Does Anxiety Increase the Risk of all-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Quality Assessment
2.3. Data Analysis
3. Results
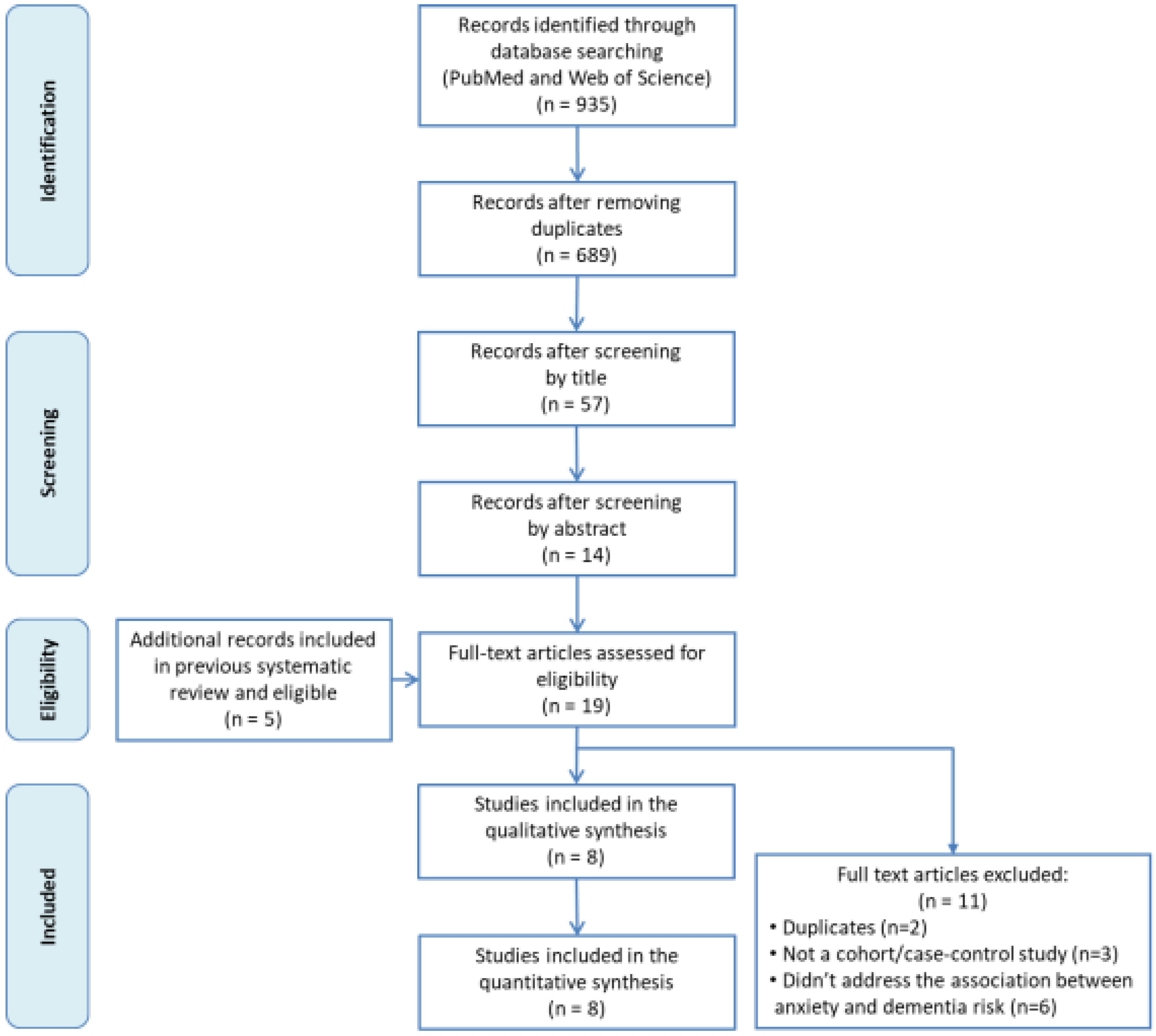
3.1. Study Selection
3.2. Description of Included Studies
3.3. Risk of Bias Assessment
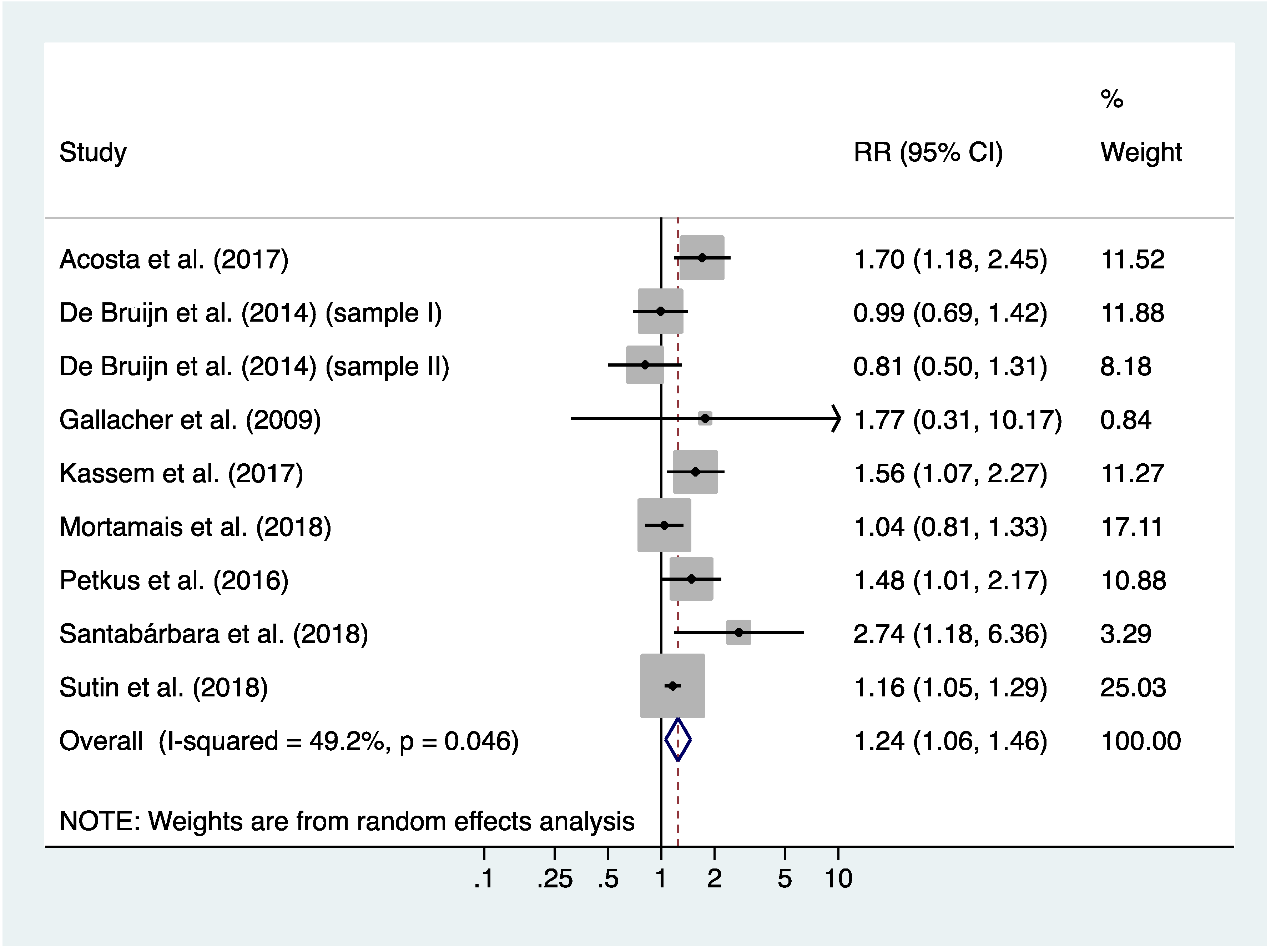
3.4. Meta-Analysis of Incidence Rates of all-Cause Dementia
3.5. Meta-Regression
3.6. Risk of Publication bias
3.7. Population Attributable Fraction
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Potential Mechanisms that Might Underlie the Link between Anxiety and All-Cause Dementia
4.4. Strengths and Limitations
4.5. Clinical and Public Health Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. First WHO Ministerial Conference on Global Action against Dementia; Meeting Report; WHO Headquarters: Geneva, Switzerland, 2015; ISBN 9789241509114. [Google Scholar]
- Birdi, R.; Stephan, B.C.M.; Robinson, L.; Davis, D. Can we influence the epidemiology of dementia? Prospectives from population based studies. Postgrad. Med. J. 2015, 91, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Ritchie, C.; Berr, C.; Artero, S.; Ancelin, M.L. Designing prevention programmes to reduce incidence of dementia: Prospective cohort study of modifiable risk factors. BMJ 2010, 341, 3885. [Google Scholar] [CrossRef] [PubMed]
- Deckers, K.; van Boxtel, M.P.; Schiepers, O.J.; de Vugt, M.; Muñoz-Sánchez, J.L.; Anstey, K.J.; Brayne, C.; Dartigues, J.F.; Engendal, K.; Kivipelto, M.; et al. Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. Int. J. Geriatr. Psychiatry 2015, 30, 234–246. [Google Scholar] [CrossRef]
- Patterson, C. World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf (accessed on 12 November 2019).
