DSC Brain Perfusion Using Advanced Deconvolution Models in the Diagnostic Work-Up of Dementia and Mild Cognitive Impairment: A Semiquantitative Comparison with HMPAO-SPECT-Brain Perfusion
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Imaging Protocol
2.3. Image Processing and Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. J. Alzheimers Assoc. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Tola-Arribas, M.A.; Yugueros, M.I.; Garea, M.J.; Ortega-Valin, F.; Ceron-Fernandez, A.; Fernandez-Malvido, B.; San Jose-Gallegos, A.; Gonzalez-Touya, M.; Botran-Velicia, A.; Iglesias-Rodriguez, V.; et al. Prevalence of dementia and subtypes in Valladolid, northwestern Spain: The DEMINVALL study. PLoS ONE 2013, 8, e77688. [Google Scholar] [CrossRef] [PubMed]
- Swan, A.; Waddell, B.; Holloway, G.; Bak, T.; Colville, S.; Khan, Z.; Pal, S. The diagnostic utility of 99mTc-HMPAO SPECT imaging: A retrospective case series from a tertiary referral early-onset cognitive disorders clinic. Dement. Geriatr. Cogn. Disord. 2015, 39, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Lahiri, D.K.; Giacobini, E. Editorial: Advances in Alzheimer therapy: Something old, something new, something borrowed, something blue. Curr. Alzheimer Res. 2005, 2, 275–279. [Google Scholar] [CrossRef]
- Karran, E.; Hardy, J. Antiamyloid therapy for Alzheimer’s disease--are we on the right road? N. Engl. J. Med. 2014, 370, 377–378. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R., Jr.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Fallmar, D.; Lilja, J.; Velickaite, V.; Danfors, T.; Lubberink, M.; Ahlgren, A.; van Osch, M.J.; Kilander, L.; Larsson, E.M. Visual Assessment of Brain Perfusion MRI Scans in Dementia: A Pilot Study. J. Neuroimaging 2015. [Google Scholar] [CrossRef]
