Risk of Psoriasis in Patients with Polycystic Ovary Syndrome: A National Population-Based Cohort Study
Abstract
1. Introduction
2. Experimental Section
2.1. Data Source
2.2. Study Participants
2.3. Main Outcome and Comorbidities
2.4. Statistical Analysis
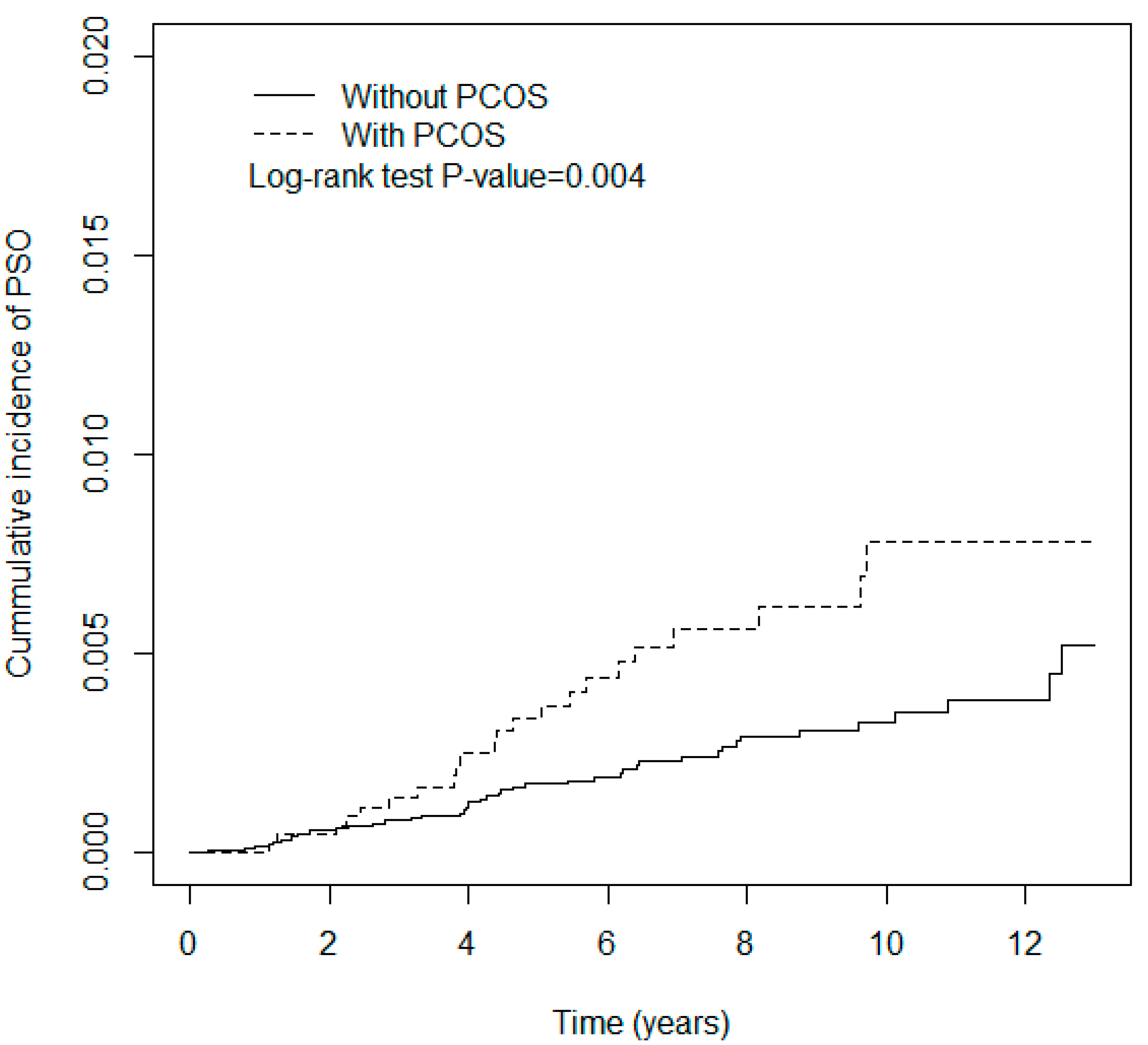
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chen, W.; Wang, J.H.; Lin, S.Z. Association between polycystic ovarian syndrome and endometrial, ovarian, and breast cancer: A population-based cohort study in Taiwan. Medicine 2018, 97, e12608. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Raychaudhuri, S.P. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J. Autoimmun. 2010, 34, J314–J321. [Google Scholar] [CrossRef] [PubMed]
- Ucak, S.; Ekmekci, T.R.; Basat, O.; Koslu, A.; Altuntas, Y. Comparison of various insulin sensitivity indices in psoriatic patients and their relationship with type of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; De Simone, C.; Morciano, A.; Tropea, A.; Sagnella, F.; Palla, C.; Scarinci, E.; Teti, A.; Caldarola, G.; D’Agostino, M.; et al. Psoriatic patients have an increased risk of polycystic ovary syndrome: Results of a cross-sectional analysis. Fertil. Steril. 2013, 99, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Gyldenlove, M.; Storgaard, H.; Holst, J.J.; Vilsboll, T.; Knop, F.K.; Skov, L. Patients with psoriasis are insulin resistant. J. Am. Acad. Dermatol. 2015, 72, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Tessari, G.; Conti, A.; Piaserico, S.; Schianchi, S.; Peserico, A.; Giannetti, A.; Girolomoni, G. Prevalence of metabolic syndrome in patients with psoriasis: A hospital-based case-control study. Br. J. Dermatol. 2007, 157, 68–73. [Google Scholar] [CrossRef]
- Takahashi, H.; Iizuka, H. Psoriasis and metabolic syndrome. J. Dermatol. 2012, 39, 212–218. [Google Scholar] [CrossRef]
- Moro, F.; Tropea, A.; Scarinci, E.; Federico, A.; De Simone, C.; Caldarola, G.; Leoncini, E.; Boccia, S.; Lanzone, A.; Apa, R. Psoriasis and polycystic ovary syndrome: A new link in different phenotypes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.F.; Hsieh, C.Y.; Lin, H.J.; Chen, Y.W.; Yang, Y.H.; Li, C.Y. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int. J. Cardiol. 2016, 215, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.H.; Hong, J.H.; See, L.C.; Yu, H.P.; Hsu, J.T.; Chou, I.J.; Chou, W.C.; Chiou, M.J.; Wang, C.C.; Kuo, C.F. