Automated Nociceptive Withdrawal Reflex Measurements Reveal Normal Reflex Thresholds and Augmented Pain Ratings in Patients with Fibromyalgia
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Health Questionnaires
2.3. Electrical Stimulation Procedure
2.4. Determination of the NWR Response
2.5. Subjective Pain Evaluation
2.6. Statistical Analysis
3. Results
3.1. Study Population Characteristics
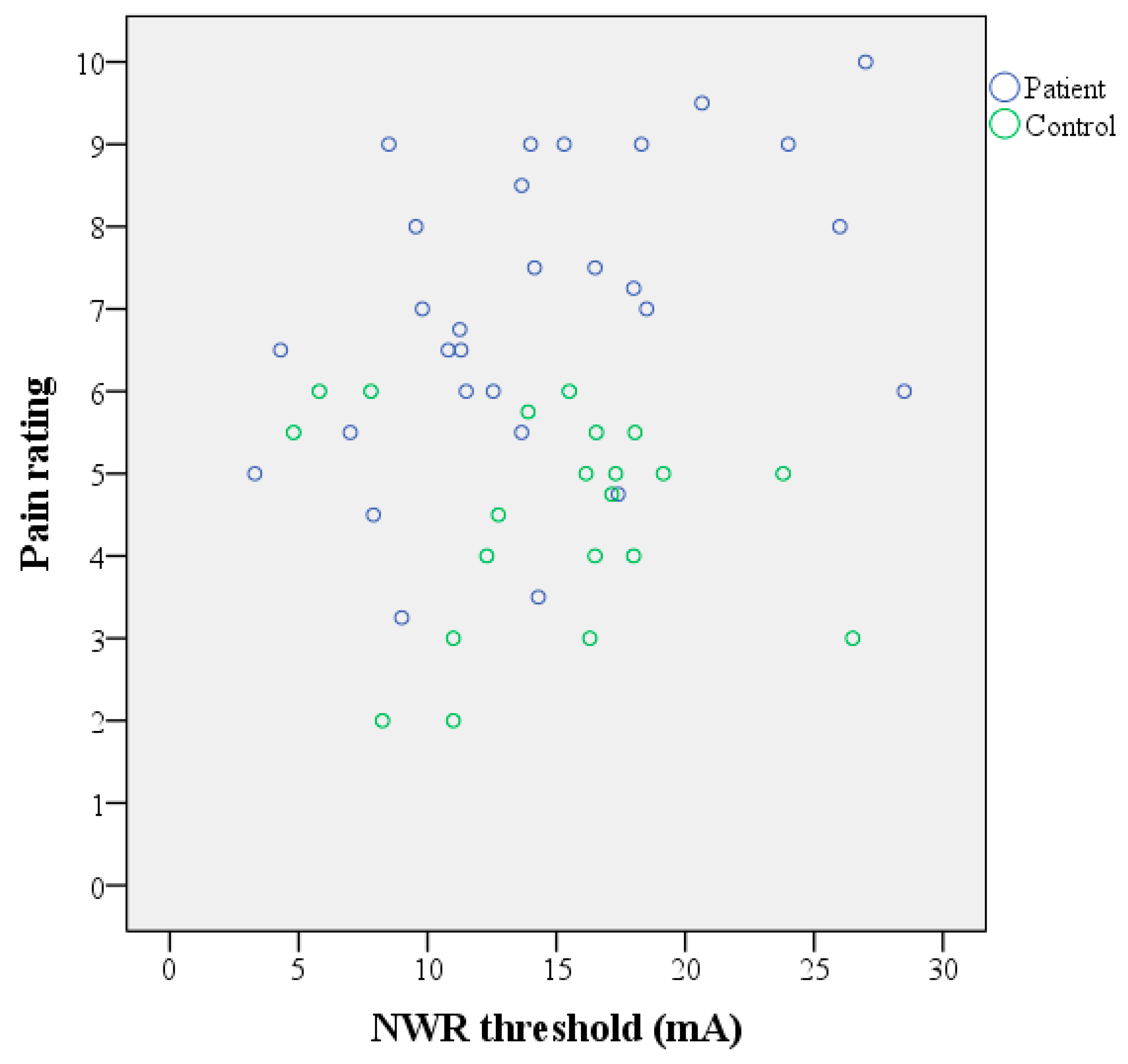
3.2. NWR Thresholds
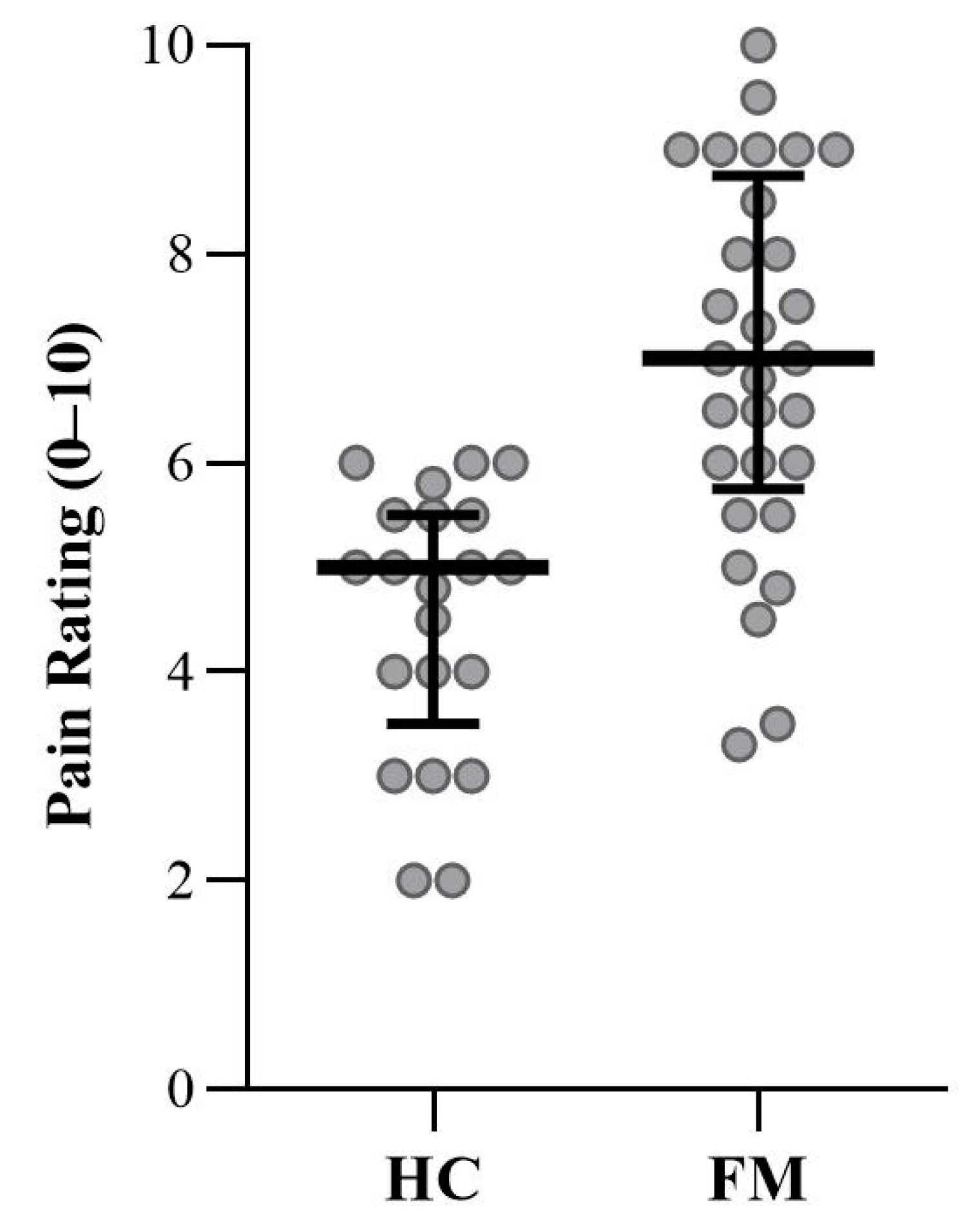
3.3. Subjective Pain Ratings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sherrington, C.S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 1910, 40, 28–121. [Google Scholar] [CrossRef]
- Kugelberg, E. Demonstration of A and C fibre components in the babinski plantar response and the pathological flexion reflex. Brain 1948, 71, 304–319. [Google Scholar] [CrossRef]
- Hagbarth, K.E. Spinal withdrawal reflexes in the human lower limbs. J. Neurol. Neurosurg. Psychiatry 1960, 23, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willer, J.C. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain 1977, 3, 69–80. [Google Scholar] [CrossRef]
- Chan, C.W.; Dallaire, M. Subjective pain sensation is linearly correlated with the flexion reflex in man. Brain Res. 1989, 479, 145–150. [Google Scholar] [CrossRef]
- Sandrini, G.; Arrigo, A.; Bono, G.; Nappi, G. The nociceptive flexion reflex as a tool for exploring pain control systems in headache and other pain syndromes. Cephalalgia 1993, 13, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Banic, B.; Petersen-Felix, S.; Andersen, O.K.; Radanov, B.P.; Villiger, P.M.; Arendt-Nielsen, L.; Curatolo, M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004, 107, 7–15. [Google Scholar] [CrossRef]
- Guieu, R.; Serratrice, G.; Pouget, J. Counter irritation test in primary fibromyalgia. Clin. Rheumatol. 1994, 13, 605–610. [Google Scholar] [CrossRef]
- Bouhassira, D.; Danziger, N.; Attal, N.; Guirimand, F. Comparison of the pain suppressive effects of clinical and experimental painful conditioning stimuli. Brain 2003, 126, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.L.; Schmidt, A.J.; Van den Hout, M.A.; Koopmans, R.; Sluijter, M.E. Chronic back pain, acute postoperative pain and the activation of diffuse noxious inhibitory controls (DNIC). Pain 1992, 50, 177–187. [Google Scholar] [CrossRef]