- Yeo, J.M.; Lim, X.; Khan, Z.; Pal, S. Systematic review of the diagnostic utility of SPECT imaging in dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 539–552. [Google Scholar] [CrossRef]
- Rausch, I.; Fuchsel, F.G.; Kuderer, C.; Hentschel, M.; Beyer, T. Radiation exposure levels of routine SPECT/CT imaging protocols. Eur. J. Radiol. 2016, 85, 1627–1636. [Google Scholar] [CrossRef]
- Eskildsen, S.F.; Gyldensted, L.; Nagenthiraja, K.; Nielsen, R.B.; Hansen, M.B.; Dalby, R.B.; Frandsen, J.; Rodell, A.; Gyldensted, C.; Jespersen, S.N.; et al. Increased cortical capillary transit time heterogeneity in Alzheimer’s disease: A DSC-MRI perfusion study. Neurobiol. Aging 2017, 50, 107–118. [Google Scholar] [CrossRef]
- Harris, G.J.; Lewis, R.F.; Satlin, A.; English, C.D.; Scott, T.M.; Yurgelun-Todd, D.A.; Renshaw, P.F. Dynamic susceptibility contrast MR imaging of regional cerebral blood volume in Alzheimer disease: A promising alternative to nuclear medicine. Am. J. Neuroradiol. 1998, 19, 1727–1732. [Google Scholar]
- Yoshiura, T.; Mihara, F.; Kuwabara, Y.; Ogomori, K.; Kaneko, K.; Tanaka, A.; Sasaki, M.; Nakagawa, M.; Koga, H.; Yamanaka, T.; et al. MR relative cerebral blood flow mapping of Alzheimer disease: Correlation with Tc-99m HMPAO SPECT. Acad. Radiol. 2002, 9, 1383–1387. [Google Scholar] [CrossRef]
- Cavallin, L.; Danielsson, R.; Oksengard, A.R.; Wahlund, L.O.; Julin, P.; Frank, A.; Engman, E.L.; Svensson, L.; Kristoffersen Wiberg, M. Can dynamic susceptibility contrast magnetic resonance imaging replace single-photon emission computed tomography in the diagnosis of patients with Alzheimer’s disease? A pilot study. Acta Radiol. 2006, 47, 977–985. [Google Scholar] [CrossRef]
- Cavallin, L.; Axelsson, R.; Wahlund, L.O.; Oksengard, A.R.; Svensson, L.; Juhlin, P.; Wiberg, M.K.; Frank, A. Voxel-based correlation between coregistered single-photon emission computed tomography and dynamic susceptibility contrast magnetic resonance imaging in subjects with suspected Alzheimer disease. Acta Radiol. 2008, 49, 1154–1161. [Google Scholar] [CrossRef]