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol. Drug Saf. 2018, 27, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Tsai, I.J.; Hsu, C.Y.; Wang, J.H.; Lin, S.Z.; Sung, F.C. Risk of hypertension after hysterectomy: A population-based study. BJOG 2018, 125, 1717–1724. [Google Scholar] [CrossRef]
- Harnod, T.; Chen, W.; Wang, J.H.; Lin, S.Z.; Ding, D.C. Association between depression risk and polycystic ovarian syndrome in young women: A retrospective nationwide population-based cohort study (1998–2013). Hum. Reprod. 2019, 34, 1830–1837. [Google Scholar] [CrossRef]
- Ding, D.C.; Tsai, I.J.; Wang, J.H.; Lin, S.Z.; Sung, F.C. Coronary artery disease risk in young women with polycystic ovary syndrome. Oncotarget 2018, 9, 8756–8764. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Jiang, Y.; Ma, H.; Chen, L.; Lai, C.; Peng, C.; He, C.; Sun, C. Association between polycystic ovary syndrome and the risk of stroke and all-cause mortality: Insights from a meta-analysis. Gynecol. Endocrinol. 2017, 33, 904–910. [Google Scholar] [CrossRef]
- Egeberg, A.; Khalid, U.; Gislason, G.H.; Mallbris, L.; Skov, L.; Hansen, P.R. Impact of Depression on Risk of Myocardial Infarction, Stroke and Cardiovascular Death in Patients with Psoriasis: A Danish Nationwide Study. Acta Derm. Venereol. 2016, 96, 218–221. [Google Scholar] [CrossRef]
- Xhaja, A.; Shkodrani, E.; Frangaj, S.; Kuneshka, L.; Vasili, E. An epidemiological study on trigger factors and quality of life in psoriatic patients. Mater. Sociomed. 2014, 26, 168–171. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, C.Y.; Chen, T.J.; Shen, J.L.; Chu, S.Y.; Wang, C.B.; Chang, Y.T. The risk of cancer in patients with psoriasis: A population-based cohort study in Taiwan. J. Am. Acad. Dermatol. 2011, 65, 84–91. [Google Scholar] [CrossRef]
- Pouplard, C.; Brenaut, E.; Horreau, C.; Barnetche, T.; Misery, L.; Richard, M.A.; Aractingi, S.; Aubin, F.; Cribier, B.; Joly, P.; et al. Risk of cancer in psoriasis: A systematic review and meta-analysis of epidemiological studies. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. 3), 36–46. [Google Scholar] [CrossRef] [PubMed]
