Benefits of High-Flow Nasal Cannula Therapy for Acute Pulmonary Edema in Patients with Heart Failure in the Emergency Department: A Prospective Multi-Center Randomized Controlled Trial
Abstract
1. Introduction
2. Experimental Section
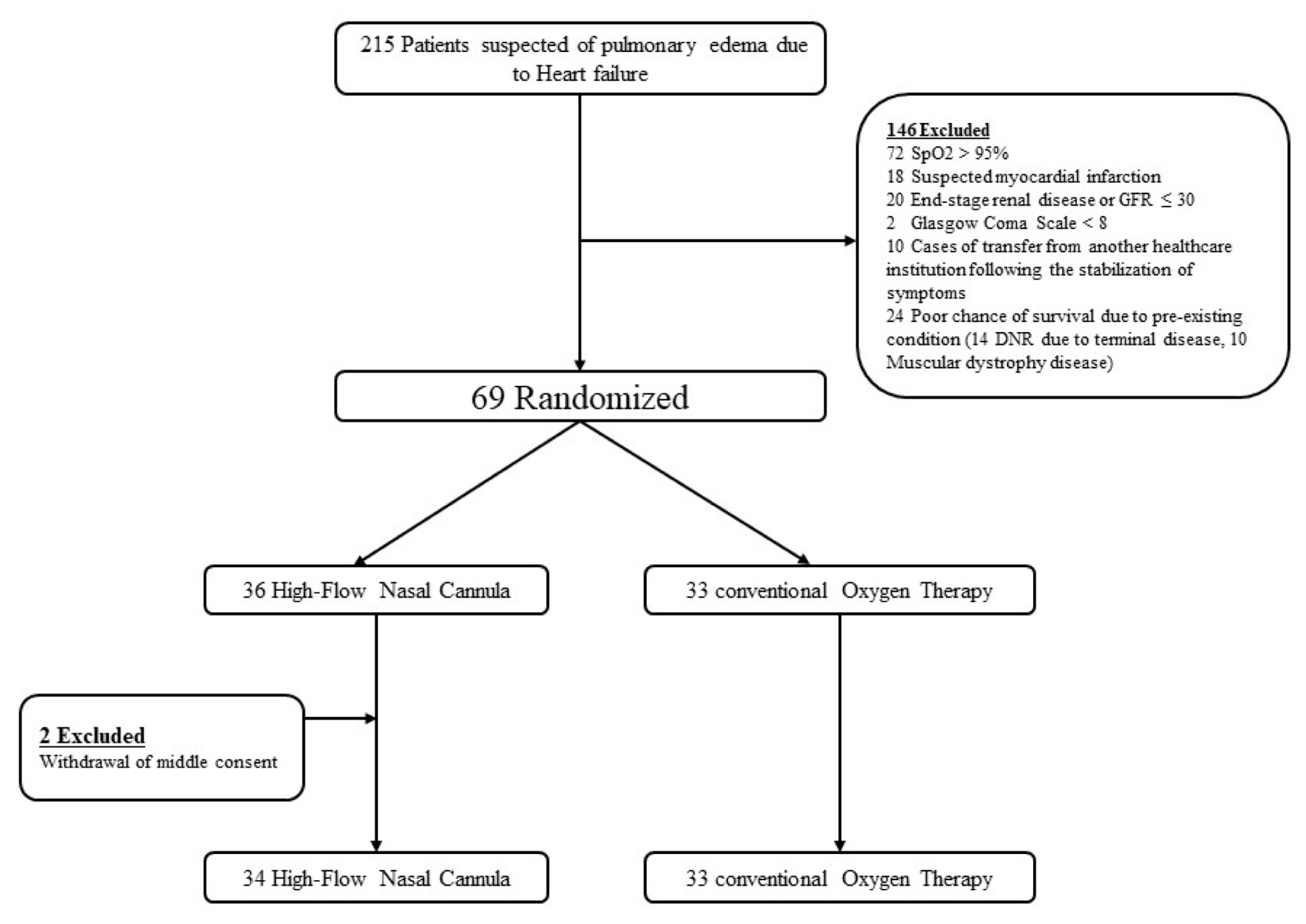
2.1. Study Design and Patients
2.2. Intervention
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Subjects
3.2. Study Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the european society of cardiology. Developed in collaboration with the heart failure association of the esc (hfa) and endorsed by the european society of intensive care medicine (esicm). Eur. Heart J. 2008, 29, 2388–2442. [Google Scholar]
- Makdee, O.; Monsomboon, A.; Surabenjawong, U.; Praphruetkit, N.; Chaisirin, W.; Chakorn, T.; Permpikul, C.; Thiravit, P.; Nakornchai, T. High-flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: A randomized controlled trial. Ann. Emerg. Med. 2017, 70, 465.e462–472.e462. [Google Scholar] [CrossRef]
- Berbenetz, N.; Wang, Y.; Brown, J.; Godfrey, C.; Ahmad, M.; Vital, F.M.; Lambiase, P.; Banerjee, A.; Bakhai, A.; Chong, M. Non-invasive positive pressure ventilation (cpap or bilevel nppv) for cardiogenic pulmonary oedema. Cochrane Database Syst. Rev. 2019, 4, CD005351. [Google Scholar] [CrossRef]
- Rudiger, A.; Harjola, V.P.; Muller, A.; Mattila, E.; Saila, P.; Nieminen, M.; Follath, F. Acute heart failure: Clinical presentation, one-year mortality and prognostic factors. Eur. J. Heart Fail. 2005, 7, 662–670. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 accf/aha guideline for the management of heart failure: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc)developed with the special contribution of the heart failure association (hfa) of the esc. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 comprehensive update of the canadian cardiovascular society guidelines for the management of heart failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef]
- Lee, C.C.; Mankodi, D.; Shaharyar, S.; Ravindranathan, S.; Danckers, M.; Herscovici, P.; Moor, M.; Ferrer, G. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: A systematic review. Respir. Med. 2016, 121, 100–108. [Google Scholar] [CrossRef]