- Matthews, F.E.; Arthur, A.; Barnes, L.E.; Bond, J.; Jagger, C.; Robinson, L.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Collaboration. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet 2013, 382, 1405–1412. [Google Scholar] [CrossRef]
- Lobo, A.; Saz, P.; Marcos, G.; Dia, J.L.; De-la-Cámara, C.; Ventura, T.; Montañes, J.A.; Lobo-Escolar, A.; Aznar, S.; ZARADEMP Workgroup. Prevalence of dementia in a southern European population in two different time periods: The ZARADEMP Project. Acta Psychiatr. Scand. 2007, 116, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, E.M.C.; Verhaaren, B.F.; Koudstaal, P.J.; Hofman, A.; Ikram, M.A.; Breteler, M.M. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 2012, 78, 1456–1463. [Google Scholar] [CrossRef]
- Qiu, C.; von Strauss, E.; Backman, L.; Winblad, B.; Fratiglioni, L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 2013, 80, 1888–1894. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Prince, M.; Albanese, E.; Guerchet, M.; Prina, M. World Alzheimer Report 2014. Dementia and Risk Reduction. An Analysis of Protective and Modifiable Risk Factors. Alzheimer’s Disease International. 2014. Available online: http://www.alz.co.uk/ research/WorldAlzheimerReport2014.pdf (accessed on 12 November 2019).
- Gimson, A.; Schlosser, M.; Huntley, J.D.; Marchant, N.L. Support for midlife anxiety diagnosis as an independent risk factor for dementia: A systematic review. BMJ Open 2018, 8, e019399. [Google Scholar] [CrossRef]
- De Bruijn, R.F.; Direk, N.; Mirza, S.S.; Hofman, A.; Koudstaal, P.J.; Tiemeier, H.; Ikram, M.A. Anxiety is not associated with the risk of dementia or cognitive decline: The Rotterdam Study. Am. J. Geriatr. Psychiatry 2014, 22, 1382–1390. [Google Scholar] [CrossRef]
- Gulpers, B.; Ramakers, I.; Hamel, R.; Köhler, S.; Oude Voshaar, R.; Verhey, F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2016, 24, 823–842. [Google Scholar] [CrossRef] [PubMed]
- Santabárbara, J.; Lipnicki, D.M.; Villagrasa, B.; Lobo, E.; Lopez-Anton, R. Anxiety and risk of dementia: Systematic review and meta-analysis of prospective cohort studies. Maturitas 2019, 119, 14–20. [Google Scholar]
- Mortamais, M.; Abdennour, M.; Bergua, V.; Tzourio, C.; Berr, C.; Gabelle, A.; Akbaraly, T.N. Anxiety and 10-Year Risk of Incident Dementia-An Association Shaped by Depressive Symptoms: Results of the Prospective Three-City Study. Front. Neurosci. 2018, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Psychological Distress, Self-Beliefs, and Risk of Cognitive Impairment and Dementia. J. Alzheimers Dis. 2018, 65, 1041–1050. [Google Scholar] [CrossRef]
- Santabárbara, J.; Lopez-Anton, R.; De la Cámara, C.; Lobo, E.; Gracia-García, P.; Villagrasa, B.; Bueno-Notivol, J.; Marcos, G.; Lobo, A. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr. Scand. 2019, 139, 6–14. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2016. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 November 2019).