- Reich, K. The concept of psoriasis as a systemic inflammation: Implications for disease management. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. 2), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Kao, W.M.; Huang, J.Y.; Hung, Y.M.; Wei, J.C. Human papillomavirus infection associated with increased risk of new-onset psoriasis: A nationwide population-based cohort study. Int. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.F.; Jen, I.A.; Chen, M.; Lan, Y.C.; Lee, C.Y.; Chuang, P.H.; Lee, Y.; Chen, Y.M. HIV Infection Increases the Risk of Incident Psoriasis: A Nationwide Population-Based Cohort Study in Taiwan. J. Acquir. Immune Defic. Syndr. 2017, 75, 493–499. [Google Scholar] [CrossRef]
- Repaci, A.; Gambineri, A.; Pasquali, R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol. Cell. Endocrinol. 2011, 335, 30–41. [Google Scholar] [CrossRef] [PubMed]
| PCOS | |||||
|---|---|---|---|---|---|
| No (N = 18,828) | Yes (N = 4707) | ||||
| Variables | n | % | n | % | p-Value 1 |
| Age, year | >0.99 | ||||
| ≤20 | 2791 | 15 | 697 | 15 | |
| 20–50 | 16,017 | 85 | 4005 | 85 | |
| >50 | 20 | 0.11 | 5 | 0.11 | |
| Comorbidities | |||||
| Asthma | 916 | 4.9 | 316 | 6.7 | <0.001 |
| Chronic obstructive pulmonary diseases | 2064 | 11 | 660 | 14 | <0.001 |
| Coronary arterial diseases | 122 | 0.65 | 40 | 0.85 | 0.162 |
| Cancer | 63 | 0.33 | 14 | 0.30 | 0.797 |
| Congestive heart failure | 40 | 0.21 | 8 | 0.17 | 0.691 |
| Chronic liver diseases | 948 | 5.0 | 375 | 8.0 | <0.001 |
| Diabetes Mellitus | 261 | 1.4 | 143 | 3.0 | <0.001 |
| Hypertension | 290 | 1.5 | 111 | 2.4 | <0.001 |
| Hyperlipidemia | 464 | 2.5 | 254 | 5.4 | <0.001 |
| Stroke | 112 | 0.59 | 32 | 0.68 | 0.573 |
| Anxiety | 52 | 0.28 | 20 | 0.42 | 0.132 |
| Depression | 740 | 3.9 | 252 | 5.4 | <0.001 |
| Sleep apnea | 19 | 0.10 | 11 | 0.23 | 0.040 |
| Psoriasis | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Events | PY | IR | Crude HR | (95%CI) | Adjusted HR ꝉ | (95%CI) |
| PCOS | |||||||
| No | 44 | 130,684 | 0.34 | 1.00 | - | 1.00 | - |
| Yes | 23 | 32,904 | 0.70 | 2.08 | (1.25,3.44) ** | 2.07 | (1.25,3.43) ** |
| Age, year | |||||||
| ≤20 | 10 | 24,622 | 0.41 | 1.00 | - | 1.00 | - |
| 20–50 | 56 | 138,783 | 0.40 | 1.00 | (0.51,1.95) | 0.98 | (0.5,1.93) |
| >50 | 1 | 183 | 5.45 | 13.82 | (1.76,108.26) * | 14.13 | (1.8,110.7) * |
| Comorbidities | |||||||
| anxiety | |||||||
| No | 67 | 163,175 | 0.41 | 1.00 | - | ||
| Yes | 0 | 413 | 0 | 0.00 | (0, Inf) | ||
| asthma | |||||||
| No | 64 | 156,490 | 0.41 | 1.00 | - | ||
