Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Method
2.2. Studied Variables
2.3. Statistical Analyses
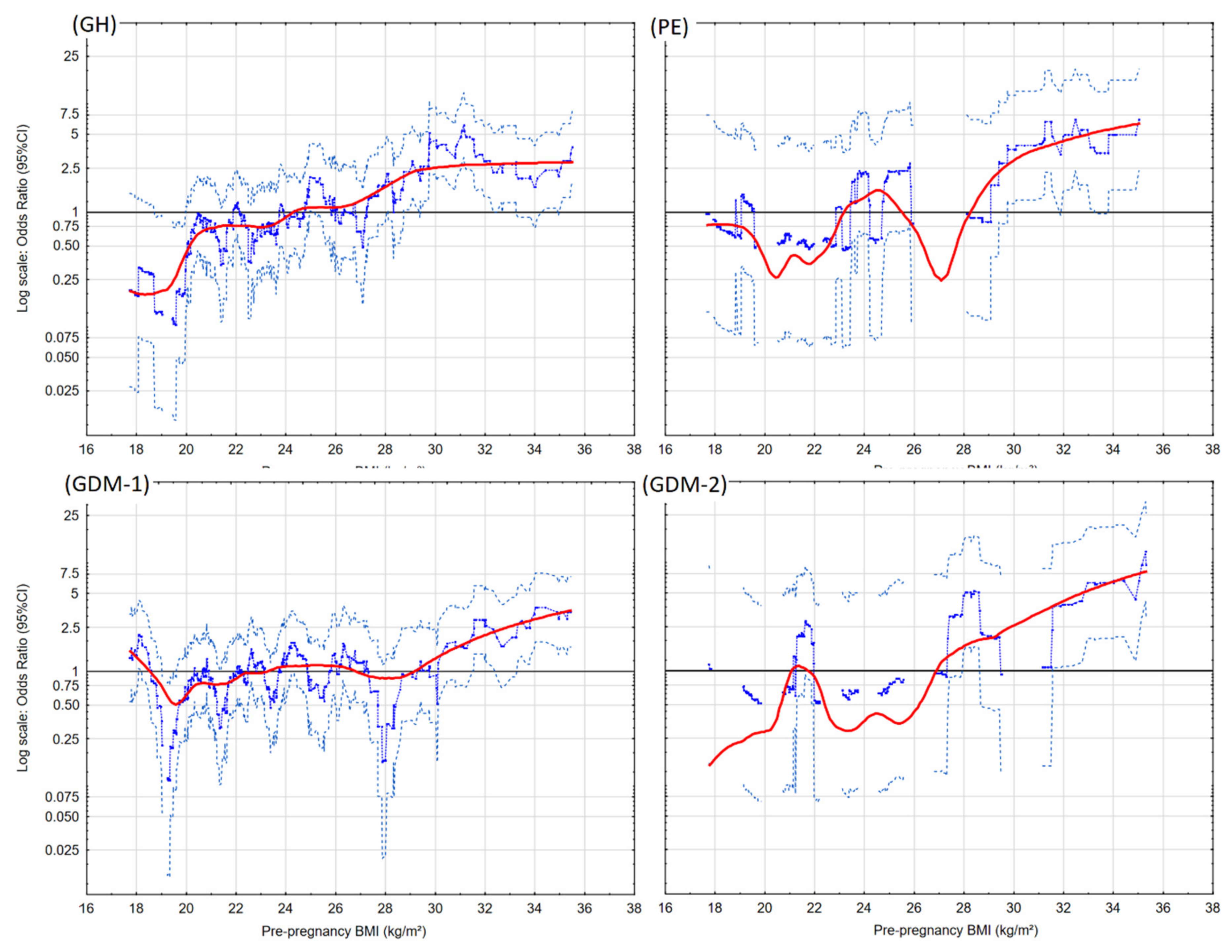
3. Results
4. Discussion
Advantages and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Neeland, I.J.; Poirier, P.; Després, J.-P. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Nkoka, O.; Ntenda, P.A.M.; Senghore, T.; Bass, P. Maternal overweight and obesity and the risk of caesarean birth in Malawi. Reprod. Health 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, D.; Gutierrez, J.; Binder, J.; Mantovani, E.; Thilaganathan, B.; Khalil, A. Impaired maternal hemodynamics in morbidly obese women: A case-control study. Ultrasound Obstet. Gynecol. 2017, 50, 761–765. [Google Scholar] [CrossRef]
- Yao, D.; Chang, Q.; Wu, Q.-J.; Gao, S.-Y.; Zhao, H.; Liu, Y.-S.; Jiang, Y.-T.; Zhao, Y.-H. Relationship between maternal central obesity and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of cohort studies. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Ramonienė, G.; Maleckienė, L.; Nadišauskienė, R.J.; Bartusevičienė, E.; Railaitė, D.R.; Mačiulevičienė, R.; Maleckas, A. Maternal obesity and obstetric outcomes in a tertiary referral center. Med. Kaunas Lith. 2017, 53, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Ruhstaller, K.E.; Bastek, J.A.; Thomas, A.; Mcelrath, T.F.; Parry, S.I.; Durnwald, C.P. The effect of early excessive weight gain on the development of hypertension in pregnancy. Am. J. Perinatol. 2016, 33, 1205–1210. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; daSilva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Cos, P.; Briedé, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS ONE 2018, 13, e0202919. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, W.; Xiong, W.; Wang, F. Effects of blood pressure level management on maternal and perinatal outcomes in pregnant women with mild to moderate gestational hypertension. Ginekol. Pol. 2020, 91, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Plows, F.J.; Stanley, L.J.; Baker, N.P.; Reynolds, M.C.; Vickers, H.M. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Niu, V.; Xu, Y.; Hao, J.; Van, S.; Huang, K.; Pan, W.; Ge, X.; Liu, G.; Huang, S.; Tao, F.; et al. Prepregnancy body mass index, gestational weight gain and hypertensive disorder complicating pregnancy: A prospective cohort study in Ma’anshan City. Wei Sheng Yan Jiu 2016, 45, 205–210. [Google Scholar] [PubMed]