- Sánchez-Meca, J.; Marín-Martínez, F.; Chacón-Moscoso, S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol. Methods 2003, 8, 448–467. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciencies, 1st ed.; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Rosenberg, M.S. The file-drawer problem revisited: A general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 2005, 59, 464–468. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updatedMarch 2011]; The Cochrane Collaboration, 2011. Available online: www.handbook.cochrane.org (accessed on 12 November 2019).
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in metaanalyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, O.S. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am. J. Epidemiol. 1974, 99, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.E. Confidence limits made easy: Interval estimation using a substitution method. Am. J. Epidemiol. 1998, 147, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Villagrasa, B.; Olaya, B.; López-Antón, R.; de la Cámara, C.; Lobo, A.; Santabárbara, J. Prevalence of anxiety disorder among older adults in spain: A meta-analysis. J. Affect. Disord. 2018, 246, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, J.; Bayer, A.; Fish, M.; Pickering, J.; Pedro, S.; Dunstan, F.; Ebrahim, S.; Ben-Shlomo, Y. Doesanxietyaffectrisk of dementia? Findingsfromthe Caerphilly Prospective Study. Psychosom. Med. 2009, 71, 659–666. [Google Scholar] [CrossRef]
- Petkus, A.J.; Reynolds, C.A.; Wetherell, J.L.; Kremen, W.S.; Pedersen, N.L.; Gatz, M. Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimers Dement. 2016, 12, 399–406. [Google Scholar] [CrossRef]
- Kassem, A.M.; Ganguli, M.; Yaffe, K.; Hanlon, J.T.; Lopez, O.L.; Wilson, J.W.; Ensrud, K.; Cauley, J.A.; Study of Osteoportic Fractures (SOF) Research Group. Anxiety symptoms and risk of dementia and mild cognitive impairment in the oldest old women. Aging Ment. Health 2018, 22, 474–482. [Google Scholar] [CrossRef]
- Acosta, I.; Borges, G.; Aguirre-Hernández, R.; Sosa, A.L.; Prince, M.; 10/66 Dementia Research Group. Neuropsychiatric symptoms as risk factors of dementia in a Mexican population: A 10/66 Dementia Research Group study. Alzheimers Dement. 2018, 14, 271–279. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Marcos, G.; Santabárbara, J.; López-Antón, R.; de la Cámara, C.; Gracia-García, P.; Lobo, E.; Pirez, G.; Menchón, J.M.; Palomo, T.; Stephan, B.C.M.; et al. Conversion to dementia in mild cognitive diagnosed with DSM-5 criteria and with Petersen’s criteria. Acta Psychiatr. Scand. 2016, 133, 378–385. [Google Scholar] [CrossRef]
- Ismail, Z.; Gatchel, J.; Bateman, D.R.; Barcelos-Ferreira, R.; Cantillon, M.; Jaeger, J.; Donovan, N.J.; Mortby, M.E. Affective and emotional dysregulation as pre-dementia risk markers: Exploring the mild behavioral impairtment sysmptoms of depression, anxiety, irritability and euphoria. Int. Psychogeriatr. 2018, 30, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimäki, M.; Sabia, S. Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry 2017, 74, 712–718. [Google Scholar] [CrossRef]
- Emdin, C.A.; Oduyato, A.; Wong, C.X.; Tran, J.; Hsiao, A.J.; Hunn, B.H. Meta-analysis of anxiety as a risk for cardiovascular disease. Am. J. Cardiol. 2016, 118, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Batelaan, N.M.; Seldernrijk, A.; Bot, M.; van Balkom, A.J.; Pennix, B.W. Anxiety and new onset of cardiovascular disease: Critical review and meta-analysis. Br. J. Psychiatry 2016, 208, 223–231. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Schneider, A.L.; Zhou, Y.; Coresh, J.; Green, E.; Gupta, N.; Knopman, D.S.; Mintz, A.; Rahmim, A.; Sharrett, A.R.; et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017, 317, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Raglan, G.B.; Schmidt, L.A.; Schulkin, J. The role of glucocorticoids and corticotropin-releasing hormone regulation on anxiety symptoms and response to treatment. Endocr. Connect. 2017, 6, R1–R7. [Google Scholar] [CrossRef]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-Pituitary-Adrenal axis modulation of glucocorticoids in the cardiovascular system. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 2000, 57, 925–935. [Google Scholar] [CrossRef]