| Yes | 3 | 7098 | 0.42 | 1.07 | (0.34,3.4) | ||
| CAD | |||||||
| No | 67 | 162,514 | 0.41 | 1.00 | - | ||
| Yes | 0 | 1074 | 0 | 0.00 | (0,Inf) | ||
| cancer | |||||||
| No | 65 | 163,144 | 0.40 | 1.00 | - | 1.00 | - |
| Yes | 2 | 444 | 4.50 | 11.70 | (2.86,47.83) *** | 11.72 | (2.87,47.9) *** |
| COPD | |||||||
| No | 60 | 147,133 | 0.41 | 1.00 | - | ||
| Yes | 7 | 16,455 | 0.43 | 1.07 | (0.49,2.35) | ||
| CHF | |||||||
| No | 67 | 163,310 | 0.41 | 1.00 | - | ||
| Yes | 0 | 278 | 0 | 0.00 | (0,Inf) | ||
| CLD | |||||||
| No | 62 | 154,714 | 0.40 | 1.00 | - | ||
| Yes | 5 | 8874 | 0.56 | 1.42 | (0.57,3.54) | ||
| DM | |||||||
| No | 65 | 160,929 | 0.40 | 1.00 | - | ||
| Yes | 2 | 2659 | 0.75 | 1.88 | (0.46,7.69) | ||
| Depression | |||||||
| No | 65 | 157,679 | 0.41 | 1.00 | - | ||
| Yes | 2 | 5909 | 0.34 | 0.84 | (0.21,3.44) | ||
| Hypertension | |||||||
| No | 67 | 161,058 | 0.42 | 1.00 | - | ||
| Yes | 0 | 2531 | 0 | 0.00 | (0,Inf) | ||
| Hyperlipidemia | |||||||
| No | 64 | 159,002 | 0.40 | 1.00 | - | ||
| Yes | 3 | 4586 | 0.65 | 1.65 | (0.52,5.24) | ||
| Stroke | |||||||
| No | 66 | 162,624 | 0.41 | 1.00 | - | ||
| Yes | 1 | 965 | 1.04 | 2.56 | (0.35,18.43) | ||
| Sleep apnea | |||||||
| No | 67 | 163,446 | 0.41 | 1.00 | - | ||
| Yes | 0 | 142 | 0 | 0.00 | (0,Inf) | ||
| PCOS | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Variables | Events | PY | IR | Events | PY | IR | Adjusted HR ꝉ | (95%CI) |
| Age, year | ||||||||
| ≤20 | 5 | 19,714 | 0.25 | 5 | 4908 | 1.02 | 4.02 | (1.16,13.9) * |
| 20–50 | 38 | 110,823 | 0.34 | 18 | 27,960 | 0.64 | 1.88 | (1.07,3.29) * |
| >50 | 1 | 148 | 6.78 | 0 | 36 | 0 | - | - |
| Comorbidities | ||||||||
| anxiety | ||||||||
| No | 44 | 130,380 | 0.34 | 23 | 32,795 | 0.70 | 2.07 | (1.25,3.43) ** |
| Yes | 0 | 304 | 0 | 0 | 109 | 0 | - | - |
| asthma | ||||||||
| No | 43 | 125,433 | 0.34 | 21 | 31,058 | 0.68 | 1.96 | (1.16,3.30) * |
| Yes | 1 | 5251 | 0.19 | 2 | 1847 | 1.08 | 5.62 | (0.51,62.4) |
| CAD | ||||||||
| No | 44 | 129,855 | 0.34 | 23 | 32,659 | 0.70 | 2.07 | (1.25,3.43) ** |
| Yes | 0 | 829 | 0 | 0 | 245 | 0 | - | - |
| cancer | ||||||||
| No | 43 | 130,330 | 0.33 | 22 | 32,814 | 0.67 | 2.03 | (1.21,3.40) ** |
| Yes | 1 | 354 | 2.83 | 1 | 91 | 11.05 | 1.44 | (0.26,68.2) |
| COPD | ||||||||
| No | 40 | 118,275 | 0.34 | 20 | 28,859 | 0.69 | 2.03 | (1.18,3.47) ** |
