Optimal Parameters of Deep Brain Stimulation in Essential Tremor: A Meta-Analysis and Novel Programming Strategy
Abstract
1. Introduction
2. Methods
2.1. Meta-Analysis
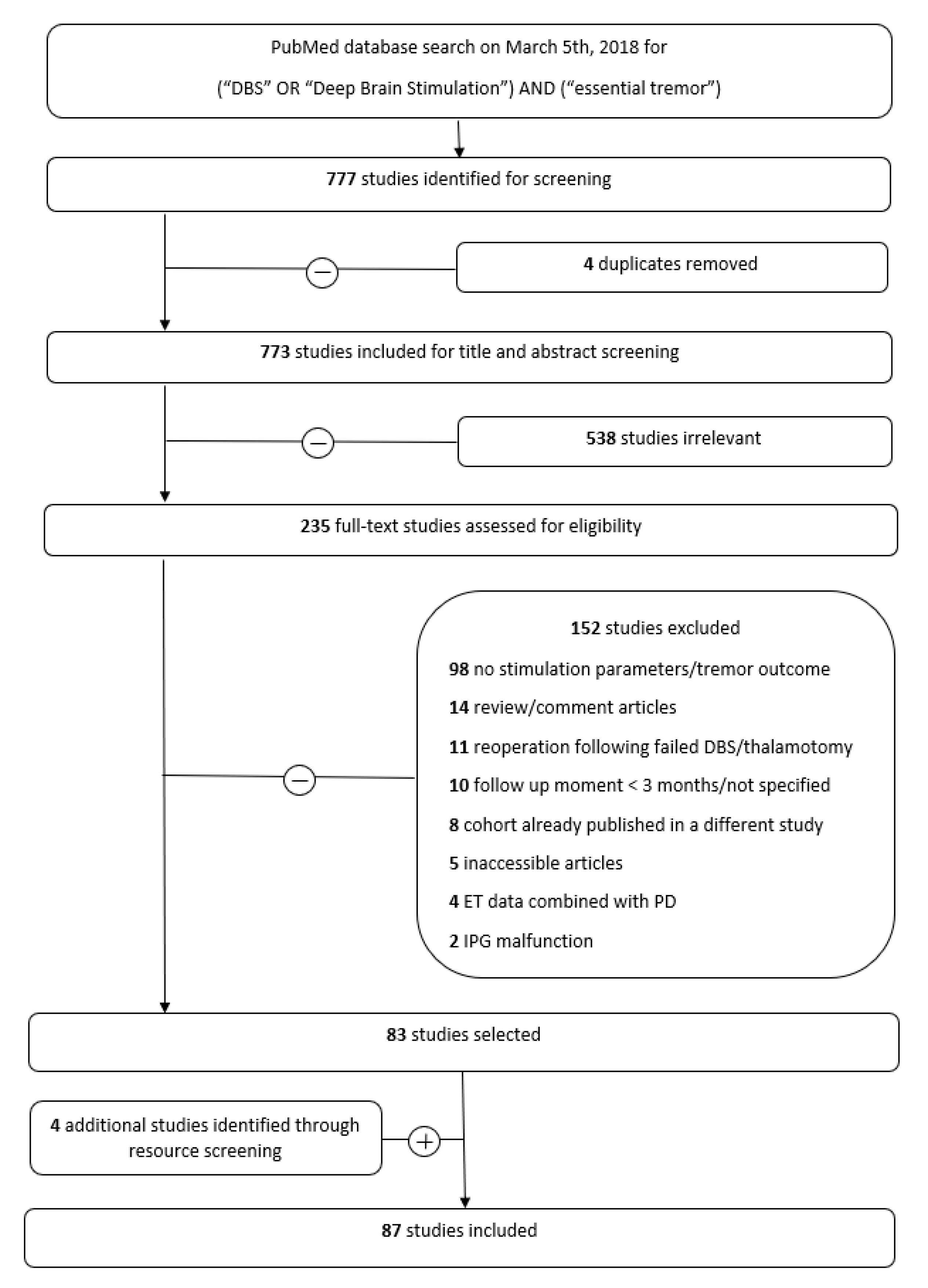
2.1.1. Search Strategy and Selection Criteria
2.1.2. Data Extraction
2.1.3. Data Synthesis
2.1.4. Comparisons
2.2. Experimental DBS Programming
2.2.1. Patients
2.2.2. Experimental Paradigm
2.2.3. Evaluation of the Experimental Settings
3. Statistical Analysis
4. Results
4.1. Meta-Analysis
4.1.1. Study Inclusion and Data Characteristics
4.1.2. DBS parameters and Tremor Outcomes
4.1.3. Correlation of Stimulation Parameters and Tremor Suppression
4.1.4. Side Effects
4.2. Experimental DBS Programming
4.2.1. Experimental Tremor Titration
4.2.2. Random DBS Parameters
5. Discussion
5.1. Meta-Analysis
The Rationale behind Conventional Stimulation Parameters
5.2. Experimental DBS Programming
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Louis, E.D. Treatment of essential tremor: Are there issues we are overlooking? Front. Neurol. 2011, 2, 91. [Google Scholar] [PubMed]
- Flora, E.D.; Perera, C.L.; Cameron, A.L.; Maddern, G.J. Deep brain stimulation for essential tremor: A systematic review. Mov. Disord. 2010, 25, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Kagnoff, M.N.; Jimenez-shahed, J.; Fekete, R.; Jankovic, J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J. Neurol. Neurosurg. Psychiatry 2014, 85, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, P.; Kendall, T. The spurious advance of antipsychotic drug therapy. Lancet 2009, 373, 4–5. [Google Scholar] [CrossRef]
- Fasano, A.; Helmich, R.C. Tremor habituation to deep brain stimulation: Underlying mechanisms and solutions. Mov. Disord. 2019, 34, 1761–1773. [Google Scholar] [CrossRef]
- Sandoe, C.; Krishna, V.; Basha, D.; Sammartino, F.; Tatsch, J.; Picillo, M.; di Biase, L.; Poon, Y.; Hamani, C.; Reddy, D.; et al. Predictors of deep brain stimulation outcome in tremor patients. Brain Stimul. 2018, 11, 592–599. [Google Scholar] [CrossRef]
- Schlaier, J.; Anthofer, J.; Steib, K.; Fellner, C.; Rothenfusser, E.; Brawanski, A.; Lange, M. Deep brain stimulation for essential tremor: Targeting the dentato-rubro-thalamic tract? Neuromodulation 2015, 18, 105–112. [Google Scholar] [CrossRef]
- Coenen, V.A.; Varkuti, B.; Parpaley, Y.; Skodda, S.; Prokop, T.; Urbach, H.; Li, M.; Reinacher, P. Postoperative neuroimaging analysis of DRT deep brain stimulation revision surgery for complicated essential tremor. Acta Neurochir. 2017, 159, 779–787. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Jenkinson, N.; Owen, S.L.; Aziz, T.Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 2007, 8, 623–635. [Google Scholar] [CrossRef]
- Holsheimer, J.; Dijkstra, E.A.; Demeulemeester, H.; Nuttin, B. Chronaxie calculated from current-duration and voltage-duration data. J. Neurosci. Methods 2000, 97, 45–50. [Google Scholar] [CrossRef]