- Rhudy, J.L.; France, C.R. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain 2007, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.A.; Lewek, M.D.; Witte, P.O.; Chmell, S.J.; Hornby, T.G. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J. Pain 2009, 10, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Biurrun Manresa, J.; Andersen, O.K. Reliable estimation of nociceptive withdrawal reflex thresholds. J. Neurosci. Methods 2015, 253, 110–115. [Google Scholar] [CrossRef]
- Jensen, M.B.; Manresa, J.A.B.; Frahm, K.S.; Andersen, O.K. Analysis of muscle fiber conduction velocity enables reliable detection of surface EMG crosstalk during detection of nociceptive withdrawal reflexes. BMC Neurosci. 2013, 14, 39. [Google Scholar] [CrossRef] [Green Version]
- Herm, C.; Silbereisen, V.; Graf, B.M.; Lassen, C.L. Long term reliability of nociceptive withdrawal reflex thresholds. J. Neurosci. Methods 2019, 320, 44–49. [Google Scholar] [CrossRef]
- Lloyd, D.P.C. Neuron patterns controlling transmission of ipsilateral hind limb reflexes in cat. J. Neurophysiol. 1943, 6, 293–315. [Google Scholar] [CrossRef]
- Hugon, M. Exteroceptive Reflexes to Stimulation of the Sural Nerve in Normal Man. In New Developments in Electromyography and Clinical Neurophysiology, Volume 3: Human Reflexes, Pathophysiology of Motor Systems, Methodology of Human Reflexes; Desmedt, J.E., Ed.; S. Karger AG: Basel, Switzerland, 1973; pp. 713–729. [Google Scholar]
- Shahani, B. Flexor reflex afferent nerve fibres in man. J. Neurol. Neurosurg. Psychiatry 1970, 33, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Ertekin, C.; Ertekin, N.; Karcioglu, M. Conduction velocity along human nociceptive reflex afferent nerve fibres. J. Neurol. Neurosurg. Psychiatry 1975, 38, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Gronroos, M.; Pertovaara, A. Capsaicin-induced central facilitation of a nociceptive flexion reflex in humans. Neurosci. Lett. 1993, 159, 215–218. [Google Scholar] [CrossRef]
- Andersen, O.K.; Jensen, L.M.; Brennum, J.; Arendt-Nielsen, L. Evidence for central summation of C and A delta nociceptive activity in man. Pain 1994, 59, 273–280. [Google Scholar] [CrossRef]
- Willer, J.C.; Albe-Fessard, D. Further studies on the role of afferent input from relatively large diameter fibers in transmission of nociceptive messages in humans. Brain Res. 1983, 278, 318–321. [Google Scholar] [CrossRef]
- Kugelberg, E.; Eklund, K.; Grimby, L. An electromyographic study of the nociceptive reflexes of the lower limb. Mechanism of the plantar responses. Brain 1960, 83, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Wiesenfeld-Hallin, Z.; Hallin, R.G.; Persson, A. Do large diameter cutaneous afferents have a role in the transmission of nociceptive messages? Brain Res. 1984, 311, 375–379. [Google Scholar] [CrossRef]