- Perna, G.; Iannone, G.; Alciati, A.; Caldirola, D. Are anxiety disorders associated with accelerated aging? A focus on neuroprogression. Neural Plast. 2016, 8457612. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Roberson, A.J.; McGuiness, T.M.; Fazeli, P.L. How neuroplasticity and cognitive reserve protect cognitive functioning. J. Psychosoc. Nurs. Ment. Health Serv. 2010, 48, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holtbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Lynch, M.D.J.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 2017, 9, 63–97. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut microbiota, cognitive frailty and dementia in older individuals: A systematic review. Clin. Interv. Aging 2018, 13, 1497–1511. [Google Scholar] [CrossRef]
- Celano, C.M.; Daunis, D.J.; Lokko, H.N.; Campbell, K.A.; Huffman, J.C. Anxiety disorders and cardiovascular disease. Curr. Psychiatry Resp. 2016, 18, 101. [Google Scholar] [CrossRef]
- Kuiper, J.S.; Zuidersma, M.; Oude Voshaar, R.C.; Zuidema, S.U.; van den Heuvel, E.R.; Stolk, R.P.; Smidt, N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2015, 22, 39–57. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical activity and risk of cognitive impairtment and dementia in elderly persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef]
- Braam, A.W.; Copeland, J.R.; Delespaul, P.A.; Beekman, A.T.; Como, A.; Dewey, M.; Fichter, M.; Holwerda, T.J.; Lawlor, B.A.; Lobo, A.; et al. Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: Results from the EURODEP concerted action. J. Affect. Disord. 2014, 155, 266–272. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kim, S.; Anstey, K.J. Dementia risk estimates associated with measures of depression: A systematic review and meta-analysis. BMJ Open 2015, 5, e008853. [Google Scholar] [CrossRef]
- He, Q.; Chen, X.; Wu, T.; Li, L.; Fei, X. Risk of dementia in long-term benzodiazepine users: Evidence from a meta-analysis of observational studies. J. Clin. Neurol. 2019, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lucchetta, R.C.; da Mata, B.P.M.; Mastroianni, P.C. Association between development of dementia and use of benzodiazepines: A systematic review and meta-analysis. Pharmacotherapy 2018, 38, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Mattishen, K.; Loye, Y.K.; Steel, N.; Fox, C.; Grossi, C.M.; Bennett, K.; Maidment, I.; Boustani, M.; Matthews, F.E.; et al. History of benzodiazepine prescriptions and risk of dementia: Possible bias due to prevalent users and covariate measurement timing in a nested case-control study. Am. J. Epidemiol. 2019, 188, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Penninkilampi, R.; Eslick, G.D. A systematic review and meta-analysis of the risk of dementia associated with benzodiazepines use, after controlling for protopathic bias. CNS Drugs 2018, 32, 485–497. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [PubMed]
- Therrien, Z.; Hunsley, J. Assessment of anxiety in older adults: A systematic review of commonly used measures. Aging Ment. Health 2012, 16, 1–16. [Google Scholar] [CrossRef]
| Authors, Year | Country | N | Follow-up, y. | Age, mean y. (SD) | Females, n (%) | Anxiety Measure | Dementia Criteria | Dementia Cases (n) | Risk Estimates (95% CI) | Statistical Model | Covariates | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acosta et al., 2018 [35] | Mexico | 1355 | 3 | 73.6 (6.4) | 1144 (62.7) | NPI-Q | DSM-IV | 129 | RR: 1.7 (1.2–2.5) | Poisson regression | Age, sex, education, MCI, delusions, hallucinations, depression, and aberrant motor behaviour | 7 |