- Fernández Alba, J.J.; Mesa Páez, C.; Vilar Sánchez, Á.; Soto Pazos, E.; González Macías, M.D.C.; Serrano Negro, E.; Paublete Herrera, M.D.C.; Moreno Corral, L.J. Overweight and obesity at risk factors for hypertensive states of pregnancy: A retrospective cohort study. Nutr. Hosp. 2018, 35, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 285–301. [Google Scholar] [CrossRef]
- Kutchi, I.; Chellammal, P.; Akila, A. Maternal obesity and pregnancy outcome: In perspective of New Asian Indian guidelines. J. Obstet. Gynaecol. India 2020, 70, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Leung, T.Y.; Sahota, D.; Cheng, Y.K.Y.; Leung, W.C.; Lo, T.K.; Poon, L.C.Y. Body mass index at 11–13 weeks’ gestation and pregnancy complications in a Southern Chinese population: A retrospective cohort study. J. Matern.-Fetal Neonatal Med. 2019, 32, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-H.; Hsieh, T.-T. Pregestational body mass index, gestational weight gain, and risks for adverse pregnancy outcomes among Taiwanese women: A retrospective cohort study. Taiwan J. Obstet. Gynecol. 2016, 55, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Zhang, W. Joint and independent associations of gestational weight gain and pre-pregnancy body mass index with outcomes of pregnancy in Chinese women: A retrospective cohort study. PLoS ONE 2015, 10, e0136850. [Google Scholar] [CrossRef]
- Cho, E.-H.; Hur, J.; Lee, K.-J. Early gestational weight gain rate and adverse pregnancy outcomes in Korean Women. PLoS ONE 2015, 10, e0140376. [Google Scholar] [CrossRef]
- Kirkham, R.; Whitbread, C.; Connors, C.; Moore, E.; Boyle, J.A.; Richa, R.; Barzi, F.; Li, S.; Dowden, M.; Oats, J.; et al. Implementation of a diabetes in pregnancy clinical register in a complex setting: Findings from a process evaluation. PLoS ONE 2017, 12, e0179487. [Google Scholar] [CrossRef]
- Liang, Y.; Li, D.-T.; Chen, M.-X.; Gong, Y.-H.; Zhang, X.; Yang, W.-Y.; Liu, Y.; Cheng, G.; Yang, D.-G. Associations of pre-pregnancy body mass index and gestational weight gain with gestational diabetes mellitus: A cohort study in Southwest China. Sichuan Da Xue Xue Bao Yi Xue Ban 2019, 50, 83–87. [Google Scholar]
- Sullivan, E.A.; Dickinson, J.E.; Vaughan, G.A.; Peek, M.J.; Ellwood, D.; Homer, C.S.E.; Knight, M.; McLintock, C.; Wang, A.; Pollock, W.; et al. Maternal super-obesity and perinatal outcomes in Australia: A national population-based cohort study. BMC Pregnancy Childbirth 2015, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Durst, J.K.; Sutton, A.L.M.; Cliver, S.P.; Tita, A.T.; Biggio, J.R. Impact of gestational weight gain on perinatal outcomes in obese women. Am. J. Perinatol. 