Distinctive Pathophysiology Underlying Constipation in Parkinson’s Disease: Implications for Cognitive Inefficiency
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Assessments
2.3. Statistical Analysis
3. Results
3.1. Evaluation of X-Rays
3.2. Evaluation of Bowel Function Questionnaires
3.3. Defining Archetypical PD Bowel Dysfunction
3.4. Cognition, Mood, and Motor Features in Relation to Bowel Dysfunction
4. Discussion
4.1. Contradictions to Single Nosological Entity Hypothesis
4.2. Mechanistic Considerations
4.3. Study Limitations and Directions for Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Zhao, E.J.; Zhang, W.; Lu, Y.; Liu, R.; Huang, X.; Ciesielski-Jones, A.J.; Justice, M.A.; Cousins, D.S.; Peddada, S. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl. Neurodegener. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.J.; Yu, S.Y.; Zuo, L.J.; Lian, T.H.; Hu, Y.; Wang, R.D.; Piao, Y.S.; Guo, P.; Liu, L.; Jin, Z.; et al. Parkinson disease with constipation: Clinical features and relevant factors. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.I.P.; Bercik, P. Epidemiology and burden of chronic constipation. Can. J. Gastroenterol. 2011, 25, 11. [Google Scholar] [CrossRef]
- El-Salhy, M.; Svensen, R.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Chronic constipation and treatment options (Review). Mol. Med. Rep. 2014, 9, 3–8. [Google Scholar] [CrossRef]
- Charlett, A.; Dobbs, R.; Weller, C.; Dobbs, S. Stasis in the gut: The source of xenobiotic in idiopathic parkinsonism. Eur. J. Clin. Pharmacol. 1997, 52, A168. [Google Scholar]
- Adams-Carr, K.L.; Bestwick, J.P.; Shribman, S.; Lees, A.; Schrag, A.; Noyce, A.J. Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, J.W.; Liu, Y.C.; Chang, C.H.; Wu, R.M. Risk of Parkinson’s disease following severe constipation: A nationwide population-based cohort study. Park. Relat. Disord. 2014, 20, 1371–1375. [Google Scholar] [CrossRef]
- Abbott, R.D.; Ross, G.W.; Petrovitch, H.; Tanner, C.M.; Davis, D.G.; Masaki, K.H.; Launer, L.J.; Curb, J.D.; White, L.R. Bowel movement frequency in late-life and incidental Lewy bodies. Mov. Disord. 2007, 22, 1581–1586. [Google Scholar] [CrossRef]