- Nagi, S.S.; Marshall, A.G.; Makdani, A.; Jarocka, E.; Liljencrantz, J.; Ridderstrom, M.; Shaikh, S.; O’Neill, F.; Saade, D.; Donkervoort, S.; et al. An ultrafast system for signaling mechanical pain in human skin. Sci. Adv. 2019, 5, eaaw1297. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Sommer, C. Fibromyalgia: A clinical update. Pain Clin. Updates 2010, 18, 1–4. [Google Scholar]
- Backryd, E.; Tanum, L.; Lind, A.L.; Larsson, A.; Gordh, T. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J. Pain Res. 2017, 10, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.A.; Clauw, D.J. Understanding fibromyalgia: Lessons from the broader pain research community. J. Pain 2009, 10, 777–791. [Google Scholar] [CrossRef] [Green Version]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Vierck, C.J., Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain 2006, 124, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Mattoo, B.; Kumar, U.; Bhatia, R. Can aberrant spinal nociception be a marker of chronicity of pain in fibromyalgia syndrome? J. Clin. Neurosci. 2019, 65, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rhudy, J.L.; DelVentura, J.L.; Terry, E.L.; Bartley, E.J.; Olech, E.; Palit, S.; Kerr, K.L. Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 2013, 154, 1045–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samour, M.S.; Nagi, S.S.; Shortland, P.J.; Mahns, D.A. Minocycline prevents muscular pain hypersensitivity and cutaneous allodynia produced by repeated intramuscular injections of hypertonic saline in healthy human participants. J. Pain 2017, 18, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Mahns, D.A.; Nagi, S.S. An investigation into the peripheral substrates involved in the tactile modulation of cutaneous pain with emphasis on the C-tactile fibres. Exp. Brain Res. 2013, 227, 457–465. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J.; Dean, L.E.; Clauw, D.J.; Goldenberg, D.L.; Fitzcharles, M.A.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT Diagnostic Criteria for Fibromyalgia. J. Pain 2019, 20, 611–628. [Google Scholar] [CrossRef] [Green Version]
- Bergman, H.; Kallmen, H. Alcohol use among Swedes and a psychometric evaluation of the alcohol use disorders identification test. Alcohol Alcohol. 2002, 37, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- LoMartire, R.; Ang, B.O.; Gerdle, B.; Vixner, L. Psychometric properties of Short Form-36 Health Survey, EuroQol 5-dimensions, and Hospital Anxiety and Depression Scale in patients with chronic pain. Pain 2020, 161, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chibnall, J.T.; Tait, R.C. The Pain Disability Index: Factor structure and normative data. Arch. Phys. Med. Rehabil. 1994, 75, 1082–1086. [Google Scholar] [CrossRef]