- Hassler, R.; Riechert, T.; Mundinger, F.; Umbach, W.; Ganglberger, J.A. Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain 1960, 83, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.S. Management of referred deep brain stimulation failures: A retrospective analysis from 2 movement disorders centers. Arch. Neurol. 2005, 62, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.T.; Liebhart, L.; Runge, M.; Pauls KA, M.; Wojtecki, L.; Schnitzler, A.; Allert, N.; Fink, G.; Sturm, V.; Maarouf, M.; et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: Habituation of tremor suppression. J. Neurol. 2011, 258, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zamora, A.; Boggs, H.; Pilitsis, J.G. Reduction in DBS frequency improves balance difficulties after thalamic DBS for essential tremor. J. Neurol. Sci. 2016, 367, 122–127. [Google Scholar] [CrossRef]
- Fasano, A.; Herzog, J.; Raethjen, J.; Rose, F.E.; Muthuraman, M.; Volkmann, J.; Falk, D.; Elble, R.; Deuschl, G. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain 2010, 133, 3635–3648. [Google Scholar] [CrossRef]
- Groppa, S.; Herzog, J.; Falk, D.; Riedel, C.; Deuschl, G.; Volkmann, J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain 2014, 137, 109–121. [Google Scholar] [CrossRef]
- Cagnan, H.; Brittain, J.S.; Little, S.; Foltynie, T.; Limousin, P.; Zrinzo, L.; Hariz, M.; Joint, C.; Fitzgerald, J.; Green, A.; et al. Phase dependent modulation of tremor amplitude in essential tremor through thalamic stimulation. Brain 2013, 136, 3062–3075. [Google Scholar] [CrossRef]
- Akbar, U.; Raike, R.S.; Hack, N.; Hess, C.W.; Skinner, J.; Martinez-Ramirez, D.; DeJesus, S.; Okun, M.S. Randomized, blinded pilot testing of nonconventional stimulation patterns and shapes in parkinson’s disease and essential tremor: Evidence for further evaluating narrow and biphasic pulses. Neuromodulation Technol. Neural Interface 2016, 19, 343–356. [Google Scholar] [CrossRef]
- Picillo, M.; Lozano, A.M.; Kou, N.; Puppi munhoz, R.; Fasano, A. Programming deep brain stimulation for parkinson’s disease: The toronto western hospital algorithms. Brain Stimul. 2016, 9, 425–437. [Google Scholar] [CrossRef]
- Koss, A.M.; Alterman, R.L.; Tagliati, M.; Shils, J.L. Calculating total electrical energy delivered by deep brain stimulation systems. Ann. Neurol. 2005, 58, 168. [Google Scholar] [CrossRef]
- Sandvik, U.; Koskinen, L.O.; Lundquist, A.; Blomstedt, P. Thalamic and subthalamic deep brain stimulation for essential tremor: Where is the optimal target? Neurosurgery 2012, 70, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E. Diffusion tractography in deep brain stimulation surgery: A review. Front. Neuroanat. 2016, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Paschen, S.; Forstenpointner, J.; Becktepe, J.; Heinzel, S.; Hellriegel, H.; Witt, K.; Helmers, A.K.; Deuschl, G. Long-term efficacy of deep brain stimulation for essential tremor: An observer-blinded study. Neurology 2019, 92, e1378–e1386. [Google Scholar] [CrossRef] [PubMed]