- Petrovitch, H.; Abbott, R.D.; Ross, G.W.; Nelson, J.; Masaki, K.H.; Tanner, C.M.; Launer, L.J.; White, L.R. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov. Disord. 2009, 24, 371–376. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Perepezko, K.; Mills, K.A.; Mari, Z.; Butala, A.; Dawson, T.M.; Pantelyat, A.; Rosenthal, L.S.; Pontone, G.M. Dopamine transporter availability reflects gastrointestinal dysautonomia in early Parkinson disease. Park. Relat. Disord. 2018, 55, 8–14. [Google Scholar] [CrossRef]
- Parkinson, J. An Essay on the Shaking Palsy; Whittingham and Rowland for Sherwood, Neely and Jones: London, UK, 1817. [Google Scholar]
- Edwards, L.L.; Pfeiffer, R.F.; Quigley, E.M.M.; Hofman, R.; Balluff, M. Gastrointestinal symptoms in Parkinson’s disease. Mov. Disord. 1991, 6, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.N.; King, D.; Billington, D.; Barrett, J.A. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad. Med. J. 1996, 72, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Bouras, E.P.; Camilleri, M.; Burton, D.D.; Thomforde, G.; McKinzie, S.; Zinsmeister, A.R. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology 2001, 120, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.; Fedorova, T.D.; Bekker, A.C.; Iversen, P.; Østergaard, K.; Krogh, K.; Borghammer, P. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson’s disease: A colon transit and volume study. J. Parkinsons. Dis. 2017, 7, 359–367. [Google Scholar] [CrossRef]
- Leentjens, A.F.G.G.; Van den Akker, M.; Metsemakers, J.F.M.; Lousberg, R.; Verhey, F.R.J. Higher Incidence of Depression Preceding the Onset of Parkinson’s Disease: A Register Study. Mov. Disord. 2003, 18, 414–418. [Google Scholar] [CrossRef]
- Dobbs, R.J.; Bowes, S.G.; Charlett, A.; Henley, M.; Frith, C.; Dickins, J.; Dobbs, S.M. Hypothesis: The bradyphrenia of parkinsonism is a nosological entity. Acta Neurol. Scand. 1993, 87, 255–261. [Google Scholar] [CrossRef]
- Dobbs, R.J.; Bowes, S.G.; Henley, M.; Charlett, A.; O’Neill, C.J.A.; Dickins, J.; Nicholson, P.W.; Dobbs, S.M. Assessment of the bradyphrenia of parkinsonism: A novel use of delayed auditory feedback. Acta Neurol. Scand. 1993, 87, 262–267. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.; Marechal, L.; Weller, C.; Charlett, A.; Taylor, D.; Dobbs, R.; Dobbs, S. Objective assessment of rapid alternating movement of hands. Neurodegener. Dis. 2017, 17, 1601. [Google Scholar]