- Schwabbauer, N.; Berg, B.; Blumenstock, G.; Haap, M.; Hetzel, J.; Riessen, R. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: Effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (niv). BMC Anesthesiol. 2014, 14, 66. [Google Scholar] [CrossRef]
- Dumas, G.; Chevret, S.; Lemiale, V.; Pène, F.; Demoule, A.; Mayaux, J.; Kouatchet, A.; Nyunga, M.; Perez, P.; Argaud, L.; et al. Oxygenation/non-invasive ventilation strategy and risk for intubation in immunocompromised patients with hypoxemic acute respiratory failure. Oncotarget 2018, 9, 33682–33693. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Bohm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [PubMed]
- Masip, J.; Roque, M.; Sanchez, B.; Fernandez, R.; Subirana, M.; Exposito, J.A. Noninvasive ventilation in acute cardiogenic pulmonary edema: Systematic review and meta-analysis. JAMA 2005, 294, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Winck, J.C.; Azevedo, L.F.; Costa-Pereira, A.; Antonelli, M.; Wyatt, J.C. Efficacy and safety of non-invasive ventilation in the treatment of acute cardiogenic pulmonary edema—A systematic review and meta-analysis. Crit. Care 2006, 10, R69. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Hill, N. Non-invasive ventilation in acute respiratory failure. Lancet 2009, 374, 250–259. [Google Scholar] [CrossRef]
- Evans, T.W. International consensus conferences in intensive care medicine: Non-invasive positive pressure ventilation in acute respiratory failure. Organised jointly by the american thoracic society, the european respiratory society, the european society of intensive care medicine, and the societe de reanimation de langue francaise, and approved by the ats board of directors, december 2000. Intensive Care Med. 2001, 27, 166–178. [Google Scholar]
- Garpestad, E.; Brennan, J.; Hill, N.S. Noninvasive ventilation for critical care. Chest 2007, 132, 711–720. [Google Scholar] [CrossRef]
- Delclaux, C.; L’Her, E.; Alberti, C.; Mancebo, J.; Abroug, F.; Conti, G.; Guerin, C.; Schortgen, F.; Lefort, Y.; Antonelli, M.; et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA 2000, 284, 2352–2360. [Google Scholar] [CrossRef]