- Gronblad, M.; Jarvinen, E.; Hurri, H.; Hupli, M.; Karaharju, E.O. Relationship of the Pain Disability Index (PDI) and the Oswestry Disability Questionnaire (ODQ) with three dynamic physical tests in a group of patients with chronic low-back and leg pain. Clin. J. Pain 1994, 10, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Group, E. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Dolan, P.; Sutton, M. Mapping visual analogue scale health state valuations onto standard gamble and time trade-off values. Social Sci. Med. 1997, 44, 1519–1530. [Google Scholar] [CrossRef]
- EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [CrossRef]
- Toorring, J.; Pedersen, E.; Klemar, B. Standardisation of the electrical elicitation of the human flexor reflex. J. Neurol. Neurosurg. Psychiatry 1981, 44, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinck, H.M.; Kuster, S.; Benecke, R.; Conrad, B. The flexor reflex—Influence of stimulus parameters on the reflex response. Electroencephalogr. Clin. Neurophysiol. 1985, 61, 287–298. [Google Scholar] [CrossRef]
- Faganel, J. An analysis of flexor reflex elicited by rhythmic and stochastic stimulation in normal man. Jugoslav. Physiol. Pharmacol. Acta 1970, 6, 145–149. [Google Scholar]
- Dimitrijevic, M.R.; Faganel, J.; Gregoric, M.; Nathan, P.W.; Trontelj, J.K. Habituation: Effects of regular and stochastic stimulation. J. Neurol. Neurosurg. Psychiatry 1972, 35, 234–242. [Google Scholar] [CrossRef] [Green Version]
- France, C.R.; Rhudy, J.L.; McGlone, S. Using normalized EMG to define the nociceptive flexion reflex (NFR) threshold: Further evaluation of standardized NFR scoring criteria. Pain 2009, 145, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hurtig, I.M.; Raak, R.I.; Kendall, S.A.; Gerdle, B.; Wahren, L.K. Quantitative sensory testing in fibromyalgia patients and in healthy subjects: Identification of subgroups. Clin. J. Pain 2001, 17, 316–322. [Google Scholar] [CrossRef]
- Yim, Y.R.; Lee, K.E.; Park, D.J.; Kim, S.H.; Nah, S.S.; Lee, J.H.; Kim, S.K.; Lee, Y.A.; Hong, S.J.; Kim, H.S.; et al. Identifying fibromyalgia subgroups using cluster analysis: Relationships with clinical variables. Eur. J. Pain 2017, 21, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Robinson, M.E.; Staud, R. Pain and Fatigue Variability Patterns Distinguish Subgroups of Fibromyalgia Patients. J. Pain 2018, 19, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Bailey, J.; Brown, C.; Jones, A.; McCabe, C.S. Sensory Function and Pain Experience in Arthritis, Complex Regional Pain Syndrome, Fibromyalgia Syndrome, and Pain-Free Volunteers: A Cross-Sectional Study. Clin. J. Pain 2019, 35, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Goubert, D.; Danneels, L.; Graven-Nielsen, T.; Descheemaeker, F.; Meeus, M. Differences in Pain Processing Between Patients with Chronic Low Back Pain, Recurrent Low Back Pain, and Fibromyalgia. Pain Physician 2017, 20, 307–318. [Google Scholar]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Mendell, L.M.; Wall, P.D. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 1965, 206, 97–99. [Google Scholar] [CrossRef]
- Treede, R.D.; Cole, J.D. Dissociated secondary hyperalgesia in a subject with a large-fibre sensory neuropathy. Pain 1993, 53, 169–174. [Google Scholar] [CrossRef]
- Filatova, E.; Latysheva, N.; Kurenkov, A. Evidence of persistent central sensitization in chronic headaches: A multi-method study. J. Headache Pain 2008, 9, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Sterling, M.; Hodkinson, E.; Pettiford, C.; Souvlis, T.; Curatolo, M. Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin. J. Pain 2008, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.; Eich, W.; Treede, R.D.; Tesarz, J. Conditioned pain modulation in patients with nonspecific chronic back pain with chronic local pain, chronic widespread pain, and fibromyalgia. Pain 2017, 158, 430–439. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.T.; Deitos, A.; Triñanes Pego, Y.; Fregni, F.; Carrillo-de-la-Peña, M.T. Defective endogenous pain modulation in fibromyalgia: A meta-analysis of temporal summation and conditioned pain modulation paradigms. J. Pain 2018, 19, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Olausson, P.; Ghafouri, B.; Ghafouri, N.; Gerdle, B. Specific proteins of the trapezius muscle correlate with pain intensity and sensitivity—An explorative multivariate proteomic study of the trapezius muscle in women with chronic widespread pain. J. Pain Res. 2016, 9, 345–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdle, B.; Ghafouri, B.; Ghafouri, N.; Bäckryd, E.; Gordh, T. Signs of ongoing inflammation in female patients with chronic widespread pain: A multivariate, explorative, cross-sectional study of blood samples. Medicine (Baltimore) 2017, 96, e6130. [Google Scholar] [CrossRef] [PubMed]