2016, 33, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, E.; Sotoudeh, G.; Dorosty-Motlagh, A.R.; Eshraghian, M.R.; Bagheri, M. Maternal obesity and energy intake as risk factors of pregnancy-induced hypertension among Iranian women. J. Health Popul. Nutr. 2014, 32, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.-M.; Kam, N.M.; Wong, L.; Tanner, C.; Shub, A.; Ekinci, E.I.; Houlihan, C. Risk factors for pregnancy outcomes in type 1 and type 2 diabetes. Intern. Med. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- von Versen-Höynck, F.; Schaub, A.M.; Chi, Y.-Y.; Chiu, K.-H.; Liu, J.; Lingis, M.; Stan Williams, R.; Rhoton-Vlasak, A.; Nichols, W.W.; Fleischmann, R.R.; et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019, 73, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Sites, C.K.; Wilson, D.; Barsky, M.; Bernson, D.; Bernstein, I.M.; Boulet, S.; Zhang, Y. Embryo cryopreservation and preeclampsia risk. Fertil. Steril. 2017, 108, 784–790. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B. The influence of various smoking categories on the risk of gestational hypertension and pre-Eclampsia. J. Clin. Med. 2020, 9, 1743. [Google Scholar] [CrossRef]
- Stepaniak, U.; Micek, A.; Waśkiewicz, A.; Bielecki, W.; Drygas, W.; Janion, M.; Kozakiewicz, K.; Niklas, A.; Puch-Walczak, A.; Pająk, A.; et al. Prevalence of general and abdominal obesity and overweight among adults in Poland. Results of the WOBASZ II study (2013-2014) and comparison with the WOBASZ study (2003–2005). Pol. Arch. Med. Wewn. 2016, 126, 662–671. [Google Scholar] [CrossRef]
- Barber, C.; Rankin, J.; Heslehurst, N. Maternal body mass index and access to antenatal care: A retrospective analysis of 619,502 births in England. BMC Pregnancy Childbirth 2017, 17, 290. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. First trimester microelements and their relationships with pregnancy outcomes and complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. Serum selenium level in early healthy pregnancy as a risk marker of pregnancy induced hypertension. Nutrients 2019, 11, 1028. [Google Scholar] [CrossRef]
- Ratajczak, M.; Skrypnik, D.; Bogdański, P.; Mądry, E.; Walkowiak, J.; Szulińska, M.; Maciaszek, J.; Kręgielska-Narożna, M.; Karolkiewicz, J. Effects of endurance and endurance-strength training on endothelial function in women with obesity: A randomized trial. Int. J. Environ. Res. Public Health 2019, 16, 4291. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. Can serum iron concentrations in early healthy pregnancy be risk marker of pregnancy-induced hypertension? Nutrients 2019, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Sajdak, S.; Marciniak, W.; Lubiński, J. First trimester serum copper or zinc levels, and risk of pregnancy-induced hypertension. Nutrients 2019, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