- Gray, A.; Goodacre, S.; Newby, D.E.; Masson, M.; Sampson, F.; Nicholl, J.; Trialists, C.P.O. Noninvasive ventilation in acute cardiogenic pulmonary edema. N. Engl. J. Med. 2008, 359, 142–151. [Google Scholar] [CrossRef]
- Boccatonda, A.; Groff, P. High-flow nasal cannula oxygenation utilization in respiratory failure. Eur. J. Intern. Med. 2019, 64, 10–14. [Google Scholar] [CrossRef]
- Roca, O.; Riera, J.; Torres, F.; Masclans, J.R. High-flow oxygen therapy in acute respiratory failure. Respir. Care 2010, 55, 408–413. [Google Scholar]
- Dysart, K.; Miller, T.L.; Wolfson, M.R.; Shaffer, T.H. Research in high flow therapy: Mechanisms of action. Respir. Med. 2009, 103, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Roca, O.; Perez-Teran, P.; Masclans, J.R.; Perez, L.; Galve, E.; Evangelista, A.; Rello, J. Patients with new york heart association class iii heart failure may benefit with high flow nasal cannula supportive therapy: High flow nasal cannula in heart failure. J. Crit. Care 2013, 28, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Kim, K.; Ju, S.; Park, H.W.; Lee, S.J.; Koh, J.S.; Hwang, S.J.; Hwang, J.Y.; Bae, J.S.; Ahn, J.H.; et al. Clinical efficacy of high-flow oxygen therapy through nasal cannula in patients with acute heart failure. J. Thorac. Dis. 2019, 11, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, D.H.; Kim, S.C.; Kang, C.; Lee, S.H.; Kang, T.S.; Lee, S.B.; Jung, S.M.; Kim, D.S. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ed. Am. J. Emerg. Med. 2015, 33, 1344–1349. [Google Scholar] [CrossRef]
- Lee, P.H.; Lee, J.E.; Kim, H.S.; Song, S.K.; Lee, H.S.; Nam, H.S.; Cheong, J.W.; Jeong, Y.; Park, H.J.; Kim, D.J.; et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann. Neurol. 2012, 72, 32–40. [Google Scholar] [CrossRef]
- Hong, H.J.; Kim, Y.D.; Cha, M.J.; Kim, J.; Lee, D.H.; Lee, H.S.; Nam, C.M.; Nam, H.S.; Heo, J.H. Early neurological outcomes according to chads2 score in stroke patients with non-valvular atrial fibrillation. Eur. J. Neurol. 2012, 19, 284–290. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Intensive Care 2015, 3, 15. [Google Scholar] [CrossRef]