- Wu, O.; Ostergaard, L.; Weisskoff, R.M.; Benner, T.; Rosen, B.R.; Sorensen, A.G. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn. Reson. Med. 2003, 50, 164–174. [Google Scholar] [CrossRef]
- Kudo, K.; Boutelier, T.; Pautot, F.; Honjo, K.; Hu, J.Q.; Wang, H.B.; Shintaku, K.; Uwano, I.; Sasaki, M. Bayesian analysis of perfusion-weighted imaging to predict infarct volume: Comparison with singular value decomposition. Magn. Reson. Med Sci. MRMS 2014, 13, 45–50. [Google Scholar] [CrossRef]
- Sasaki, M.; Kudo, K.; Boutelier, T.; Pautot, F.; Christensen, S.; Uwano, I.; Goodwin, J.; Higuchi, S.; Ito, K.; Yamashita, F. Assessment of the accuracy of a Bayesian estimation algorithm for perfusion CT by using a digital phantom. Neuroradiology 2013, 55, 1197–1203. [Google Scholar] [CrossRef]
- Mouridsen, K.; Christensen, S.; Gyldensted, L.; Ostergaard, L. Automatic selection of arterial input function using cluster analysis. Magn. Reson. Med. 2006, 55, 524–531. [Google Scholar] [CrossRef]
- Ostergaard, L.; Weisskoff, R.M.; Chesler, D.A.; Gyldensted, C.; Rosen, B.R. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn. Reson. Med. 1996, 36, 715–725. [Google Scholar] [CrossRef]
- Boutelier, T.; Kudo, K.; Pautot, F.; Sasaki, M. Bayesian hemodynamic parameter estimation by bolus tracking perfusion weighted imaging. IEEE Trans. Med. Imaging 2012, 31, 1381–1395. [Google Scholar] [CrossRef]
- Hara, S.; Tanaka, Y.; Hayashi, S.; Inaji, M.; Maehara, T.; Hori, M.; Aoki, S.; Ishii, K.; Nariai, T. Bayesian Estimation of CBF Measured by DSC-MRI in Patients with Moyamoya Disease: Comparison with (15)O-Gas PET and Singular Value Decomposition. Am. J. Neuroradiol. 