| Controls | Cases | ||
|---|---|---|---|
| Characteristics | n (%) | n (%) | p * |
| Normotensives (n = 775) | GH cases (n = 113) | ||
| Birth Weight (g) | 3416.5 (511.7); 3449.0 | 3174.1 (734.3); 3200.0 | 0.001 |
| <10th Percentile | 42 (5.4%) | 21 (18.6%) | <0.001 |
| 10–90th Percentile | 652 (84.1%) | 75 (66.4%) | ** |
| > 90th Percentile | 81 (10.5%) | 17 (15%) | 0.038 |
| Gestational Age (Week) | 38.9 (1.6); 39.0 | 38.3 (2.2); 39.0 | 0.016 |
| Birth < 37th Week | 41 (5.3%) | 11 (9.7%) | 0.060 |
| Birth < 34th Week | 10 (1.3%) | 7 (6.2%) | 0.003 |
| Normotensives (n = 775) | PE cases (n = 24) | ||
| Birth Weight (g) | 3416.5 (511.7); 3449.0 | 2294.2 (927.5); 2445.0 | <0.001 |
| <10th Percentile | 42 (5.4%) | 9 (37.5%) | <0.001 |
| 10–90th Percentile | 652 (84.1%) | 14 (58.3%) | ** |
| >90th Percentile | 81 (10.5%) | 1 (4.2%) | 1.000 |
| Gestational Age (Week) | 38.9 (1.6); 39.0 | 35.1 (3.7); 36.0 | <0.001 |
| Birth < 37th Week | 41 (5.3%) | 13 (54.2%) | <0.001 |
| Birth < 34th Week | 10 (1.3%) | 6 (25%) | <0.001 |
| Without diabetes (n = 766) | Diabetes GDM-1 (n = 125) | ||
| Birth Weight (g) | 3347.2 (588.3); 3400.0 | 3416.1 (547.8); 3400.0 | 0.470 |
| <10th Percentile | 59 (7.7%) | 11(8.8%) | 0.491 |
| 10–90th Percentile | 633 (82.6%) | 93 (74.4%) | ** |
| >90th Percentile | 74 (9.7%) | 21 (16.8%) | 0.014 |
| Gestational Age (Week) | 38.7 (2.0); 39.0 | 38.7 (1.5); 39.0 | 0.125 |
| Birth < 37th Week | 54 (7%) | 9 (7.2%) | 0.952 |
| Birth < 34th Week | 21 (2.7%) | 1 (0.8%) | 0.346 |
| Without diabetes (n = 766) | Diabetes GDM-2 (n = 21) | ||
| Birth Weight (g) | 3347.2 (588.3); 3400.0 | 3358.6 (837.8); 3300.0 | 0.771 |
| <10th Percentile | 59 (7.7%) | 2 (9.5%) | 0.651 |
| 10–90th Percentile | 633 (82.6%) | 15 (71.4%) | ** |
| >90th Percentile | 74 (9.7%) | 4 (19%) | 0.137 |
| Gestational Age (Week) | 38.7 (2.0); 39.0 | 38.0 (2.2); 38.0 | 0.037 |
| Birth < 37th Week | 54 (7%) | 2 (9.5%) | 0.656 |
| Birth < 34th Week | 21 (2.7%) | 1 (4.8%) | 0.453 |
| Controls | Cases | ||
|---|---|---|---|
| Characteristics | n (%) | n (%) | p * |
| Normotensives (n = 775) | GH cases (n = 113) | ||
| Pre-pregnancy BMI (kg/m2) | 23.3 (4.1); 22.5 | 26.7 (5.3); 25.5 | <0.001 |
| Obesity | 58 (7.5%) | 31 (27.4%) | <0.001 |
| Overweight | 139 (17.9%) | 30 (26.5%) | |