- Hernandez, G.; Roca, O.; Colinas, L. High-flow nasal cannula support therapy: New insights and improving performance. Crit. Care 2017, 21, 62. [Google Scholar] [CrossRef]
- Fraser, J.F.; Spooner, A.J.; Dunster, K.R.; Anstey, C.M.; Corley, A. Nasal high flow oxygen therapy in patients with copd reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: A randomised crossover trial. Thorax 2016, 71, 759–761. [Google Scholar] [CrossRef]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.; et al. Rapid measurement of b-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Stevens, T.L.; Sandberg, S.M.; Heublein, D.M.; Nelson, S.M.; Jougasaki, M.; Redfield, M.M.; Burnett, J.C., Jr. The potential of brain natriuretic peptide as a biomarker for new york heart association class during the outpatient treatment of heart failure. J. Card. Fail. 2002, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Parke, R.; McGuinness, S.; Eccleston, M. Nasal high-flow therapy delivers low level positive airway pressure. Br. J. Anaesth. 2009, 103, 886–890. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 72) | Conventional O2 Therapy Group | High-Flow Nasal Cannula Group | p-Value |
|---|---|---|---|---|
| Mean ± SD or n (%) | (n = 33, 49.3%) | (n = 34, 50.7%) | ||
| Age (years) | 76 ± 9 | 76 ± 9 | 77 ± 8 | 0.530 |
| Male sex, number (%) | 28(41.79) | 13(39.39) | 15(44.12) | 0.695 |
| Comorbidity (%) | ||||
| Hypertension | 40(59.70) | 18(54.55) | 22(64.71) | 0.396 |
| Diabetes mellitus | 25(37.31) | 11(33.33) | 14(41.18) | 0.506 |
| Cardiovascular disease | 9(13.43) | 6(18.18) | 3(8.82) | 0.305 |
| Chronic kidney disease | 4(5.97) | 2(6.06) | 2(5.88) | 0.999 |
| Systolic blood pressure (mmHg) | 148.96 ± 33.81 | 140.24 ± 22.41 | 157.47 ± 40.61 | 0.035 * |
| Diastolic blood pressure (mmHg) | 86.67 ± 24.98 | 81.33 ± 13.71 | 91.85 ± 31.78 | 0.084 * |
| Heart rate (bpm) | 95.51 ± 22.61 | 92.61 ± 19.33 | 98.32 ± 25.37 | 0.304 |
| Body temperature (℃) | 36.10 ± 3.26 | 35.43 ± 4.54 | 36.75 ± 0.56 | 0.106 |
| Laboratory data ** | ||||
| White blood cell count (μL) | 10855.22 ± 6803.40 | 10052.42 ± 5724.55 | 11634.41 ± 7715.25 | 0.345 |
| Hemoglobin (g/dL) | 11.66 ± 2.17 | 11.34 ± 1.99 | 11.976 ± 2.32 | 0.235 |