2019, 40, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Steketee, R.M.; Bron, E.E.; Meijboom, R.; Houston, G.C.; Klein, S.; Mutsaerts, H.J.; Mendez Orellana, C.P.; de Jong, F.J.; van Swieten, J.C.; van der Lugt, A.; et al. Early-stage differentiation between presenile Alzheimer’s disease and frontotemporal dementia using arterial spin labeling MRI. Eur. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Binnewijzend, M.A.; Kuijer, J.P.; van der Flier, W.M.; Benedictus, M.R.; Moller, C.M.; Pijnenburg, Y.A.; Lemstra, A.W.; Prins, N.D.; Wattjes, M.P.; van Berckel, B.N.; et al. Distinct perfusion patterns in Alzheimer’s disease, frontotemporal dementia and dementia with Lewy bodies. Eur. Radiol. 2014, 24, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Horowitz, S.; Ragin, A.; Chen, Y.; Walker, M.; Carroll, T.J. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: Evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn. Reson. Med. 2007, 58, 1232–1241. [Google Scholar] [CrossRef]
- Henriksen, O.M.; Jensen, L.T.; Krabbe, K.; Guldberg, P.; Teerlink, T.; Rostrup, E. Resting brain perfusion and selected vascular risk factors in healthy elderly subjects. PLoS ONE 2014, 9, e97363. [Google Scholar] [CrossRef]
- Menzel, C.; Hufnagel, A.; Grunwald, F.; Pavics, L.; Reichmann, K.; Elger, C.E.; Biersack, H.J. The relevance of interictal rCBF brain SPECT in temporal lobe epilepsy: Diagnostical value and effects of spatial resolution. Ann. Nucl. Med. 1995, 9, 215–223. [Google Scholar] [CrossRef]
- Rodriguez, G.; Vitali, P.; Calvini, P.; Bordoni, C.; Girtler, N.; Taddei, G.; Mariani, G.; Nobili, F. Hippocampal perfusion in mild Alzheimer’s disease. Psychiatry Res. 2000, 100, 65–74. [Google Scholar] [CrossRef]
- Burton, E.J.; Karas, G.; Paling, S.M.; Barber, R.; Williams, E.D.; Ballard, C.G.; McKeith, I.G.; Scheltens, P.; Barkhof, F.; O’Brien, J.T. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. NeuroImage 2002, 17, 618–630. [Google Scholar] [CrossRef]