| Normal Pre-Pregnancy BMI | 534 (68.9%) | 51 (45.1%) | |
| Underweight | 44 (5.7%) | 1 (0.9%) | |
| GWG (kg) | 13.4 (5.3); 13.0 | 14.6 (8); 14,0 | 0.115 |
| GWG Above Recommendation | 263 (33.9%) | 62 (54.9%) | <0.001 |
| GWG in Range of Recommendation ** | 301 (38.8%) | 29 (25.7%) | |
| GWG Below Recommendation | 211 (27.2%) | 22 (19.5%) | |
| Normotensives (n = 775) | PE cases (n = 24) | ||
| Pre-Pregnancy BMI (kg/m2) | 23.3 (4.1); 22.5 | 26.5 (6.2); 25.0 | 0.008 |
| Obesity | 58 (7.5%) | 9 (37.5%) | <0.001 |
| Overweight | 139 (17.9%) | 4 (16.7%) | |
| Normal Pre-Pregnancy BMI | 534 (68.9%) | 9 (37.5%) | |
| Underweight | 44 (5.7%) | 2 (8.3%) | |
| GWG (kg) | 13.4 (5.3); 13.0 | 15.1(8.2); 14.3 | 0.612 |
| GWG Above Recommendation | 263 (33.9%) | 11 (45.8%) | 0.258 |
| GWG in Range of Recommendation ** | 301 (38.8%) | 8 (33.3%) | |
| GWG Below Recommendation | 211 (27.2%) | 5 (20.8%) | |
| Without diabetes (n = 766) | Diabetes GDM-1 (n = 125) | ||
| Pre-Pregnancy BMI (kg/m2) | 23.5 (4.2); 22.6 | 25.0 (5.2); 23.9 | 0.004 |
| Obesity | 66 (8.6%) | 25 (20.0%) | 0.007 |
| Overweight | 147 (19.2%) | 21 (16.8%) | |
| Normal Pre-Pregnancy BMI | 515 (67.2%) | 71 (56.8%) | |
| Underweight | 38 (5.0%) | 8 (6.4%) | |
| GWG (kg) | 14.1 (5.5); 14.0 | 11.2 (6.7); 11.0 | <0.001 |
| GWG Above Recommendation | 298 (38.9%) | 33 (26.4%) | <0.001 |
| GWG in Range of Recommendation ** | 292 (38.1%) | 39 (31.2%) | |
| GWG Below Recommendation | 176 (23.0%) | 53 (42.4%) | |
| Without diabetes (n = 766) | Diabetes GDM-2 (n = 21) | ||
| Pre-Pregnancy BMI (kg/m2) | 23.5 (4.2); 22.6 | 27.9 (7.0); 28.0 | 0.003 |
| Obesity | 66 (8.6%) | 7 (33.3%) | 0.001 |
| Overweight | 147 (19.2%) | 5 (23.8%) | |
| Normal Pre-Pregnancy BMI | 515 (67.2%) | 8 (38.1%) | |
| Underweight | 38 (5.0%) | 1 (4.8%) | |
| GWG (kg) | 14.1 (5.5); 14.0 | 9.0 (5.0); 9.0 | <0.001 |
| GWG Above Recommendation | 298 (38.9%) | 5 (23.8%) | 0.041 |
| GWG in Range of Recommendation ** | 292 (38.1%) | 7 (33.3%) | |
| GWG Below Recommendation | 176 (23.0%) | 9 (42.9%) |
| The Odds Ratios of the Diseases for Weight Categories | ||||
|---|---|---|---|---|
| Cases/ Controls | OR (95% CI:); p | AOR-c * (95% CI:); p | ||
| Gestational Hypertension (GH) ** | ||||
| Obesity | 31/58 | 5.60 (3.32–9.43); < 0.001 | 4.94 (2.77–8.81); < 0.001 | |
| Overweight | 30/139 | 2.26 (1.39–3.68); 0.001 | 2.09 (1.21–3.60); 0.008 | |
| Underweight | 1/44 | 0.24 (0.03–1.76); 0.16 | 0.29 (0.04–2.17); 0.226 | |
| Normal Pre-Pregnancy BMI | 51/534 | 1 | 1 | |
| GWG Above the Range | 62/263 | 2.45 (1.53–3.92); < 0.001 | 1.71 (1.01–2.89); 0.045 | |