- Kirollos, C.; O’neill, C.J.A.; Dobbs, R.J.; Charlett, A.; Bowes, S.G.; Weller, C.; Purkiss, A.G.; Hunt, W.B.; Dobbs, S.M. Quantification of the cardinal signs of parkinsonism and of associated disability in spouses of sufferers. Age Ageing 1993, 22, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.D.; Charlett, A.; Weller, C.; Dobbs, S.M.; Taylor, D.; Bjarnason, I.; Dobbs, R.J. Quantifying rigidity of Parkinson’s disease in relation to laxative treatment: A service evaluation. Br. J. Clin. Pharmacol. 2016, 82, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Calne, D.B.; Snow, B.J.; Lee, C. Criteria for diagnosing Parkinson’s disease. Ann. Neurol. 1992, 32, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Abrahamsson, H.; Antov, S.; Bosaeus, I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal x-ray in healthy subjects and constipated patients. Scand. J. Gastroenterol. 1988, 23, 72–80. [Google Scholar] [CrossRef]
- Sadik, R.; Abrahamsson, H.; Stotzer, P.-O. Gender Differences in Gut Transit Shown with a Newly Developed Radiological Procedure. Scand. J. Gastroenterol. 2003, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sadik, R.; Stotzer, P.-O.; Simrén, M.; Abrahamsson, H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol. Motil. 2007, 20, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, H.; Antov, S. Accuracy in assessment of colonic transit time with particles: How many markers should be used? Neurogastroenterol. Motil. 2010, 22, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Strid, H.; Simrén, M.; Störsrud, S.; Stotzer, P.-O.; Sadik, R.; Stotzer, P. Effect of heavy exercise on gastrointestinal transit in endurance athletes. Scand. J. Gastroenterol. 2011, 46, 673–677. [Google Scholar] [CrossRef]
- Törnblom, H.; Van Oudenhove, L.; Sadik, R.; Abrahamsson, H.; Tack, J.; Simrén, M. Colonic transit time and IBS symptoms: What’s the link? Am. J. Gastroenterol. 2012, 107, 754–760. [Google Scholar] [CrossRef]
- Leech, S.C.; McHugh, K.; Sullivan, P.B. Evaluation of a method of assessing faecal loading on plain abdominal radiographs in children. Pediatr. Radiol. 1999, 29, 255–258. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.; Graafmans, D.; Nievelstein, R.; Beek, E. Systematic assessment of constipation on plain abdominal radiographs in children. Pediatr. Radiol. 2006, 36, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, D.J.; Flanagan, R.; Barshop, K.; Kuo, B.; Staller, K. Colonic Stool Burden a Useful Surrogate for Slow Transit Constipation as Determined by a Radiopaque Transit Study. Am. J. Gastroenterol. 