 (4.1%) | 0 (0%) | 2 (3.4%) | |
| US findings | ||||
| Laterality of tumor | 0.047 | |||
| Unilateral | 40 (81.6%) | 5 (50%) | 45 (76.3%) | |
| Bilateral | 9 (18.4%) | 5 (50%) | 14 (23.7%) | |
| Maximum diameter of lesion, mm(range) | 63.6 (17.0–200.0) | 75.8 (27.0–168.0) | 65.5 (17.0–200.0) | 0.322 |
| Maximum diameter of largest solid, mm (range) | 10.1 (0–86) | 45.7 (0–74) | 16.2 (0–86) | <0.001 |
| More than 10 cyst locules | 0.055 | |||
| No | 46 (93.9%) | 7 (70%) | 53 (89.8%) | |
| Yes | 3 (6.1%) | 3 (30%) | 6 (10.2%) | |
| Number of papillary projection | <0.001 | |||
| 0 | 41 (83.7%) | 1 (10%) | 42 (71.2%) | |
| 1 | 3 (6.1%) | 2 (20%) | 5 (8.5%) | |
| 2 | 0 (0%) | 1 (10%) | 1 (1.7%) | |
| 3 | 1 (2%) | 0 (0%) | 1 (1.7%) | |
| >3 | 4 (8.2%) | 6 (60%) | 10 (16.9%) | |
| Acoustic shadow | 1.000 | |||
| No | 40 (81.6%) | 9 (90%) | 49 (83.1%) | |
| Yes | 9 (18.4%) | 1 (10%) | 10 (16.9%) | |
| Ascites | 0.002 | |||
| No | 48 (98%) | 6 (60%) | 54 (91.5%) | |
| Yes | 1 (2%) | 4 (40%) | 5 (8.5%) | |
| B-mode | NA | |||
| Unilocular | 24 (49%) | 1 (10%) | 25 (42.4%) | |
| Multilocular | 14 (28.6%) | 1 (10%) | 15 (25.4%) | |
| Unilocular-solid | 3 (6.1%) | 2 (20%) | 5 (8.5%) | |
| Multilocular-solid | 8 (16.3%) | 3 (30%) | 11 (18.7%) | |
| Solid | 0 (0%) | 3 (30%) | 3 (5%) | |
| Color doppler | NA | |||
| 0 | 41 (83.7%) | 2 (20%) | 43 (72.9%) | |
| 1 | 7 (14.3%) | 2 (20%) | 9 (15.2%) | |
| 2 | 1 (2%) | 5 (50%) | 6 (10.2%) | |
| 3 | 0 (0%) | 1 (10%) | 1 (1.7%) |
| Total (%) | |
|---|---|
| Benign | 44 (81.4) |
| Endometrioma | 18 (33.3) |
| Fibroma | 1 (1.9) |
| Simple cyst | 4 (7.4) |
| Mature cystic teratoma | 8 (14.8) |
| Mucinous cystadenofibroma | 2 (3.7) |
| Mucinous cystadenoma | 1 (1.9) |
| Paratubal cyst | 1 (1.9) |
| Serous cystadenoma | 7 (12.8) |
| Serous cystadenofibroma | 2 (3.7) |
| Borderline and malignancy | 10 (18.6) |
| Mucinous borderline | 2 (3.7) |
| High-grade serous carcinoma | 3 (5.4) |
| High-grade neuroendocrine carcinoma | 1 (1.9) |
| Low-grade endometrioid carcinoma | 1 (1.9) |
| High-grade endometrioid carcinoma | 1 (1.9) |
| High-grade seromucinous carcinoma | 1 (1.9) |
| Poorly differentiated carcinoma | 1 (1.9) |
| Cut-off Point | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | Accuracy | AUC |
|---|---|---|---|---|---|---|---|---|
| 5% | 0.9 | 0.755 | 0.429 | 0.974 | 3.680 | 0.132 | 0.780 | 0.828 |
| 10% | 0.9 | 0.816 | 0.500 | 0.976 | 4.900 | 0.123 | 0.831 | 0.858 |
| 15% | 0.9 | 0.837 | 0.529 | 0.976 | 5.513 | 0.120 | 0.848 | 0.868 |
| 47.3% * | 0.9 | 0.980 | 0.900 | 0.980 | 44.100 | 0.102 | 0.966 | 0.940 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.Y.; Park, B.K.; Lee, Y.Y.; Kim, T.-J. Validation of IOTA-ADNEX Model in Discriminating Characteristics of Adnexal Masses: A Comparison with Subjective Assessment. J. Clin. Med. 2020, 9, 2010. https://doi.org/10.3390/jcm9062010
Jeong SY, Park BK, Lee YY, Kim T-J. Validation of IOTA-ADNEX Model in Discriminating Characteristics of Adnexal Masses: A Comparison with Subjective Assessment. Journal of Clinical Medicine. 2020; 9(6):2010. https://doi.org/10.3390/jcm9062010
Chicago/Turabian StyleJeong, Soo Young, Byung Kwan Park, Yoo Young Lee, and Tae-Joong Kim. 2020. "Validation of IOTA-ADNEX Model in Discriminating Characteristics of Adnexal Masses: A Comparison with Subjective Assessment" Journal of Clinical Medicine 9, no. 6: 2010. https://doi.org/10.3390/jcm9062010
APA StyleJeong, S. Y., Park, B. K., Lee, Y. Y., & Kim, T.-J. (2020). Validation of IOTA-ADNEX Model in Discriminating Characteristics of Adnexal Masses: A Comparison with Subjective Assessment. Journal of Clinical Medicine, 9(6), 2010. https://doi.org/10.3390/jcm9062010