- Mak, E.; Su, L.; Williams, G.B.; O’Brien, J.T. Neuroimaging characteristics of dementia with Lewy bodies. Alzheimers Res. Ther. 2014, 6, 18. [Google Scholar] [CrossRef]
- Harris, R.J.; Cloughesy, T.F.; Hardy, A.J.; Liau, L.M.; Pope, W.B.; Nghiemphu, P.L.; Lai, A.; Ellingson, B.M. MRI perfusion measurements calculated using advanced deconvolution techniques predict survival in recurrent glioblastoma treated with bevacizumab. J. Neuro Oncol. 2015, 122, 497–505. [Google Scholar] [CrossRef]
- Alvarez-Linera, J. 3T MRI: Advances in brain imaging. Eur. J. Radiol. 2008, 67, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
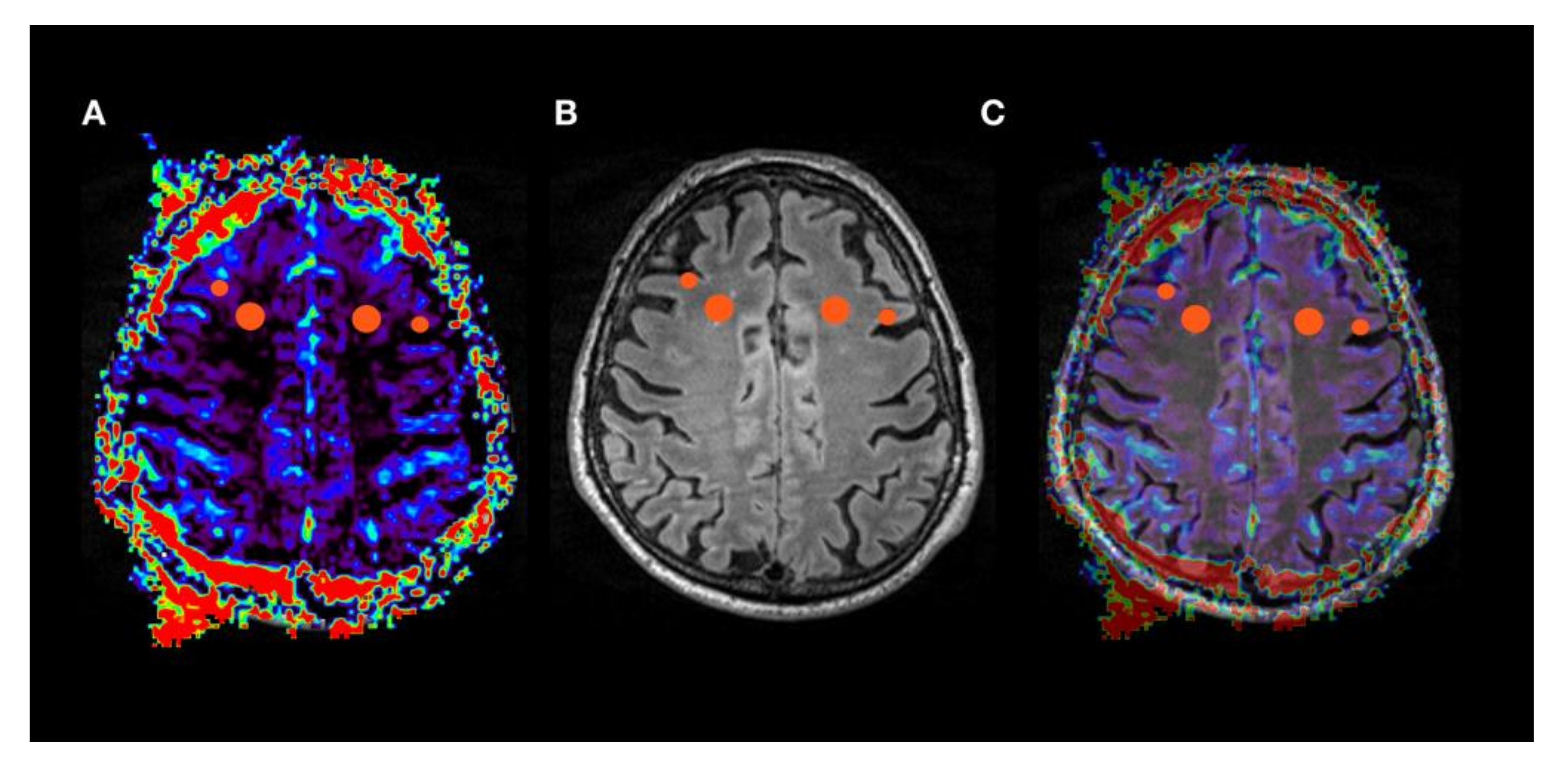
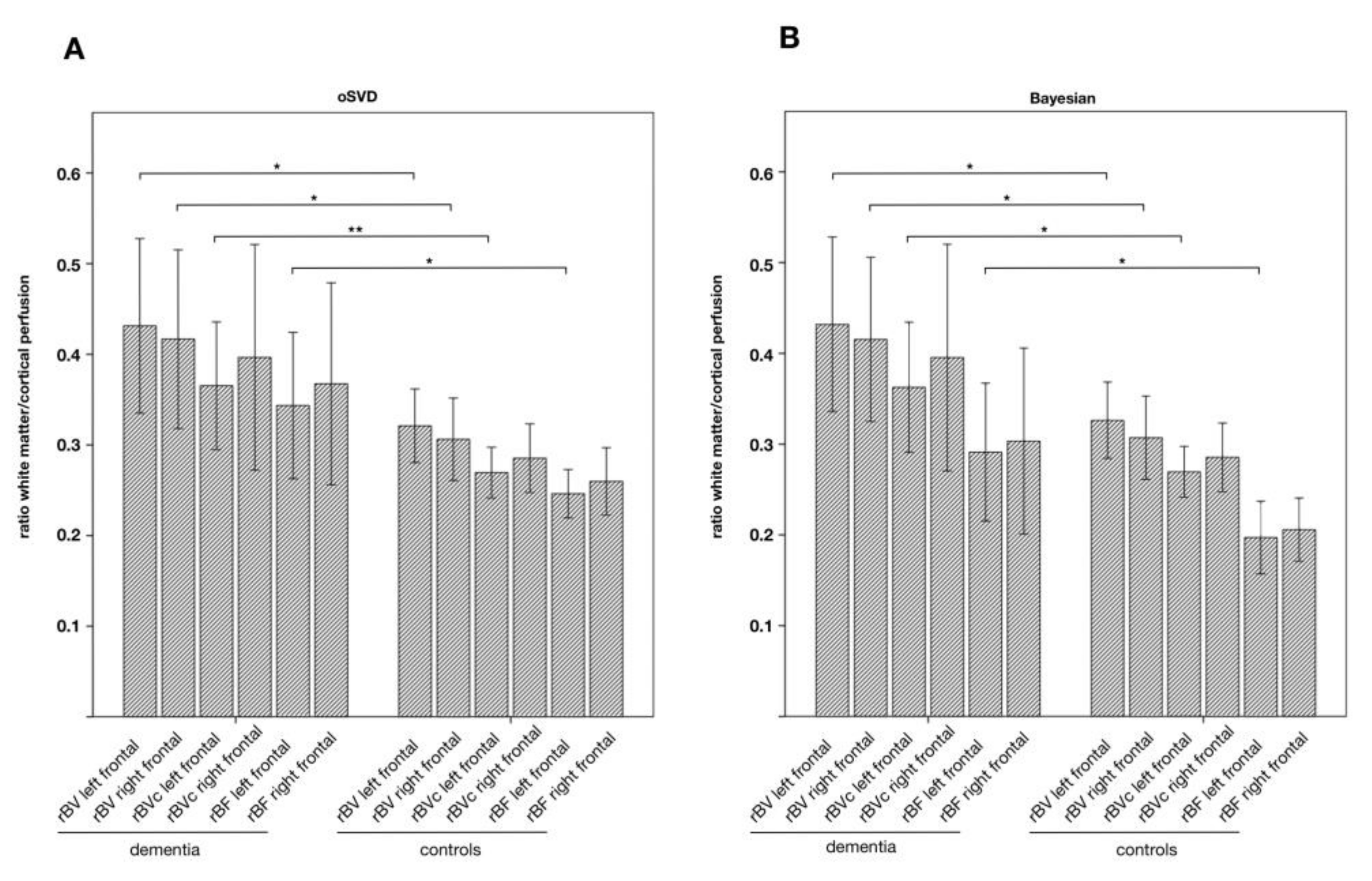
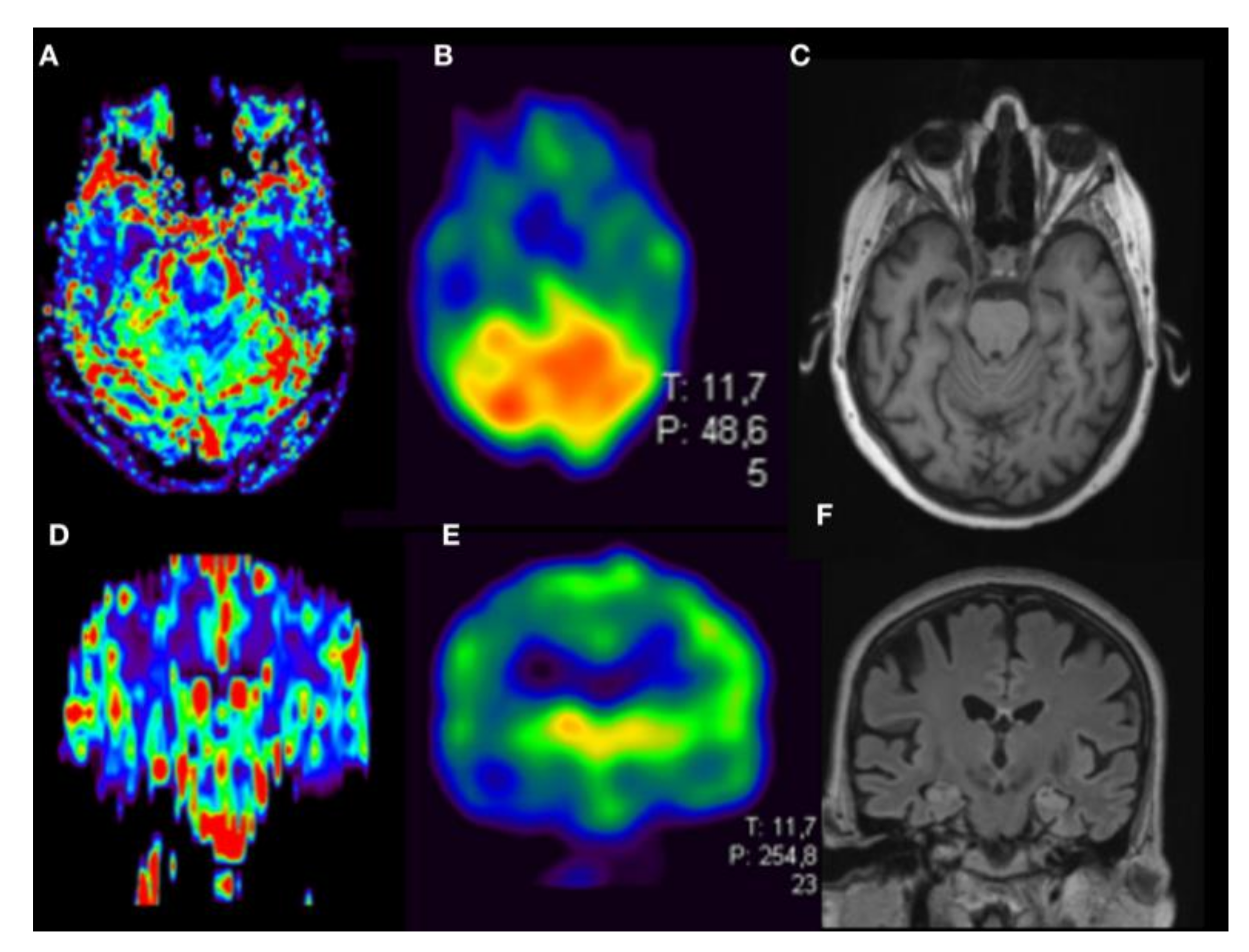
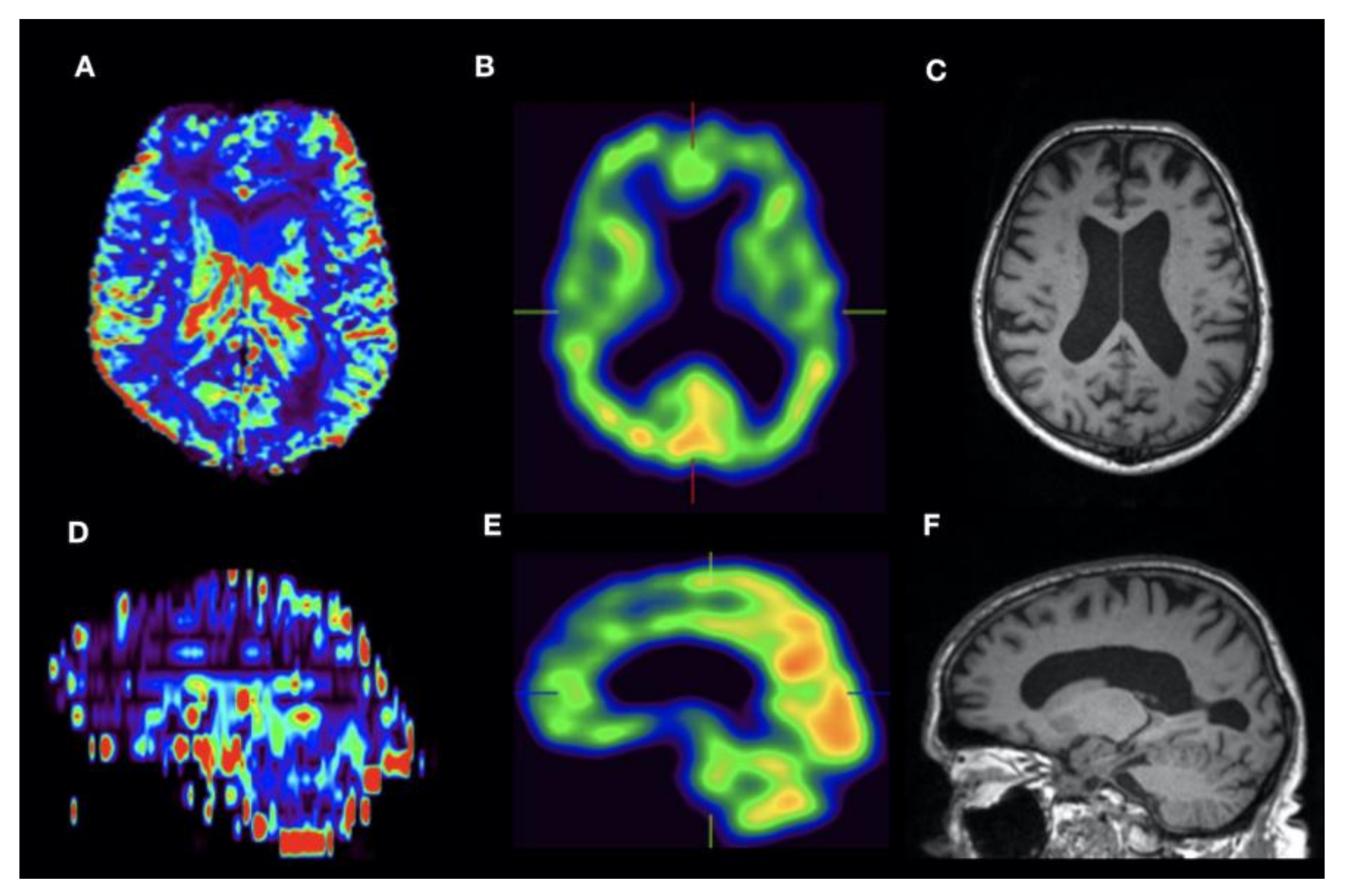
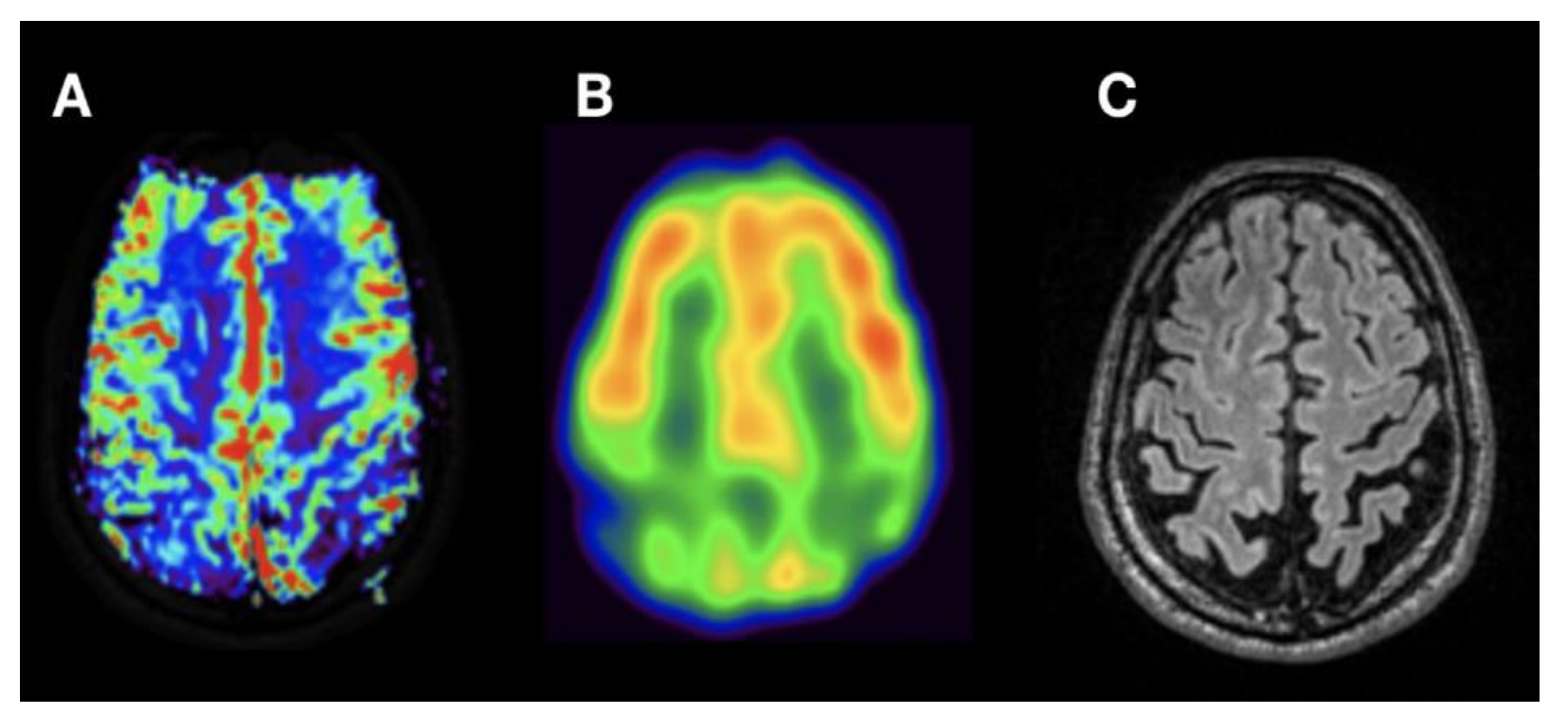
| Patient No | Age | Gender | Suspected Diagnosis | MMST |
|---|---|---|---|---|
| 1 | 69 | f | FTD | 27 |
| 2 | 57 | m | FTD | 27 |
| 3 | 50 | m | AD | 27 |
| 4 | 79 | f | FTD | 23 |
| 5 | 55 | m | MCI | 30 |
| 6 | 83 | m | FTD | 19 |
| 7 | 70 | f | FTD | 29 |
| 8 | 78 | f | DLB | 20 |
| 9 | 53 | m | CBD | 24 |
| 10 | 63 | m | MCI | 29 |
| 11 | 54 | f | AD | 10 |
| 12 | 73 | f | DLB | 28 |
| 13 | 78 | f | MCI | 25 |
| 14 | 58 | f | MCI | 28 |
| 15 | 62 | f | FTD | 14 |
| 16 | 69 | m | FTD | 30 |
| 17 | 68 | m | AD | 26 |
| 18 | 77 | m | AD | 27 |
| 19 | 56 | m | MCI | 28 |
| 20 | 58 | m | MCI | 29 |
| 21 | 73 | f | VD | 27 |
| 22 | 74 | f | MCI | 27 |
| 23 | 60 | m | AD | 30 |
| 24 | 69 | m | VD | 28 |
| 25 | 54 | m | MCI | 26 |
| 26 | 63 | m | MCI | 26 |
| 27 | 69 | f | AD | 22 |
| MNI Cortex | MNI White Matter | |||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
| left frontal | −36 | 2 | 53 | −12 | 7 | 51 |
| left parietal | −28 | −68 | 41 | −19 | −53 | 41 |
| left temporal | −53 | −7 | −22 | −42 | −1 | −22 |