2019, 114, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, S.M.; Charlett, A.; John Dobbs, R.; Weller, C.; Iguodala, O.; Smee, C.; Lawson, A.J.; Taylor, D.; Bjarnason, I. Antimicrobial surveillance in idiopathic parkinsonism: Indication-specific improvement in hypokinesia following Helicobacter pylori eradication and non-specific effect of antimicrobials for other indications in worsening rigidity. Helicobacter 2013, 18, 187–196. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Beck, A.T.; Beamesderfer, A. Assessment of depression: The depression inventory. Mod. Probl. Pharm. 1974, 7, 151–169. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Stiasny-Kolster, K.; Mayer, G.; Schäfer, S.; Möller, J.C.; Heinzel-Gutenbrunner, M.; Oertel, W.H. The REM sleep behavior disorder screening questionnaire-A new diagnostic instrument. Mov. Disord. 2007, 22, 2386–2393. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s Disease? Evidence from 3 cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Grathwohl, S.A.; Steiner, J.A.; Britschgi, M.; Brundin, P. Mind the gut: Secretion of α-synuclein by enteric neurons. J. Neurochem. 2013, 125, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, R.J.; Charlett, A.; Dobbs, S.M.; Weller, C.; Ibrahim, M.A.A.; Iguodala, O.; Smee, C.; Plant, J.M.; Lawson, A.J.; Taylor, D.; et al. Leukocyte-subset counts in idiopathic parkinsonism provide clues to a pathogenic pathway involving small intestinal bacterial overgrowth. A surveillance study. Gut Pathog. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef]
- Tan, A.H.; Mahadeva, S.; Thalha, A.M.; Gibson, P.R.; Kiew, C.K.; Yeat, C.M.; Ng, S.W.; Ang, S.P.; Chow, S.K.; Tan, C.T.; et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, R.J.; Dobbs, S.M.; Weller, C.; Charlett, A.; Bjarnason, I.T.; Curry, A.; Ellis, D.S.; Ibrahim, M.A.A.; Mccrossan, M.V.; Donohue, J.O.; et al. Helicobacter Hypothesis for Idiopathic Parkinsonism: Before and Beyond. Helicobacter 2008, 13, 309–322. [Google Scholar] [CrossRef]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Abenavoli, L.; Vonghia, L.; Cardone, S.; Leso, V.; Cossari, A.; et al. State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008, 62, 1063–1069. [Google Scholar] [CrossRef]