- Gerdle, B.; Wåhlén, K.; Ghafouri, B. Plasma protein patterns are strongly correlated with pressure pain thresholds in women with chronic widespread pain and in healthy controls-an exploratory case-control study. Medicine (Baltimore) 2020, 99, e20497. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef]
- Boehme, R.; van Ettinger-Veenstra, H.; Olausson, H.; Gerdle, B.; Nagi, S.S. Anhedonia to gentle touch in fibromyalgia: Normal sensory processing but abnormal evaluation. Brain Sci. 2020, 10, 306. [Google Scholar] [CrossRef]
- Nagi, S.S.; Mahns, D.A. Mechanical allodynia in human glabrous skin mediated by low-threshold cutaneous mechanoreceptors with unmyelinated fibres. Exp. Brain Res. 2013, 231, 139–151. [Google Scholar] [CrossRef]
- Dunn, J.S.; Nagi, S.S.; Mahns, D.A. Regionally diffuse muscle pain-hypersensitivity in humans during acute muscle pain. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Arendt-Nielsen, L.; Brennum, J.; Sindrup, S.; Bak, P. Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nagi, S.S.; McGlone, F.; Mahns, D.A. Psychophysical investigations into the role of low-threshold C fibres in non-painful affective processing and pain modulation. PLoS ONE 2015, 10, e0138299. [Google Scholar] [CrossRef] [PubMed]
- Vallbo, Å.B.; Olausson, H.; Wessberg, J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999, 81, 2753–2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| FM (n = 29) | HC (n = 21) | Statistic | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | SD | Mean | 95% CI | SD | p-Value | |
| Age (Years) | 38.9 | 34.4, 43.4 | 11.7 | 41.2 | 36.2, 46.2 | 11.0 | 0.49 |
| Blood Pressure Systolic (mm Hg) | 122 | 117, 126 | 12.8 | 114 | 110, 117 | 8.0 | 0.02 |
| Blood Pressure Diastolic (mm Hg) | 81 | 77, 85 | 10.8 | 76 | 72, 80 | 8.4 | 0.10 |
| Height (m) | 1.65 | 1.63, 1.68 | 0.06 | 1.69 | 1.67, 1.72 | 0.06 | 0.03 |
| Weight (kg) | 81.2 | 73.3, 89.2 | 19.8 | 69.4 | 64.0, 74.8 | 11.8 | 0.02 |
| BMI (kg/m2) | 29.5 | 26.9, 32.1 | 6.5 | 24.3 | 22.6, 26.0 | 3.7 | <0.01 |
| Current Pain Intensity | 5.5 | 4.7, 6.3 | 2.1 | 0.0 | NA | 0.0 | <0.001 |
| HADS Total | 13.3 | 10.7, 15.8 | 6.5 | 3.8 | 2.2, 5.4 | 3.4 | <0.001 |
| PDI | 37.3 | 32.8, 41.8 | 11.3 | 7.8 | 6.9, 8.7 | 2.0 | <0.001 |
| EQ-5D-VAS | 52.7 | 44.9, 60.5 | 19.7 | 86.3 | 83.2, 89.3 | 6.4 | <0.001 |
| A. NWR Threshold | B. Pain Rating | C. Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | SD | t-Test | Median | IQR | U-Test | Rho | 95% CI | p-Value | |
| FM (n = 29) | 14.4 | 11.9, 16.8 | 6.4 | p = 0.85 | 7 | 3 | p < 0.001 | 0.460 | 0.113, 0.707 | 0.012 |
| HC (n = 21) | 14.7 | 12.2, 17.2 | 5.4 | 5 | 2 | −0.101 | −0.510, 0.345 | 0.664 | ||
| All (n = 50) | 0.154 | −0.129, 0.414 | 0.286 | |||||||