| Hematocrit (%) | 36.32 ± 6.73 | 34.99 ± 6.26 | 37.62 ± 6.99 | 0.109 |
| Platelet count (103/μL) | 216.97 ± 86.38 | 210.18 ± 92.31 | 223.56 ± 81.04 | 0.530 |
| Blood urea nitrogen (mg/dL) | 30.90 ± 17.85 | 25.86 ± 12.86 | 35.80 ± 20.66 | 0.021 * |
| Creatinine (mg/dL) | 1.59 ± 1.07 | 1.40 ± 0.90 | 1.79 ± 1.20 | 0.139 |
| Albumin (g/dL) | 3.65 ± 0.53 | 3.66 ± 0.53 | 3.64 ± 0.53 | 0.881 |
| Aspartate aminotransferase (IU/L) | 38.27 ± 21.99 | 38.76 ± 20.15 | 37.79 ± 23.94 | 0.859 |
| Alkaline phosphatase (IU/L) | 26.73 ± 25.84 | 27.73 ± 27.07 | 25.77 ± 24.96 | 0.759 |
| Total bilirubin (mg/dL) | 1.14 ± 1.40 | 1.13 ± 1.31 | 1.15 ± 1.51 | 0.962 |
| Sodium (mmol/L) | 137.40 ± 5.44 | 137.55 ± 6.04 | 137.27 ± 4.87 | 0.835 |
| Potassium (mmol/L) | 4.44 ± 0.80 | 4.15 ± 0.75 | 4.73 ± 0.76 | 0.003 * |
| Chloride (mmol/L) | 102.46 ± 5.97 | 103.15 ± 6.79 | 101.79 ± 5.07 | 0.351 |
| Creatine kinase (U/L) | 114.33 ± 87.24 | 105.73 ± 64.76 | 122.68 ± 104.92 | 0.428 |
| Creatine kinase myocardial bandisoenzyme (mcg/L) | 4.19 ± 3.17 | 3.35 ± 2.72 | 5.02 ± 3.38 | 0.030 * |
| Troponin-I (mcg/L) | 0.0842 ± 0.099 | 0.0674 ± 0.081 | 0.101 ± 0.112 | 0.169 |
| Pro-brain natriuretic peptide (pg/mL) | 8383.51 ± 14132.58 | 2662.59 ± 2092.40 | 13936.17 ± 18185.67 | 0.001 * |
| PaO2/FiO2 (initial) | 337.4 3± 82.50 | 342.43 ± 94.17 | 332.58 ± 70.45 | 0.371 |
| Echocardiography—previous ED visit | ||||
| Ejection fraction (%) | 46.10 ± 15.18 | 44.52 ± 15.30 | 47.65 ± 15.13 | 0.403 |
| Valve disease | 17(25.37) | 7(21.21) | 10(29.41) | 0.441 |
| Furosemide | 67(100.00) | 33(100.00) | 34(100.00) | 0.999 |
| Dobutamine | 48(71.64) | 25(75.76) | 23(67.65) | 0.462 |
| Variable | Total (n = 72) | Conventional O2 Therapy Group | High-Flow Nasal Cannula Group | p-Value |
|---|---|---|---|---|
| Mean ± SD or n (%) | (n = 33, 49.3%) | (n = 34, 50.7%) | ||
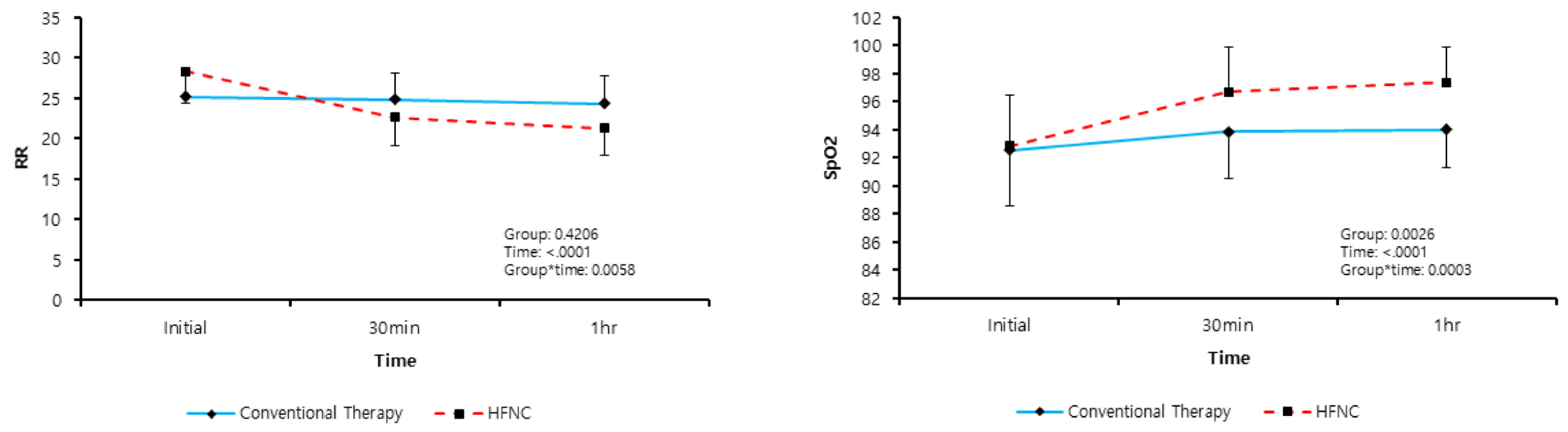
| Respiratory rate (bpm) | ||||
| Initial | 26.78 ± 3.99 | 25.18 ± 3.51 | 28.32 ± 3.86 | 0.001 * |
| 30 min | 23.75 ± 3.50 | 24.85 ± 3.19 | 22.68 ± 3.49 | 0.010 * |
| 60 min | 22.79 ± 3.72 | 24.30 ± 3.55 | 21.32 ± 3.32 | 0.001 * |
| SpO2 (%) | ||||
| Initial | 91.41 ± 5.89 | 92.55 ± 3.78 | 90.31 ± 7.29 | 0.120 |