- Dobbs, S.M.; Dobbs, R.J.; Weller, C.; Charlett, A.; Augustin, A.; Taylor, D.; Ibrahim, M.A.A.; Bjarnason, I. Peripheral aetiopathogenic drivers and mediators of Parkinson’s disease and co-morbidities: Role of gastrointestinal microbiota. J. Neurovirol. 2016, 22, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, M.; Liu, X.; Qu, H.; Chen, Q.; Qian, W.; Wei, D.; Xu, W.; Ma, B.; Wu, W. Clinical characteristics and peripheral T cell subsets in Parkinson’s disease patients with constipation. Int. J. Clin. Exp. Pathol. 2015, 8, 2495–2504. [Google Scholar] [PubMed]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P.; Nguyen, J.M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Spiegel, J.; Dillmann, U.; Grundmann, D.; Bürmann, J.; Faßbender, K.; Schäfer, K.H.; Unger, M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Park. Relat. Disord. 2018, 50, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Zhu, F.; Li, C.; Gong, J.; Zhu, W.; Gu, L.; Li, N. The risk of Parkinson’s disease in inflammatory bowel disease: A systematic review and meta-analysis. Dig. Liver Dis. 2019, 51, 38–42. [Google Scholar] [CrossRef]
- Hui, K.Y.; Fernandez-Hernandez, H.; Hu, J.; Schaffner, A.; Pankratz, N.; Hsu, N.Y.; Chuang, L.S.; Carmi, S.; Villaverde, N.; Li, X.; et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 2018, 10, eaai7795. [Google Scholar] [CrossRef]
- Bialecka, M.; Kurzawski, M.; Klodowska-Duda, G.; Opala, G.; Juzwiak, S.; Kurzawski, G.; Tan, E.K.; Drozdzik, M. CARD15 variants in patients with sporadic Parkinson’s disease. Neurosci. Res. 2007, 57, 473–476. [Google Scholar] [CrossRef]
- Ma, Q.; An, X.; Li, Z.; Zhang, H.; Huang, W.; Cai, L.; Hu, P.; Lin, Q.; Tzeng, C.M. P268S in NOD2 associates with susceptibility to Parkinson’s disease in Chinese population. Behav. Brain Funct. 2013, 9, 19. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, J.; Krummey, S.; Lu, W.; Cai, H.; Lenardo, M.J. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol. 2011, 12, 1063–1070. [Google Scholar] [CrossRef]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018, 11, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; Sarah, D.M. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Panarese, A.; Pesce, F.; Porcelli, P.; Riezzo, G.; Iacovazzi, P.A.; Leone, C.M.; De Carne, M.; Rinaldi, C.M.; Shahini, E. Chronic functional constipation is strongly linked to Vitamin D deficiency. World J. Gastroenterol. 2019, 25, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kilkkinen, A.; Rissanen, H.; Marniemi, J.; Sääksjärvi, K.; Heliövaara, M. Serum vitamin D and the risk of Parkinson disease. Arch. Neurol. 2010, 67, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef]