| Yes | 4 | 12,409 | 0.32 | 3 | 4046 | 0.74 | 2.27 | (0.57,10.2) |
| CHF | ||||||||
| No | 44 | 130,439 | 0.34 | 23 | 32,871 | 0.70 | 2.07 | (1.25,3.43) ** |
| Yes | 0 | 245 | 0 | 0 | 33 | 0 | - | - |
| CLD | ||||||||
| No | 42 | 124,509 | 0.34 | 20 | 30,205 | 0.66 | 1.96 | (1.15,3.34) * |
| Yes | 2 | 6175 | 0.32 | 3 | 2699 | 1.11 | 2.78 | (0.46,16.9) |
| DM | ||||||||
| No | 43 | 128,984 | 0.33 | 22 | 31,945 | 0.69 | 2.05 | (1.23,3.43) ** |
| Yes | 1 | 1700 | 0.59 | 1 | 959 | 1.04 | 1.75 | (0.11,28.0) |
| Depression | ||||||||
| No | 42 | 126,183 | 0.33 | 23 | 32,859 | 0.70 | 2.18 | (1.31,3.62) ** |
| Yes | 2 | 4501 | 0.44 | 0 | 45 | 0 | - | - |
| Hypertension | ||||||||
| No | 44 | 128,876 | 0.34 | 23 | 32,182 | 0.71 | 2.07 | (1.25,3.44) ** |
| Yes | 0 | 1808 | 0 | 0 | 722 | 0 | - | - |
| Hyperlipidemia | ||||||||
| No | 43 | 127,796 | 0.34 | 21 | 31,206 | 0.67 | 1.99 | (1.18,3.36) ** |
| Yes | 1 | 2888 | 0.35 | 2 | 1698 | 1.18 | 3.09 | (0.27,34.1) |
| Stroke | ||||||||
| No | 44 | 129,947 | 0.34 | 22 | 32,677 | 0.67 | 1.98 | (1.19,3.30) ** |
| Yes | 0 | 737 | 0 | 1 | 227 | 4.40 | - | - |
| Sleep apnea | ||||||||
| No | 44 | 130,587 | 0.34 | 23 | 32,859 | 0.70 | 2.07 | (1.25,3.43) ** |
| Yes | 0 | 97 | 0 | 0 | 45 | 0 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-H.; Wu, C.-H.; Chen, M.-L.; Yip, H.-T.; Lee, C.-I.; Lee, M.-S.; Wei, J.C.-C. Risk of Psoriasis in Patients with Polycystic Ovary Syndrome: A National Population-Based Cohort Study. J. Clin. Med. 2020, 9, 1947. https://doi.org/10.3390/jcm9061947
Lee T-H, Wu C-H, Chen M-L, Yip H-T, Lee C-I, Lee M-S, Wei JC-C. Risk of Psoriasis in Patients with Polycystic Ovary Syndrome: A National Population-Based Cohort Study. Journal of Clinical Medicine. 2020; 9(6):1947. https://doi.org/10.3390/jcm9061947
Chicago/Turabian StyleLee, Tsung-Hsien, Cheng-Hsuan Wu, Ming-Li Chen, Hei-Tung Yip, Chun-I Lee, Maw-Sheng Lee, and James Cheng-Chung Wei. 2020. "Risk of Psoriasis in Patients with Polycystic Ovary Syndrome: A National Population-Based Cohort Study" Journal of Clinical Medicine 9, no. 6: 1947. https://doi.org/10.3390/jcm9061947
APA StyleLee, T.-H., Wu, C.-H., Chen, M.-L., Yip, H.-T., Lee, C.-I., Lee, M.-S., & Wei, J. C.-C. (2020). Risk of Psoriasis in Patients with Polycystic Ovary Syndrome: A National Population-Based Cohort Study. Journal of Clinical Medicine, 9(6), 1947. https://doi.org/10.3390/jcm9061947