- Seier, M.; Hiller, A.; Quinn, J.; Murchison, C.; Brodsky, M.; Anderson, S. Alternating thalamic deep brain stimulation for essential tremor: A trial to reduce habituation. Mov. Disord. Clin. Pract. 2018, 5, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Cagnan, H.; Pedrosa, D.; Little, S.; Pogosyan, A.; Cheeran, B.; Aziz, T.; Green, A.; Fitzgerald, J.; Foltynie, T.; Limousin, P.; et al. Stimulating at the right time: Phase-specific deep brain stimulation. Brain 2017, 140, 132–145. [Google Scholar]
- Gildenberg, P.L. Evolution of neuromodulation. Stereotact Funct. Neurosurg. 2005, 83, 71–79. [Google Scholar] [CrossRef]
- Huang, H.; Watts, R.L.; Montgomery, E.B. Effects of deep brain stimulation frequency on bradykinesia of Parkinson’s disease. Mov. Disord. 2014, 29, 203–206. [Google Scholar] [CrossRef]
- Blomstedt, P.; Hariz, G.M.; Hariz, M.I.; Koskinen, L.O. Thalamic deep brain stimulation in the treatment of essential tremor: A long-term follow-up. Br. J. Neurosurg. 2007, 21, 504–509. [Google Scholar] [CrossRef]
- Histed, M.H.; Bonin, V.; Reid, R.C. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 2009, 63, 508–522. [Google Scholar] [CrossRef]
- Pastor, J.; Vega-zelaya, L. A new potential specifically marks the sensory thalamus in anaesthetised patients. Clin. Neurophysiol. 2019, 130, 1926–1936. [Google Scholar] [CrossRef]
- Montgomery, E.B. Deep Brain Stimulation Programming: Principles and Practice; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Hartmann, C.J.; Hirschmann, J.; Vesper, J.; Wojtecki, L.; Butz, M.; Schnitzler, A. Distinct cortical responses evoked by electrical stimulation of the thalamic ventral intermediate nucleus and of the subthalamic nucleus. NeuroImage Clin. 2018, 20, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Butson, C.R.; Lempka, S.F.; Cooper, S.E.; Mcintyre, C.C. Patient-specific models of deep brain stimulation: Influence of field model complexity on neural activation predictions. Brain Stimul. 2010, 3, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.; Mcintyre, C.C. Analyzing the tradeoff between electrical complexity and accuracy in patient-specific computational models of deep brain stimulation. J. Neural Eng. 2016, 13, 036023. [Google Scholar] [CrossRef] [PubMed]
- Chiken, S.; Nambu, A. Mechanism of deep brain stimulation: Inhibition, excitation, or disruption? Neuroscientist 2016, 22, 313–322. [Google Scholar] [CrossRef]
- Jakobs, M.; Fomenko, A.; Lozano, A.M.; Kiening, K.L. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation-a systematic review on established indications and outlook on future developments. EMBO Mol. Med. 2019, 11, e9575. [Google Scholar] [CrossRef]