- Murphy, S.E.; Wright, L.C.; Browning, M.; Cowen, P.J.; Harmer, C.J. A role for 5-HT4 receptors in human learning and memory. Psychol. Med. 2019, 1–9. [Google Scholar] [CrossRef]
- Dobbs, R.J.; Lubel, D.D.; Charlett, A.; Bowes, S.G.; O’Neill, C.J.; Weller, C.; Dobbs, S.M. Hypothesis: Age-Associated Changes in Gait Represent, in Part, a Tendency Towards Parkinsonism—PubMed. Age Ageing 1992, 21, 221–225. [Google Scholar] [CrossRef]
- Dobbs, R.J.; Charlett, A.; Purkiss, A.G.; Dobbs, S.M.; Weller, C.; Peterson, D.W. Association of circulating TNF-α and IL-6 with ageing and parkinsonism. Acta Neurol. Scand. 1999, 100, 34–41. [Google Scholar] [CrossRef]
| Characteristic | Mean (95% Data Interval) * | Mean Difference * (95% CI) | ||
|---|---|---|---|---|
| PD n = 58 | Control n = 71 | |||
| Demographic | Age (years) | 67.6 (50.2, 85.0) | 64.8 (43.6, 86.0) | 2.9 (–0.7, 6.3) |
| Gender (male) | 58.62 a | 42.25 a | –0.16 (–0.33, 0.01) c | |
| Height (cm) | 169.1 (148.8, 189.5) | 171.2 (151.4, 191.0) | –2.1 (–5.7, 1.5) | |
| Weight (kg) | 70.9 (42.9, 98.9) | 73.6 (45.1, 102.2) | –2.7 (–7.8, 2.3) | |
| Time since diagnosis (years) | 5 (0, 21) b | na | na | |
| Laxatives † (% taking) | 69.1 a | 14.5 a | 0.55 (0.40, 0.69) c | |
| Anti-Parkinsonian medication ⱡ (% taking) | 83.0 a | na | na | |
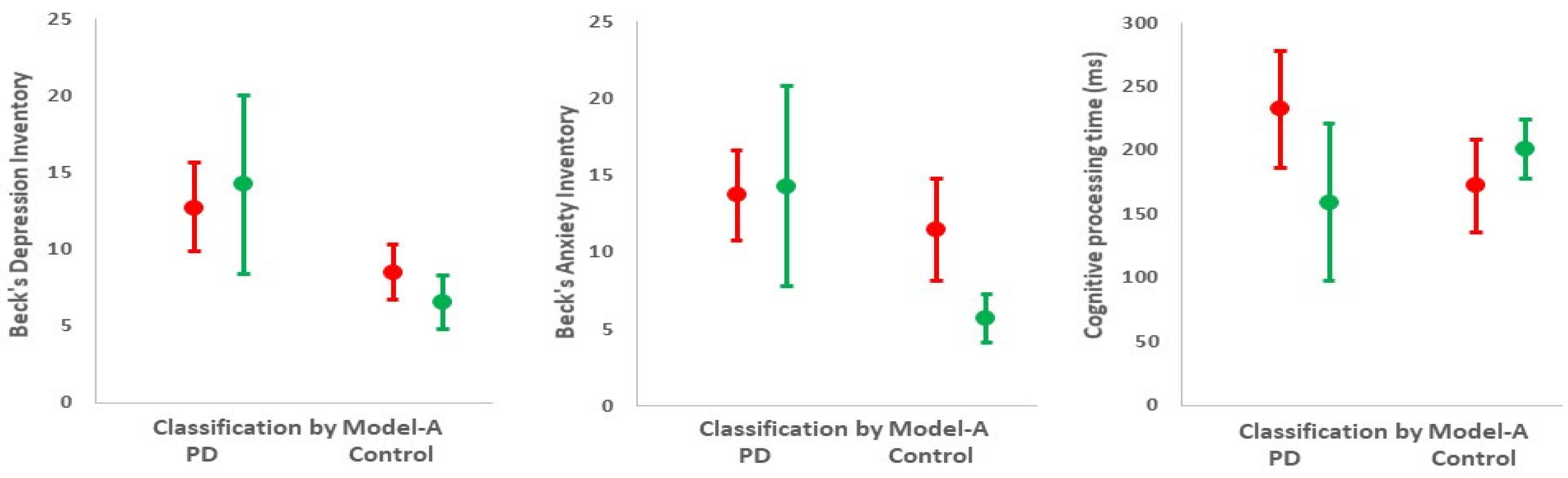
| Phenotype | Mini-mental score | 30 (17, 30) b | 30 (28, 30) b | –0.33 (–0.25, –0.41) d |
| Depression score | 10 (1, 29) b | 5 (0, 27) b | 5 (3, 4) e | |
| Anxiety score | 11 (0, 30) b | 5 (0, 28) b | 6 (3, 8) e | |
| REM score | 4 (0, 10) b | 2 (0, 9) b | 2 (1, 3) e | |
| Mean stride length (mm) | 1118 (701, 1535) | 1306 (897, 1733) | –187 (–251, –124) | |
| Angular velocity of forearm inwards (°/s) | 525 (132, 919) | 802 (455, 1149) | –276 (–343, –208) | |
| Angular velocity of forearm outwards (°/s) | 501 (179, 823) | 752 (443, 1061) | –251 (–308, –193) | |
| Angular velocity of foot upwards (°/s) | 32 (–7, 70) | 54 (5, 104) | –23 (–31, –15) | |
| Angular velocity of foot downwards (°/s) | 46 (–7, 99) | 73 (7, 138) | –27 (–37, –16) | |
| Flexor rigidity § on worse side (Nm·10−3) | 482 (71, 1367) b | 240 (5, 931) b | 256 (114, 399) e | |
| Posture by UPDRS 3.13 subscore | 1 (0, 4) b | 0 (0, 1) b | 1 (0.79, 1.21) e | |
| Tremor (% with) | 64.29 a | 15.49 a | 0.49 (0.34, 0.64) c | |
| Unwarned reaction time (ms) | 680 (459, 1446) b | 610 (410, 841) b | 71 (12, 130) e | |
| Warned reaction time (ms) | 480 (208, 1192) b | 399 (221, 776) b | 81 (13, 148) e | |