| de Bruijn et al., 2014 (sample I) [13] | Netherlands | 2708 | 17 | 68.6 (8.5) | 1495 (55.2) | HADS | DSM-III-R | 358 | HR: 0.99 (0.69–1.41) | Cox regression | Age, sex, educational level (low), ApoE-ε4 and depressive symptoms. | 9 |
| de Bruijn et al., 2014 (sample II) [13] | Netherlands | 3079 | 9 | 75.5 (6.2) | 1810 (59.1) | DSM-IV | DSM-III-R | 248 | HR: 0.81 (0.50–1.30) | Cox regression | Age, sex, educational level (low), ApoE-ε4 and depressive disorder. | 8 |
| Gallacher et al., 2009 [32] | United Kingdom | 755 | 17 | NR (NR) | 0 (0) | STAI-trait scale | DSM-IV | NR | OR: 1.77 (0.31–10.2) | Logistic regression | Age, Vascular risk factors, GHQ and NART | 6 |
| Kassem et al., 2017 [34] | United States | 1425 | 5 | 82.8 (3.1) | 1425 (100) | GAS | DSM-IV | 233 | OR: 1.56 (1.07–2.26) | Logistic regression | Age, education, marital status, health behaviours, medical history, psychotropic medications, depression, poor sleep. | 6 |
| Mortamais et al. 2018 [16] | France | 5234 | 10 | 73.4 (5.2) | 3069 (58.5) | STAI-trait scale | DSM-IV | 378 | HR: 1.04 (0.81–1.32) | Cox regression | Age, sex, center, smoking habits, alcohol intake, education, living alone, body mass index, history of vascular pathology, hypertension, diabetes, dyslipidemia, incapacity, MMSE at baseline and depressive symptoms. | 7 |
| Petkus et al., 2015 [33] | Sweden | 1082 | 28 | 60.8 (11.1) | 612 (56.6) | STAI-state scale | DSM-III, IV | 172 | HR: 1.48 (1.01–2.18) | Cox mixed Effects regression | Age, sex, education, physical illness, depression (average and symptoms), neuroticism | 8 |
| Santabárbara et al., 2018 [18] | Spain | 4057 | 4.5 | 72.1 (9.1) | 2229 (54.9) | GMS-AGECAT | DSM-IV | 138 | SHR: 2.74 (1.18–6.35) | Fine and Gray Regression | Age (as timescale), sex, educational level, marital status, living alone, vascular disease, hypertension, diabetes, health status, depression and cognitive status. | 7 |
| Sutin et al., 2018 [17] | United States | 9913 | 8 | 67.03 (9.16) | 5948 (60) | Beck Anxiety Inventory | TICSm | 397 | HR: 1.16 (1.04–1.28) | Cox regression | Age, sex, race, ethnicity, education, depressive symptoms, history of a mental disorder, obesity, diabetes, hypertension, smoking and physical activity | 6 |
| b | 95% CI | p value | |
|---|---|---|---|
| Age (75 + years) * | −0.09 | (−0.77; 0.59) | 0.749 |
| Female (%) | 0.004 | (−0.011; 0.020) | 0.519 |
| Sample size (per 1000 persons) | −0.03 | (−0.10; 0.04) | 0.398 |
| Follow-up (years) | −0.006 | (−0.039; 0.027) | 0.681 |
| Methodological quality (score) | −0.09 | (−0.32; 0.14) | 0.382 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santabárbara, J.; Lipnicki, D.M.; Olaya, B.; Villagrasa, B.; Bueno-Notivol, J.; Nuez, L.; López-Antón, R.; Gracia-García, P. Does Anxiety Increase the Risk of all-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies. J. Clin. Med. 2020, 9, 1791. https://doi.org/10.3390/jcm9061791
Santabárbara J, Lipnicki DM, Olaya B, Villagrasa B, Bueno-Notivol J, Nuez L, López-Antón R, Gracia-García P. Does Anxiety Increase the Risk of all-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies. Journal of Clinical Medicine. 2020; 9(6):1791. https://doi.org/10.3390/jcm9061791
Chicago/Turabian StyleSantabárbara, Javier, Darren M. Lipnicki, Beatriz Olaya, Beatriz Villagrasa, Juan Bueno-Notivol, Lucia Nuez, Raúl López-Antón, and Patricia Gracia-García. 2020. "Does Anxiety Increase the Risk of all-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies" Journal of Clinical Medicine 9, no. 6: 1791. https://doi.org/10.3390/jcm9061791
APA StyleSantabárbara, J., Lipnicki, D. M., Olaya, B., Villagrasa, B., Bueno-Notivol, J., Nuez, L., López-Antón, R., & Gracia-García, P. (2020). Does Anxiety Increase the Risk of all-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies. Journal of Clinical Medicine, 9(6), 1791. https://doi.org/10.3390/jcm9061791