| GWG in the Range | 29/301 | 1 | 1 | |
| GWG Below the Range | 22/211 | 1.08 (0.61–1.94); 0.790 | 1.02 (0.54–1.91); 0.950 | |
| Preeclampsia (PE) ** | ||||
| Obesity | 9/58 | 9.21 (3.52–24.11); < 0.001 | 8.61 (3.05–24.36); < 0.001 | |
| Overweight | 4/139 | 1.71 (0.52–5.63); 0.379 | 1.91 (0.53–6.87); 0.324 | |
| Underweight | 2/44 | 2.70 (0.57–12.87); 0.213 | 2.95 (0.57–15.16); 0.195 | |
| Normal Pre-Pregnancy BMI | 9/534 | 1 | 1 | |
| GWG Above the Range | 11/263 | 1.57 (0.62–3.97); 0.337 | 1.14 (0.41–3.12); 0.803 | |
| GWG in the Range | 8/301 | 1 | 1 | |
| GWG Below the Range | 5/211 | 0.89 (0.29–2.76); 0.842 | 0.80 (0.24–2.65); 0.716 | |
| Gestational Diabetes GDM-1 *** | ||||
| Obesity | 25/66 | 2.75 (1.63–4.64); < 0.001 | 2.99 (1.71–5.25); < 0.001 | |
| Overweight | 21/147 | 1.04 (0.62–1.74); 0.893 | 1.24 (0.71–2.14); 0.452 | |
| Underweight | 8/38 | 1.53 (0.69–3.41); 0.301 | 1.53 (0.66–3.52); 0.322 | |
| Normal Pre-Pregnancy BMI | 71/515 | 1 | 1 | |
| GWG Above the Range | 33/298 | 0.83 (0.51–1.36); 0.454 | 0.74 (0.44–1.24); 0.245 | |
| GWG in the Range | 39/292 | 1 | 1 | |
| GWG Below the Range | 53/176 | 2.26 (1.43–3.55); < 0.001 | 2.53 (1.58–4.06); < 0.001 | |
| Gestational Diabetes GDM-2 *** | ||||
| Obesity | 7/66 | 6.83 (2.4–19.44); < 0.001 | 11.88 (3.67–38.48); < 0.001 | |
| Overweight | 5/147 | 2.19 (0.71–6.79); 0.175 | 2.29 (0.58–9.06); 0.238 | |
| Underweight | 1/38 | 1.69 (0.21–13.9); 0.624 | 2.66 (0.3–23.74); 0.381 | |
| Normal Pre-Pregnancy BMI | 8/515 | 1 | 1 | |
| GWG Above the Range | 5/298 | 0.70 (0.22–2.23); 0.546 | 0.76 (0.21–2.77); 0.672 | |
| GWG in the Range | 7/292 | 1 | 1 | |
| GWG Below the Range | 9/176 | 2.13 (0.78–5.83); 0.14 | 3.43 (1.02–11.5); 0.046 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, M.; Więckowska, B.; Sajdak, S. Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus. J. Clin. Med. 2020, 9, 1980. https://doi.org/10.3390/jcm9061980
Lewandowska M, Więckowska B, Sajdak S. Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus. Journal of Clinical Medicine. 2020; 9(6):1980. https://doi.org/10.3390/jcm9061980
Chicago/Turabian StyleLewandowska, Małgorzata, Barbara Więckowska, and Stefan Sajdak. 2020. "Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus" Journal of Clinical Medicine 9, no. 6: 1980. https://doi.org/10.3390/jcm9061980
APA StyleLewandowska, M., Więckowska, B., & Sajdak, S. (2020). Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus. Journal of Clinical Medicine, 9(6), 1980. https://doi.org/10.3390/jcm9061980