| Cognitive processing time (ms) | 209 (7, 458) b | 204 (–14, 406) b | 8 (–32, 48) e | |
| Characteristic | Effect of PD * | Additional Effect of Age † | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
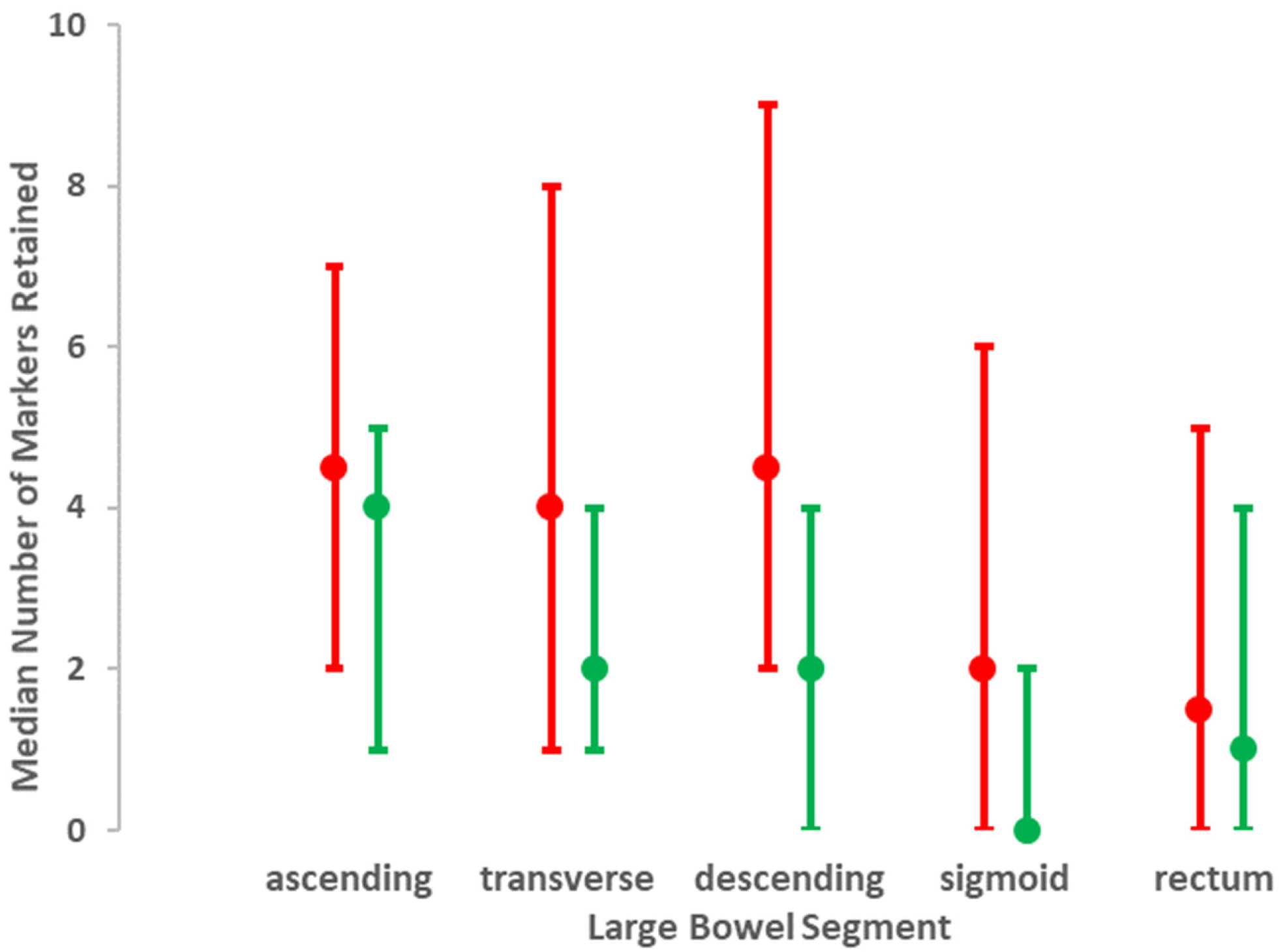
| Number of markers retained | ||||
| right colon | 1.36 (0.99, 1.87) | 0.06 | 1.01 (1.00, 1.03) | 0.2 |
| transverse colon | 2.37 (1.58, 3.56) | 0.001 | 1.02 (1.00, 1.04) | 0.1 |
| descending colon | 2.02 (1.30, 3.13) | 0.002 | 1.01 (0.99, 1.03) | 0.4 |
| sigmoid | 2.03 (1.22, 3.39) | 0.007 | 1.01 (0.98, 1.04) a | 0.5 |
| rectum | 1.18 (0.75, 1.86) | 0.5 | 1.010 (0.99, 1.04) | 0.4 |
| Total | 2.12 (1.46, 3.06) | 0.001 | 1.02 (1.00, 1.04) a | 0.1 |
| Segmental delay (presence/absence) | ||||
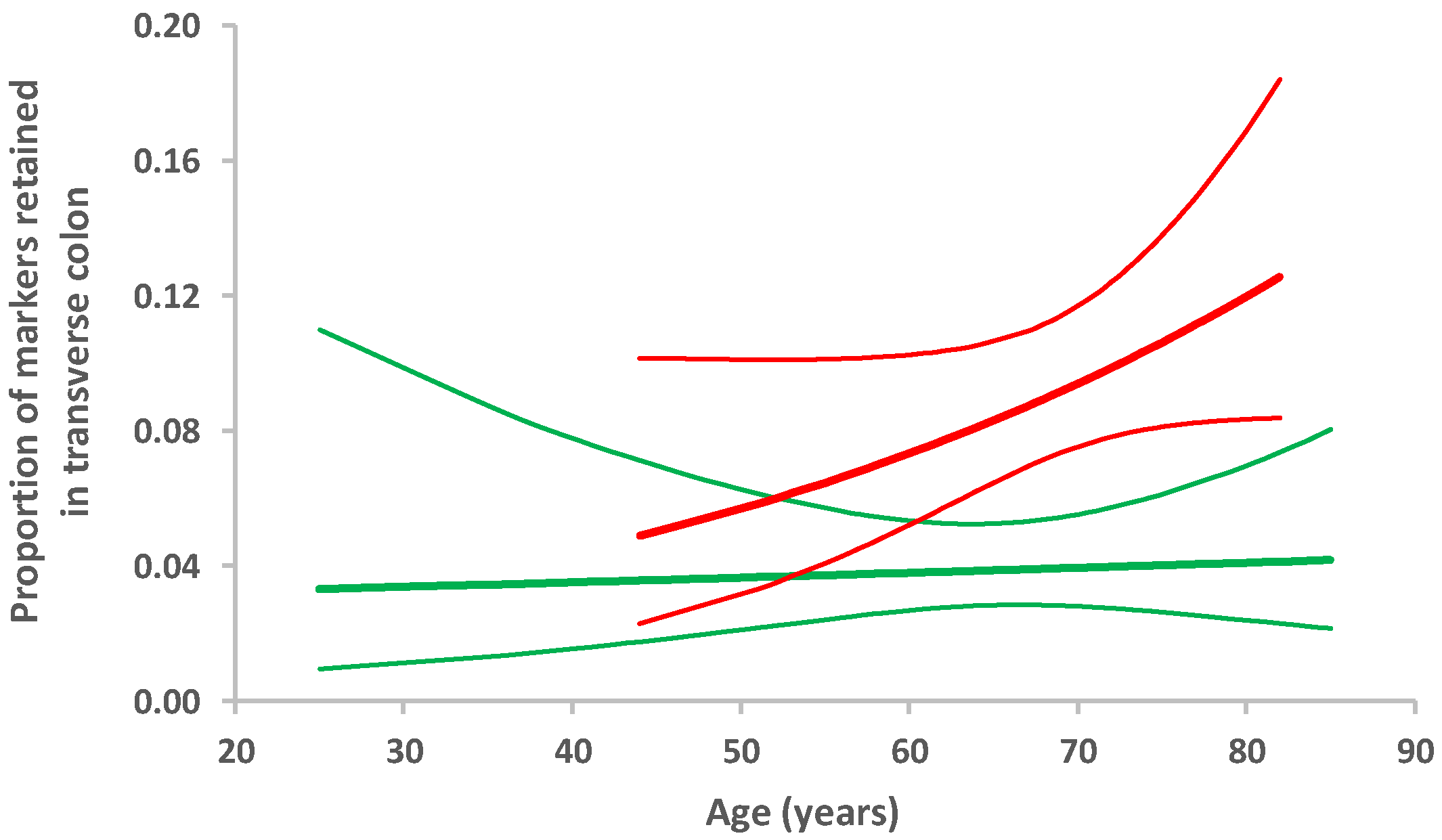
| right colon | 7.17 (0.83, 62.13) | 0.07 | 1.08 (1.00, 1.21) | 0.2 |
| transverse colon | ⱡ | 1.04 (0.96, 1.12) | 0.4 | |
| descending colon | 3.39 (0.99, 11.54) | 0.05 | 1.01 (0.95, 1.08) | 0.7 |
| sigmoid | 3.59 (0.90, 14.39) | 0.07 | 1.00 (0.94, 1.07) | 0.9 |
| rectum | 1.31 (0.32, 5.33) | 0.7 | 1.13 (1.01, 1.26) | 0.03 |
| any | 4.92 (2.01, 12.05) | 0.001 | 1.07 (1.02, 1.13) | 0.01 |
| Relative mean (95% CI) | p-value | Relative mean (95% CI) | p-value | |
| Diameter (cm) | ||||
| right colon | 0.92 (0.79, 1.07) | 0.3 | 1.00 (0.79, 1.07) b | 0.05 |
| transverse colon | 1.17 (0.97, 1.41) | 0.1 | 1.01 (1.00, 1.02) | 0.07 |
| descending colon | 1.21 (0.97, 1.50) | 0.09 | 1.00 (0.99, 1.02) | 0.6 |
| sigmoid | 1.13 (0.82, 1.55) | 0.5 | 1.02 (0.99, 1.04) | 0.2 |
| rectum | 1.28 (0.85, 1.92) | 0.2 | 1.00 (0.98, 1.02) | 0.8 |
| Difference in medians (95% CI) | p-value | Difference in medians (95% CI) | p-value | |
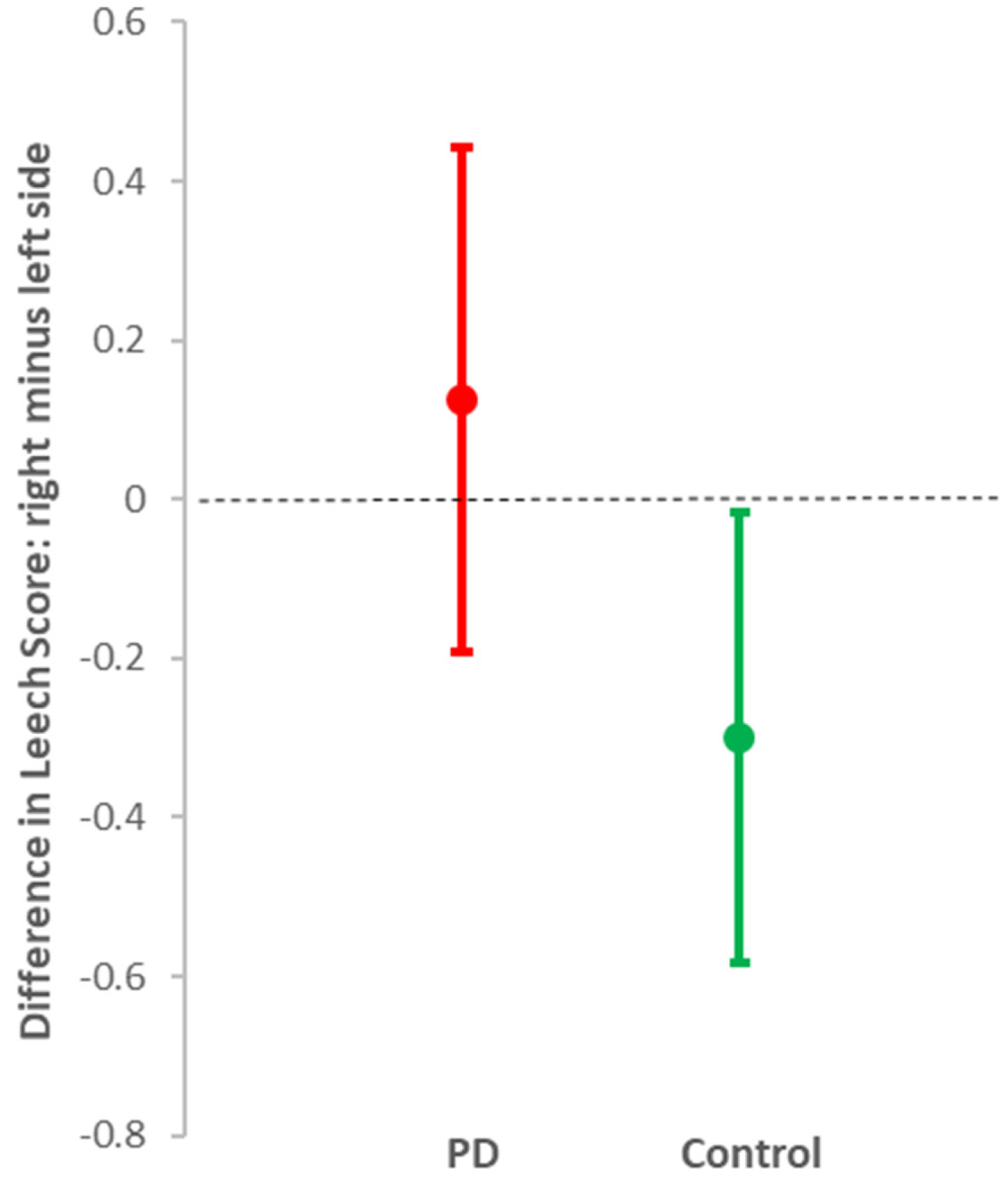
| Leech Sscore for faecal loading | ||||
| right side colon | 1 (0.28, 1.72) | 0.007 | 0 (−0.04, 0.04) | 1 |
| left side colon | 1 (0.54, 1.46) | 0.001 | 0 (−0.02, 0.02) | 1 |
| rectosigmoid colon | 0 (−0.76, 0.76) | 1 | 0 (−0.04, 0.04) | 1 |
| total | 1 (0.25, 2.25) | 0.1 | 0 (−0.06, 0.06) | 1 |
| Characteristic | Effect of PD | |
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Rome III Functional bowel disorders | ||
| C1. Irritable bowel syndrome | 0.59 (0.18, 1.74), | 0.3 |
| C2. Functional bloating | 1.78 (0.80, 3.95) | 0.2 |
| C3. Functional constipation | 4.41 (1.87, 10.40) | 0.001 |
| C4. Functional diarrhoea | 1.58 (0.40, 6.17) | 0.5 |