| 30 min | 95.69 ± 3.31 | 94.15 ± 3.26 | 97.18 ± 2.65 | <0.001 * |
| 60 min | 95.94 ± 3.27 | 94.12 ± 3.25 | 97.71 ± 2.14 | <0.001 * |
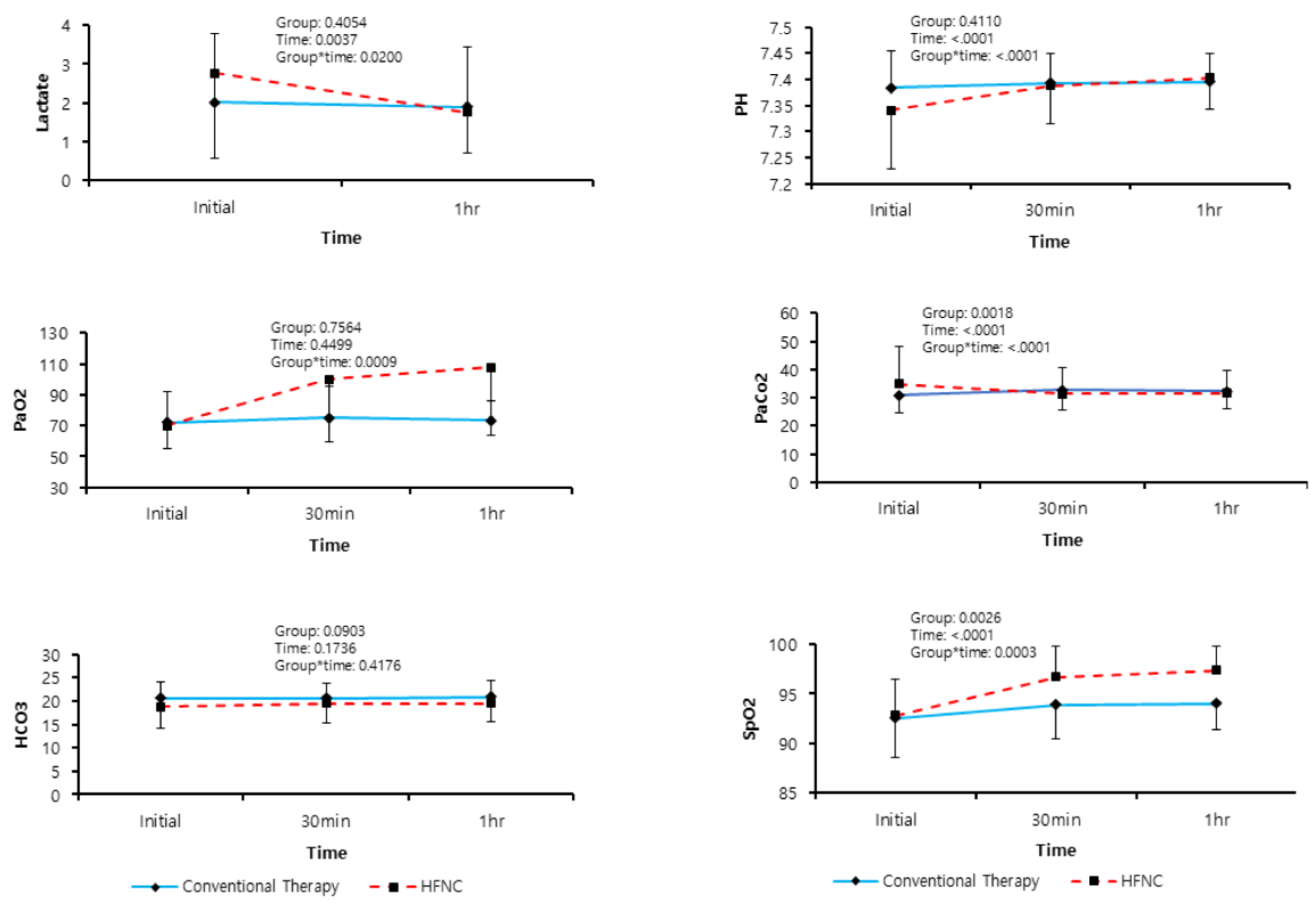
| Arterial Blood Gas Analysis | ||||
| pH, initial | 7.36 ± 0.09 | 7.38 ± 0.07 | 7.34 ± 0.11 | 0.063 |
| pH, 30 min | 7.39 ± 0.07 | 7.39 ± 0.06 | 7.39 ± 0.07 | 0.788 |
| pH, 60 min | 7.40 ± 0.06 | 7.40 ± 0.06 | 7.40 ± 0.06 | 0.595 |
| PaO2, initial | 70.86 ± 17.32 | 71.91 ± 19.78 | 69.84 ± 14.79 | 0.629 |
| PaO2 30 min | 87.79 ± 34.46 | 75.23 ± 19.87 | 99.98 ± 41.00 | 0.003 * |
| PaO2 60 min | 90.62 ± 36.79 | 73.25 ± 13.02 | 107.47 ± 44.15 | <0.001 * |
| PaCO2, initial | 32.85 ± 10.44 | 30.89 ± 6.18 | 34.76 ± 13.17 | 0.129 |
| PaCO2, 30 min | 31.97 ± 8.21 | 32.61 ± 7.13 | 31.35 ± 9.20 | 0.532 |
| PaCO2, 60 min | 31.91 ± 7.22 | 32.30 ± 6.22 | 31.54 ± 8.14 | 0.670 |
| SpO2 (%), initial | 92.69 ± 3.79 | 92.55 ± 4.01 | 92.83 ± 3.63 | 0.765 |
| SpO2, 30 min | 95.30 ± 3.55 | 93.86 ± 3.38 | 96.71 ± 3.17 | 0.001 * |
| SpO2, 60 min | 95.71 ± 3.07 | 93.99 ± 2.64 | 97.38 ± 2.51 | <0.001 * |
| Lactate (mmol/L) | ||||
| Initial | 2.39 ± 2.02 | 2.01 ± 1.78 | 2.77 ± 2.20 | 0.126 |
| 60 min | 1.82 ± 1.31 | 1.89 ± 1.55 | 1.75 ± 1.04 | 0.666 |
| Echocardiography After ED visit | ||||
| Ejection fraction (%) | 40.15 ± 13.12 | 40.36 ± 15.23 | 39.94 ± 10.92 | 0.896 |
| Valve disease | 18(26.87) | 8(24.24) | 10(29.41) | 0.633 |
| Intubation | 2(2.99) | 1(3.03) | 1(2.94) | 0.999 |
| ICU admission | 18(26.87) | 8(24.24) | 10(29.41) | 0.633 |
| Group Post-Hoc p-Value | Time Post-Hoc p-Value | Group * Time Post Hoc | ||||
|---|---|---|---|---|---|---|
| Conventional vs. HFNC | Conventional vs. HFNC | p-Value | ||||
| Respiratory Rate | ||||||
| Initial | 0.001 * | Initial vs. 30 min | 0.250 | <0.001 * | C vs. HFNC and Initial vs. 30 min | <0.001 * |
| 30 min | 0.010 * | Initial vs. 1 h | 0.037 * | <0.001 * | C vs. HFNC and Initial vs. 1 h | <0.001 * |
| 1 h | 0.001 * | 30 min vs. 1 h | 0.064 | <0.001 * | C vs. HFNC and 30 min vs. 1 h | 0.051 |
| Arterial Blood Gas Analysis (ABGA), pH | ||||||
| Initial | 0.064 | Initial vs. 30 min | 0.327 | <0.001 * | C vs. HFNC and Initial vs. 30 min | 0.002 * |