| A. NWR Threshold (mA) | B. Pain Rating | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | 1st Limb Mean (95% CI) [SD] | 2nd Limb Mean (95% CI) [SD] | Percent Change Mean (95% CI) [SD] | t-Test | 1st Limb Mdn (IQR) | 2nd Limb Mdn (IQR) | Percent Change Mean (95% CI) [SD] | t-Test |
| FM (n = 17) | 11.7 (8.8, 14.5) [5.5] | 13.5 (10.8, 16.3) [5.3] | 47 (−14, 108) [118] | p = 0.29 | 7 (3) | 7 (3) | 23 (−6, 51) [55] | p = 0.08 |
| HC (n = 12) | 14.2 (10.8, 17.5) [5.3] | 14.1 (11.8, 16.3) [3.5] | 12 (−18, 42) [47] | 5 (2) | 4 (3) | −6 (−24, 12) [28] | ||
| A. Intensity (mA) | B. Impedance (kOhm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | 1st Limb Mean (95% CI) [SD] | t-Test | n | 2nd Limb Mean (95% CI) [SD] | t-Test | n | 1st Limb Mean (95% CI) [SD] | t-Test | n | 2nd Limb Mean (95% CI) [SD] | t-Test |
| FM | 28 | 10.4 (8.6, 12.3) [4.8] | p = 0.23 | 24 | 11.9 (10.2, 13.7) [4.2] | p = 0.97 | 26 | 38.1 (29.8, 46.3) [20.4] | p = 0.73 | 22 | 32.4 (22.7, 42.1) [21.8] | p = 0.38 |
| HC | 21 | 12.1 (10.0, 14.2) [4.5] | 21 | 11.9 (10.6, 13.2) [2.8] | 20 | 36.0 (24.0, 47.4) [25.1] | 20 | 38.8 (27.4, 50.1) [24.4] | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ydrefors, J.; Karlsson, T.; Wentzel Olausson, U.; Ghafouri, B.; Johansson, A.-C.; Olausson, H.; Gerdle, B.; Nagi, S.S. Automated Nociceptive Withdrawal Reflex Measurements Reveal Normal Reflex Thresholds and Augmented Pain Ratings in Patients with Fibromyalgia. J. Clin. Med. 2020, 9, 1992. https://doi.org/10.3390/jcm9061992
Ydrefors J, Karlsson T, Wentzel Olausson U, Ghafouri B, Johansson A-C, Olausson H, Gerdle B, Nagi SS. Automated Nociceptive Withdrawal Reflex Measurements Reveal Normal Reflex Thresholds and Augmented Pain Ratings in Patients with Fibromyalgia. Journal of Clinical Medicine. 2020; 9(6):1992. https://doi.org/10.3390/jcm9061992
Chicago/Turabian StyleYdrefors, Johannes, Tomas Karlsson, Ulrika Wentzel Olausson, Bijar Ghafouri, Ann-Charlotte Johansson, Håkan Olausson, Björn Gerdle, and Saad S. Nagi. 2020. "Automated Nociceptive Withdrawal Reflex Measurements Reveal Normal Reflex Thresholds and Augmented Pain Ratings in Patients with Fibromyalgia" Journal of Clinical Medicine 9, no. 6: 1992. https://doi.org/10.3390/jcm9061992
APA StyleYdrefors, J., Karlsson, T., Wentzel Olausson, U., Ghafouri, B., Johansson, A.-C., Olausson, H., Gerdle, B., & Nagi, S. S. (2020). Automated Nociceptive Withdrawal Reflex Measurements Reveal Normal Reflex Thresholds and Augmented Pain Ratings in Patients with Fibromyalgia. Journal of Clinical Medicine, 9(6), 1992. https://doi.org/10.3390/jcm9061992