| Bristol stool scale | ||
| Last motion | 0.96 (0.75, 1.23) | 0.7 |
| Median over last 3 months | 1.09 (0.78, 1.51) | 0.6 |
| Single aspects | ||
| Number of times bowels opened/day † | 1.13 (0.75, 1.72) | 0.6 |
| Number of days bowels opened/fortnight | 0.83 (0.72, 0.96) | 0.01 |
| Straining (% of days when bowels opened) † | 1.02 (1.00, 1.03) | 0.02 |
| Satisfactory emptying (% of days when bowels opened) † | 0.99 (0.97, 1.00) | 0.01 |
| Predictors | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| Model A: from all available measures | ||
| Segmental delay in transverse colon (1 = yes, 0 = no) | 38.67 (2.17, 689.73) | 0.01 |
| Rome III: functional constipation | 4.57 (1.89, 11.04) | 0.001 |
| Constant | 0.43 (0.27, 0.68) | 0.001 |
| Model B: from colonic transit time test X-ray | ||
| Segmental delay in transverse colon (1 = yes, 0 = no) | 17.69 (0.96, 327.04) | 0.05 |
| Total number of markers retained | 1.04 (1.01, 1.07) | 0.02 |
| Constant | 0.35 0.19, 0.66) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucker, R.M.; Ryan, S.; Hayee, B.H.; Bjarnason, I.; Augustin, A.D.; Umamahesan, C.; Taylor, D.; Weller, C.; Dobbs, S.M.; Dobbs, R.J.; et al. Distinctive Pathophysiology Underlying Constipation in Parkinson’s Disease: Implications for Cognitive Inefficiency. J. Clin. Med. 2020, 9, 1916. https://doi.org/10.3390/jcm9061916
Tucker RM, Ryan S, Hayee BH, Bjarnason I, Augustin AD, Umamahesan C, Taylor D, Weller C, Dobbs SM, Dobbs RJ, et al. Distinctive Pathophysiology Underlying Constipation in Parkinson’s Disease: Implications for Cognitive Inefficiency. Journal of Clinical Medicine. 2020; 9(6):1916. https://doi.org/10.3390/jcm9061916
Chicago/Turabian StyleTucker, Rosalind M., Suzanne Ryan, Bu’ Hussain Hayee, Ingvar Bjarnason, Aisha D. Augustin, Chianna Umamahesan, David Taylor, Clive Weller, Sylvia M Dobbs, R John Dobbs, and et al. 2020. "Distinctive Pathophysiology Underlying Constipation in Parkinson’s Disease: Implications for Cognitive Inefficiency" Journal of Clinical Medicine 9, no. 6: 1916. https://doi.org/10.3390/jcm9061916
APA StyleTucker, R. M., Ryan, S., Hayee, B. H., Bjarnason, I., Augustin, A. D., Umamahesan, C., Taylor, D., Weller, C., Dobbs, S. M., Dobbs, R. J., & Charlett, A. (2020). Distinctive Pathophysiology Underlying Constipation in Parkinson’s Disease: Implications for Cognitive Inefficiency. Journal of Clinical Medicine, 9(6), 1916. https://doi.org/10.3390/jcm9061916