| 30 min | 0.788 | Initial vs. 1 h | 0.330 | <0.001 * | C vs. HFNC and Initial vs. 1 h | 0.003 * |
| 1 h | 0.595 | 30 min vs. 1 h | 0.714 | 0.043 * | C vs. HFNC and 30 min vs. 1 h | 0.241 |
| ABGA, PaCO2 | ||||||
| Initial | 0.130 | Initial vs. 30 min | 0.073 | 0.001 * | C vs. HFNC and Initial vs. 30 min | <0.001 * |
| 30 min | 0.532 | Initial vs. 1 h | 0.250 | 0.009 | C vs. HFNC and Initial vs. 1 h | 0.008 |
| 1 h | 0.670 | 30 min vs. 1 h | 0.648 | 0.779 | C vs. HFNC and 30 min vs. 1 h | 0.602 |
| ABGA, PaO2 | ||||||
| Initial | 0.629 | Initial vs. 30 min | 0.520 | <0.001 * | C vs. HFNC and Initial vs. 30 min | <0.001 * |
| 30 min | 0.003 * | Initial vs. 1 h | 0.809 | <0.001 * | C vs. HFNC and Initial vs. 1 h | <0.001 * |
| 1 h | <0.001 * | 30 min vs. 1 h | 0.518 | 0.015 * | C vs. HFNC and 30 min vs. 1 h | 0.030 * |
| ABGA, HCO3 | ||||||
| Initial | 0.056 | Initial vs. 30 min | 0.871 | 0.089 | C vs. HFNC and Initial vs. 30 min | 0.188 |
| 30 min | 0.220 | Initial vs. 1 h | 0.469 | 0.055 | C vs. HFNC and Initial vs. 1 h | 0.398 |
| 1 h | 0.106 | 30 min vs. 1 h | 0.289 | 0.831 | C vs. HFNC and 30 min vs. 1 h | 0.543 |
| ABGA, SpO2 | ||||||
| Initial | 0.765 | Initial vs. 30 min | 0.020 * | <0.001 * | C vs. HFNC and Initial vs. 30 min | 0.001 * |
| 30 min | 0.001 * | Initial vs. 1 h | 0.008 * | <0.001 * | C vs. HFNC and Initial vs. 1 h | <0.001 * |
| 1 h | <0.001 * | 30 min vs. 1 h | 0.715 | 0.062 | C vs. HFNC and 30 min vs. 1 h | 0.288 |
| Lactate | ||||||
| Initial | 0.126 | Initial vs. 60 min | 0.661 | <0.001 * | C vs. HFNC and Initial vs. 60 min | 0.020 * |
| 60 min | 0.664 | |||||
| Conventional O2 Therapy Group | HFNC Group | p-Value † | |
|---|---|---|---|
| Estimated Mean (SE) | Estimated Mean (SE) | ||
| Respiratory Rate | |||
| 30 min–Initial | −0.644(0.274) | −5.346(0.27) | < 0.001 * |
| 1 h–Initial | −1.189(0.386) | −6.999(0.38) | <0.001 * |
| 1 h–30 min | −0.546(0.29) | −1.353(0.285) | 0.051 |
| Arterial Blood Gas Analysis (ABGA), pH | |||
| 30 min–Initial | 0.019(0.006) | 0.038(0.006) | 0.027 * |
| 1 h–Initial | 0.021(0.007) | 0.053(0.007) | 0.004 * |
| 1 h–30 min | 0.003(0.007) | 0.015(0.01) | 0.241 |
| ABGA, PaCO2 | |||