| right frontal | 24 | 11 | 53 | 17 | 6 | 53 |
| right parietal | 28 | −68 | 41 | 19 | −53 | 41 |
| right temporal | 53 | −7 | −22 | 42 | −1 | −22 |
| oSVD | Patients | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| rBV | rBVc | rBF | MTT [ms] | rBV | rBVc | rBF | MTT [ms] | |
| left frontal | 0.43 * ± 0.24 | 0.37 ** ± 0.17 | 0.34 * ± 0.2 | 4720 ± 1430 | 0.32 ± 0.08 | 0.27 ± 0.06 | 0.25 ± 0.06 | 4930 ± 870 |
| right frontal | 0.42 * ± 0.24 | 0.40 ± 0.21 | 0.37 ± 0.18 | 4860 ±1520 | 0.31 ± 0.1 | 0.29 ± 0.08 | 0.26 ± 0.08 | 4920 ± 850 |
| left parietal | 0.41 ± 0.18 | 0.41 ± 0.2 | 0.40 ± 0.2 | 5990 ± 3710 | 0.44 ± 0.14 | 0.37 ± 0.12 | 0.36 ± 0.13 | 4760 ± 750 |
| right parietal | 0.52 ± 0.25 | 0.44 ± 0.28 | 0.44 ± 0.25 | 5190 ± 2280 | 0.43 ± 0.13 | 0.35 ± 0.1 | 0.35 ± 0.1 | 5050 ± 770 |
| left temporal | 0.58 ± 0.26 | 0.49 ± 0.17 | 0.51 ± 0.15 | 4810 ± 1930 | 0.49 ± 0.14 | 0.46 ± 0.1 | 0.46 ± 0.12 | 4880 ± 940 |
| right temporal | 0.47 ± 0.21 | 0.43 ± 0.16 | 0.44 ± 0.14 | 5540 ± 2520 | 0.43 ± 0.17 | 0.42 ± 0.12 | 0.39 ± 0.12 | 5000 ± 1050 |
| Bayesian | patients | controls | ||||||
| left frontal | 0.43 * ± 0.24 | 0.36* ± 0.18 | 0.29 * ± 0.19 | 3300 ± 1320 | 0.33 ± 0.09 | 0.27 ± 0.06 | 0.20 ± 0.08 | 3290 ± 1680 |
| right frontal | 0.42 * ± 0.22 | 0.40 ± 0.31 | 0.30 ± 0.15 | 3940 ± 3150 | 0.31 ± 0.1 | 0.29 ± 0.08 | 0.21 ± 0.07 | 3370 ± 1240 |
| left parietal | 0.42 ± 0.17 | 0.40 ± 0.2 | 0.33 ± 0.18 | 4990 ± 4070 | 0.44 ± 0.15 | 0.37 ± 0.12 | 0.26 ± 0.08 | 3080 ± 920 |
| right parietal | 0.52 ± 0.24 | 0.44 ± 0.28 | 0.39 ± 0.31 | 3690 ± 1980 | 0.43 ± 0.13 | 0.35 ± 0.1 | 0.26 ± 0.06 | 3320 ± 910 |
| left temporal | 0.50 ± 0.21 | 0.48 ± 0.18 | 0.44 ± 0.2 | 3840 ± 1890 | 0.49 ± 0.15 | 0.46 ± 0.1 | 0.40 ± 0.15 | 3650 ± 1080 |
| right temporal | 0.52 ± 0.2 | 0.42 ± 0.16 | 0.39 ± 0.17 | 4440 ± 2450 | 0.43 ± 0.17 | 0.42 ± 0.1 | 0.36 ± 0.13 | 4250 ± 3060 |