- Little, S.; Beudel, M.; Zrinzo, L.; Foltynie, T.; Limousin, P.; Hariz, M.; Neal, S.; Cheeran, B.; Cagnan, H.; Gratwicke, J.; et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 717–721. [Google Scholar] [CrossRef]
- Beudel, M.; Sadnicka, A.; Edwards, M.; De jong, B.M. Linking pathological oscillations with altered temporal processing in parkinsons disease: Neurophysiological mechanisms and implications for neuromodulation. Front. Neurol. 2019, 10, 462. [Google Scholar] [CrossRef]
- Cooper, S.E.; Kuncel, A.M.; Wolgamuth, B.R.; Rezai, A.R.; Grill, W.M. A model predicting optimal parameters for deep brain stimulation in essential tremor. J. Clin. Neurophysiol. 2008, 25, 265–273. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Loui, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G. Tremor Task Force of the International Parkinson and Movement Disorder Society Consensus statement on the classification of tremors. From the task force on tremor of the international parkinson and movement disorder society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Deuschl, G.; Bergman, H. Pathophysiology of nonparkinsonian tremors. Mov. Disord. 2002, 17, 41–48. [Google Scholar] [CrossRef]
- Middlebrooks, E.H.; Holanda, V.M.; Tuna, I.S.; Deshpande, H.D.; Bredel, M.; Almeida, L.; Walker, H.C.; Guthrie, B.L.; Foote, K.D.; Okun, M.S. A method for pre-operative single-subject thalamic segmentation based on probabilistic tractography for essential tremor deep brain stimulation. Neuroradiology 2018, 60, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, D.J.; Reck, C.; Florin, E.; Pauls, M.; Maarouf, M.; Wojtecki, L.; Dafsari, S.H.; Sturm, V.; Schnitzler, A.; Fink, G.R.; et al. Essential tremor and tremor in Parkinson’s disease are associated with distinct ‘tremor clusters’ in the ventral thalamus. Exp. Neurol. 2012, 237, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.Q. Ethics and social risks in brain stimulation. Brain Stimul. 2013, 6, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.; Bain, P.; Forjaz, M.J.; Haubenberger, D.; Testa, C.; Goetz, C.G.; Leentjens, A.F.G.; Martinez-Martin, P.; Pavy-Le Traon, A.; Post, B.; et al. Task force report: Scales for screening and evaluating tremor: Critique and recommendations. Mov. Disord. 2013, 28, 1793–1800. [Google Scholar] [CrossRef]
- Willsie, A.; Dorval, A. Fabrication and initial testing of the DBS: A novel deep brain stimulation electrode with thousands of individually controllable contacts. Biomed. Microdevices 2015, 17, 56. [Google Scholar] [CrossRef]
- Timmermann, L.; Jain, R.; Chen, L.; Maarouf, M.; Barbe, M.T.; Allert, N.; Brucke, T.; Kaiser, I.; Beirer, S.; Sejio, F.; et al. 134 VANTAGE trial: Three-year outcomes of a prospective, multicenter trial evaluating deep brain stimulation with a new multiple-source, constant-current rechargeable system in parkinson disease. Neurosurgery 2016, 63, 155. [Google Scholar] [CrossRef]