| 30 min–Initial | 1.003(0.766) | −2.712(0.755) | 0.001 * |
| 1 h–Initial | 0.688(0.885) | −2.521(0.872) | 0.013 * |
| 1 h–30 min | −0.315(0.687) | 0.191(0.677) | 0.602 |
| ABGA, PaO2 | |||
| 30 min–Initial | 3.6(5.143) | 29.866(5.067) | 0.001 * |
| 1 h–Initial | 1.622(5.444) | 37.355(5.363) | <0.001 * |
| 1 h–30 min | −1.979(3.042) | 7.488(2.997) | 0.030 * |
| ABGA, HCO3 | |||
| 30 min–Initial | 0.195(0.394) | 0.47(0.388) | 0.625 |
| 1 h–Initial | 0.568(0.372) | 0.543(0.366) | 0.963 |
| 1 h–30 min | 0.373(0.349) | 0.074(0.344) | 0.543 |
| ABGA, SpO2 | |||
| 30 min–Initial | 1.223(0.453) | 3.957(0.446) | <0.001 * |
| 1 h–Initial | 1.354(0.353) | 4.621(0.347) | <0.001 * |
| 1 h–30 min | 0.13(0.355) | 0.665(0.35) | 0.288 |
| Lactate | |||
| 60 min–Initial | −0.342(0.18) | −0.801(0.177) | 0.076 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.R.; Beom, J.; Lee, H.S.; You, J.S.; Chung, H.S.; Chung, S.P. Benefits of High-Flow Nasal Cannula Therapy for Acute Pulmonary Edema in Patients with Heart Failure in the Emergency Department: A Prospective Multi-Center Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1937. https://doi.org/10.3390/jcm9061937
Ko DR, Beom J, Lee HS, You JS, Chung HS, Chung SP. Benefits of High-Flow Nasal Cannula Therapy for Acute Pulmonary Edema in Patients with Heart Failure in the Emergency Department: A Prospective Multi-Center Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(6):1937. https://doi.org/10.3390/jcm9061937
Chicago/Turabian StyleKo, Dong Ryul, Jinho Beom, Hye Sun Lee, Je Sung You, Hyun Soo Chung, and Sung Phil Chung. 2020. "Benefits of High-Flow Nasal Cannula Therapy for Acute Pulmonary Edema in Patients with Heart Failure in the Emergency Department: A Prospective Multi-Center Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 6: 1937. https://doi.org/10.3390/jcm9061937
APA StyleKo, D. R., Beom, J., Lee, H. S., You, J. S., Chung, H. S., & Chung, S. P. (2020). Benefits of High-Flow Nasal Cannula Therapy for Acute Pulmonary Edema in Patients with Heart Failure in the Emergency Department: A Prospective Multi-Center Randomized Controlled Trial. Journal of Clinical Medicine, 9(6), 1937. https://doi.org/10.3390/jcm9061937