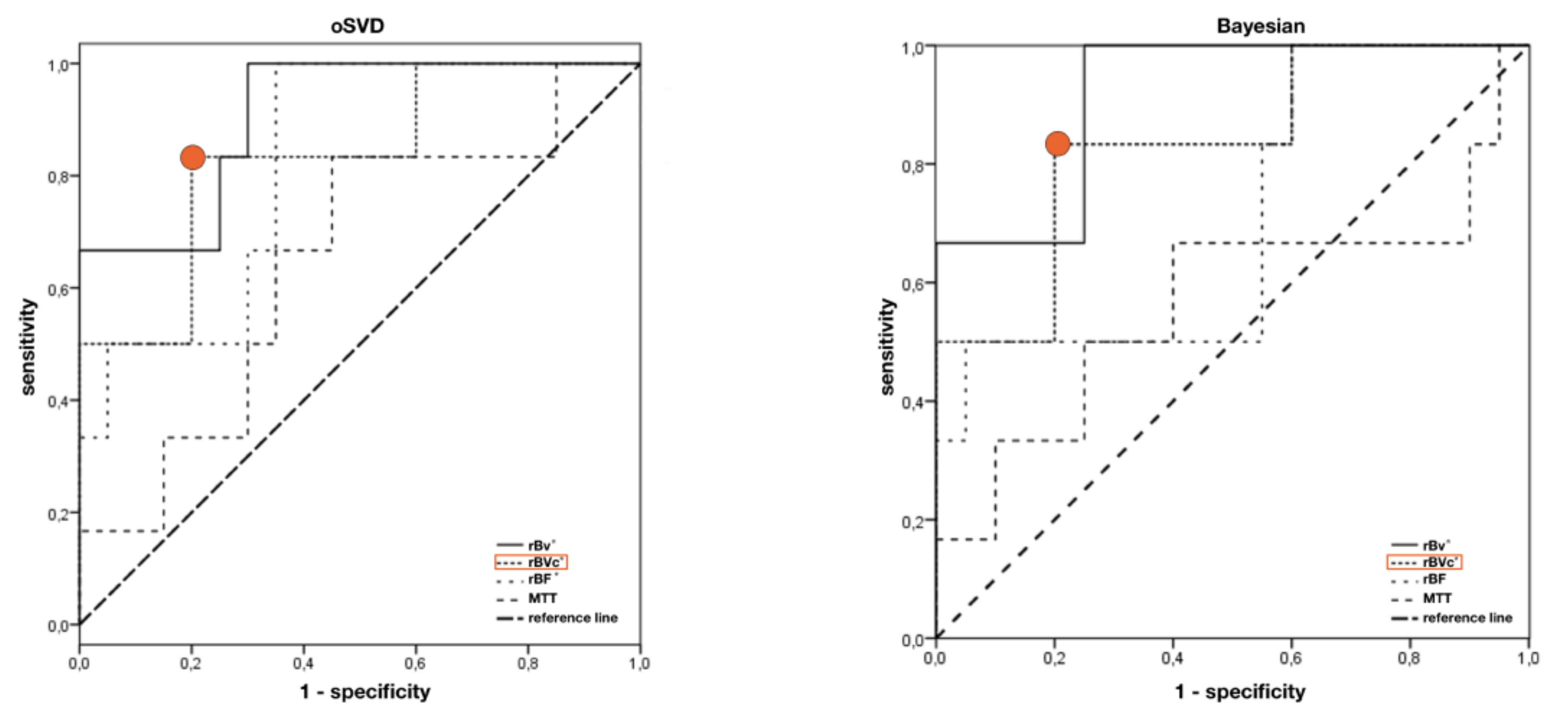
| oSVD | Bayesian | |||||||
|---|---|---|---|---|---|---|---|---|
| rBV | rBVc | rBF | MTT | rBV | rBVc | rBF | MTT | |
| left frontal | ||||||||
| Cut off ratio | 0.4 | 0.36 | 0.29 | 1.18 | 0.41 | 0.36 | 0.21 | 1.39 |
| Sensitivity | 83% | 83% | 83% | 83% | 83% | 83% | 83% | 67% |
| specificity | 75% | 80% | 65% | 55% | 75% | 80% | 45% | 60% |
| AUC | 0.908 * | 0.833 * | 0.825 * | 0.650 | 0.917 * | 0.833 * | 0.708 | 0.567 |
| right frontal | ||||||||
| Cut off ratio | 0.33 | 0.3 | 0.27 | 1.18 | 0.33 | 0.30 | 0.21 | 0.92 |
| Sensitivity | 60% | 60% | 60% | 80% | 60% | 60% | 60% | 60% |
| specificity | 42.9% | 43% | 47.6% | 47.6% | 42.9% | 42.9% | 47.6% | 14.4% |
| AUC | 0.667 | 0.657 | 0.667 | 0.514 | 0.667 | 0.657 | 0.543 | 0.581 |
| left parietal | ||||||||
| Cut off | 0.66 | 0.91 | 0.87 | - | 0.66 | 0.91 | 0.77 | - |
| Sensitivity | 50% | 50% | 50% | - | 50% | 50% | 50% | - |
| specificity | 95.8% | 100% | 100% | - | 58% | 100% | 100% | - |
| AUC | 0.479 | 0.521 | 0.542 | 0 | 0.479 | 0.521 | 0.688 | 0.021 |
| right parietal | ||||||||
| Cut off ratio | 0.65 | 0.97 | 0.91 | - | 0.67 | 0.97 | 0.83 | - |
| Sensitivity | 66.7% | 66.7% | 66.7% | - | 66.7% | 66.7% | 66.7% | - |
| specificity | 82.6% | 100% | 100% | - | 87%% | 100% | 100% | - |
| AUC | 0.609 | 0.667 | 0.667 | 0.203 | 0.609 | 0.681 | 0.739 | 0.072 |
| left temporal | ||||||||
| Cut off ratio | 0.73 | 0.55 | 0.85 | 1.03 | 0.77 | 0.55 | 0.42 | 1.47 |
| Sensitivity | 50% | 50% | 25% | 75% | 50% | 50% | 75% | 75% |
| specificity | 77.3% | 72.7% | 100% | 50% | 77.3% | 72.7% | 59.1% | 54.5% |
| AUC | 0.591 | 0.545 | 0.466 | 0.568 | 0.602 | 0.557 | 0.625 | 0.602 |
| right temporal | ||||||||
| Cut off ratio | 0.47 | 0.43 | 0.45 | 1.03 | 0.47 | 0.43 | 0.35 | 1.4 |
| Sensitivity | 75% | 75% | 75% | 75% | 100% | 75% | 75% | 50% |