| Patient | Age (Years) | Gender | Disease Duration (years) | DBS Duration | DBS Target | DBS Contacts | Baseline | Best–Subjective | Best–Objective | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | P | F | TDAmp | V | P | F | TDAmp | V | P | F | TDAmp | |||||||
| ET1 | 53 | male | Since youth | 2 | Vim | −1 | 2 | 90 | 185 | 0.87 | 3.2 | 180 | 80 | 0.32 | 3.3 | 200 | 95 | 0.20 |
| ET3 | 77 | male | 22 | 1 | Vim | −10 | 3 | 90 | 185 | 1.03 | 3.5 | 180 | 145 | 0.27 | 3.9 | 150 | 160 | 0.24 |
| ET4 | 73 | male | 33 | 20 | Vim | −0 | 0.8 | 60 | 180 | 0.14 | 1.6 | 70 | 165 | 0.11 | 2.1 | 170 | 85 | 0.11 |
| ET5 | 69 | male | 52 | 1 | Vim | −1/2+ | 2.8 | 60 | 185 | 0.07 | 2.7 | 210 | 170 | 0.07 | 3.2 | 140 | 175 | 0.07 |
| Average tremor improvement (%) compared to baseline for Vim-DBS according to subjective (53%) and objective (58%) measurements. | ||||||||||||||||||
| ET2 | 78 | male | Since youth | 3 | ZI | −3 | 3.3 | 60 | 185 | 0.94 | 3.6 | 60 | 160 | 0.36 | 3.9 | 70 | 150 | 0.28 |
| ET6 | 70 | male | 27 | 4 | ZI | −9/10+ | 1.7 | 60 | 180 | n.a. | 1.5 | 90 | 155 | 0.43 | 1.5 | 90 | 155 | 0.43 |
| ET7 | 72 | female | Since youth | 6 | ZI | −1 | 1.8 | 90 | 180 | 1.07 | 2.2 | 140 | 140 | 0.20 | 2,2 | 140 | 140 | 0.20 |
| ET8 | 51 | female | 48 | 6 | ZI | −1/2+ | 1 | 90 | 185 | 1.81 | 2.2 | 160 | 180 | 0.11 | 1.5 | 220 | 180 | 0.06 |
| Average tremor improvement (%) compared to baseline for ZI-DBS according to subjective (79%) and objective (82%) measurements. | ||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, I.D.; Laar, T.v.; Oterdoom, D.L.M.; Drost, G.; van Dijk, J.M.C.; Beudel, M. Optimal Parameters of Deep Brain Stimulation in Essential Tremor: A Meta-Analysis and Novel Programming Strategy. J. Clin. Med. 2020, 9, 1855. https://doi.org/10.3390/jcm9061855
Bogdan ID, Laar Tv, Oterdoom DLM, Drost G, van Dijk JMC, Beudel M. Optimal Parameters of Deep Brain Stimulation in Essential Tremor: A Meta-Analysis and Novel Programming Strategy. Journal of Clinical Medicine. 2020; 9(6):1855. https://doi.org/10.3390/jcm9061855
Chicago/Turabian StyleBogdan, I. Daria, Teus van Laar, D.L. Marinus Oterdoom, Gea Drost, J. Marc C. van Dijk, and Martijn Beudel. 2020. "Optimal Parameters of Deep Brain Stimulation in Essential Tremor: A Meta-Analysis and Novel Programming Strategy" Journal of Clinical Medicine 9, no. 6: 1855. https://doi.org/10.3390/jcm9061855
APA StyleBogdan, I. D., Laar, T. v., Oterdoom, D. L. M., Drost, G., van Dijk, J. M. C., & Beudel, M. (2020). Optimal Parameters of Deep Brain Stimulation in Essential Tremor: A Meta-Analysis and Novel Programming Strategy. Journal of Clinical Medicine, 9(6), 1855. https://doi.org/10.3390/jcm9061855