| specificity | 50% | 59.1% | 63.6% | 36.4% | 45.5% | 59.1% | 54.5% | 63.6% |
| AUC | 0.659 | 0.591 | 0.716 | 0.466 | 0.625 | 0.602 | 0.648 | 0.432 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, M.A.; Engelhorn, T.; Lang, S.; Luecking, H.; Hoelter, P.; Fröhlich, K.; Ritt, P.; Maler, J.M.; Kuwert, T.; Kornhuber, J.; et al. DSC Brain Perfusion Using Advanced Deconvolution Models in the Diagnostic Work-Up of Dementia and Mild Cognitive Impairment: A Semiquantitative Comparison with HMPAO-SPECT-Brain Perfusion. J. Clin. Med. 2020, 9, 1800. https://doi.org/10.3390/jcm9061800
Schmidt MA, Engelhorn T, Lang S, Luecking H, Hoelter P, Fröhlich K, Ritt P, Maler JM, Kuwert T, Kornhuber J, et al. DSC Brain Perfusion Using Advanced Deconvolution Models in the Diagnostic Work-Up of Dementia and Mild Cognitive Impairment: A Semiquantitative Comparison with HMPAO-SPECT-Brain Perfusion. Journal of Clinical Medicine. 2020; 9(6):1800. https://doi.org/10.3390/jcm9061800
Chicago/Turabian StyleSchmidt, Manuel A., Tobias Engelhorn, Stefan Lang, Hannes Luecking, Philip Hoelter, Kilian Fröhlich, Philipp Ritt, Juan Manuel Maler, Torsten Kuwert, Johannes Kornhuber, and et al. 2020. "DSC Brain Perfusion Using Advanced Deconvolution Models in the Diagnostic Work-Up of Dementia and Mild Cognitive Impairment: A Semiquantitative Comparison with HMPAO-SPECT-Brain Perfusion" Journal of Clinical Medicine 9, no. 6: 1800. https://doi.org/10.3390/jcm9061800
APA StyleSchmidt, M. A., Engelhorn, T., Lang, S., Luecking, H., Hoelter, P., Fröhlich, K., Ritt, P., Maler, J. M., Kuwert, T., Kornhuber, J., & Doerfler, A. (2020). DSC Brain Perfusion Using Advanced Deconvolution Models in the Diagnostic Work-Up of Dementia and Mild Cognitive Impairment: A Semiquantitative Comparison with HMPAO-SPECT-Brain Perfusion. Journal of Clinical Medicine, 9(6), 1800. https://doi.org/10.3390/jcm9061800





