Comparison of 99mTc-Labeled Colloid SPECT/CT and Planar Lymphoscintigraphy in Sentinel Lymph Node Detection in Patients with Melanoma: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
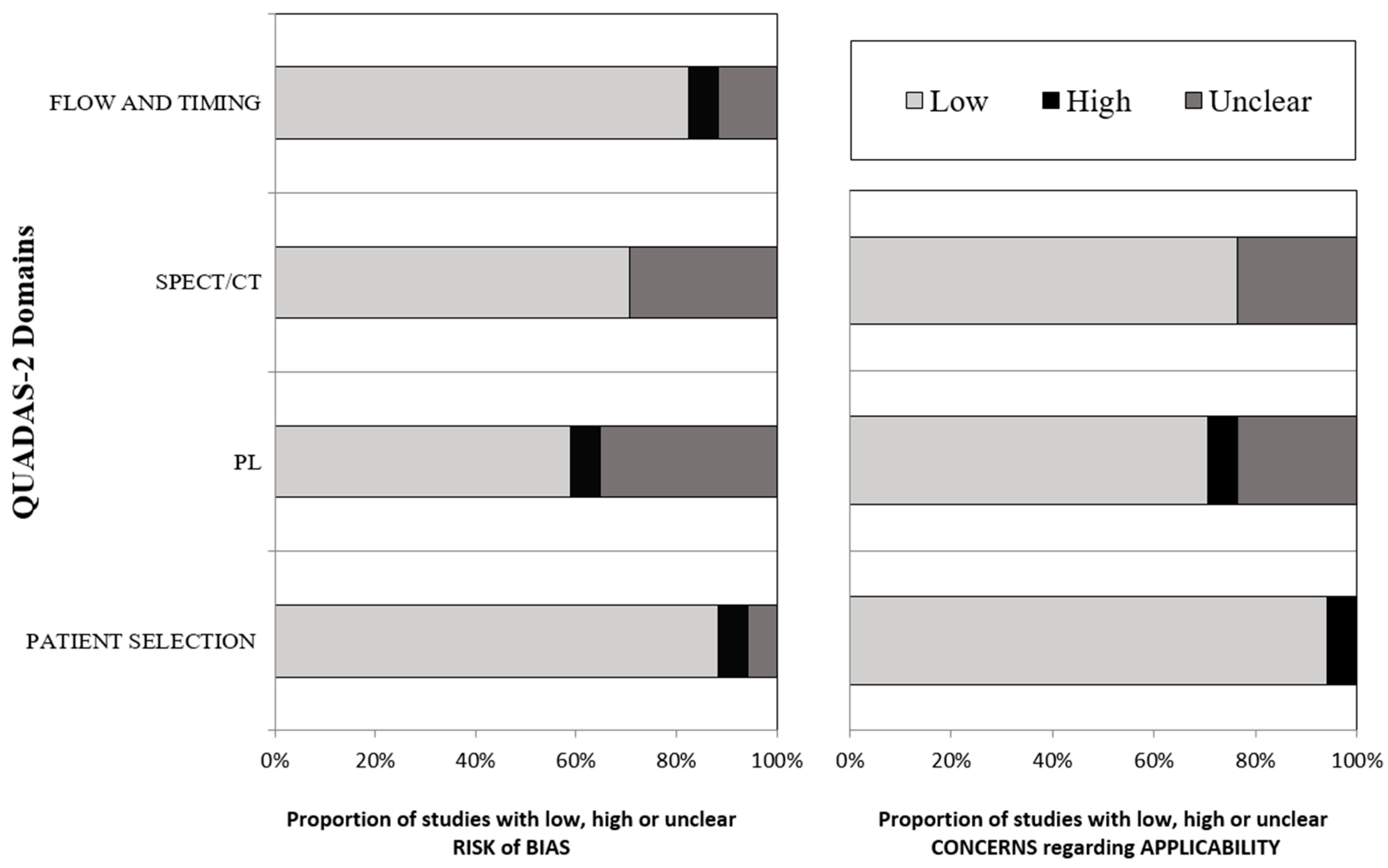
2.4. Methodological Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search and Eligibility Assessment
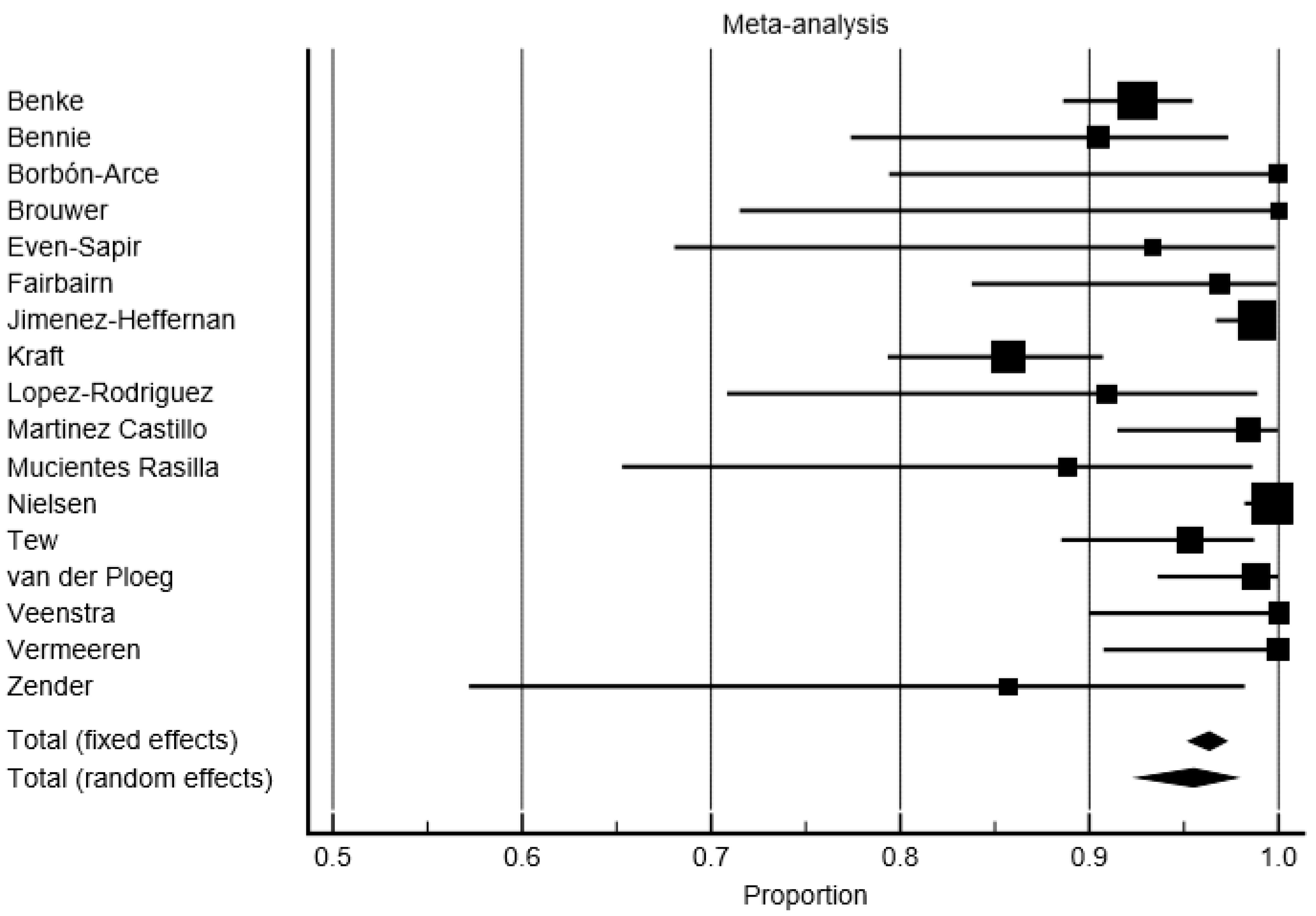
3.2. Detection Rate
3.3. Comparison of Number of SLNs Detected by SPECT/CT and PL
3.4. Average Proportion of Patients with Additional SLNs Detected by SPECT/CT or PL
3.5. Impact on Surgery of SPECT/CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moncayo, V.M.; Aarsvold, J.N.; Alazraki, N.P. Lymphoscintigraphy and sentinel nodes. J. Nucl. Med. 2015, 56, 901–907. [Google Scholar] [CrossRef]
- Arnone, G. Ruolo Clinico Dell’imaging Integrato nel Melanoma; Mattioli 1885: Fidenza, Italy, 1998. [Google Scholar]
- Sim, F.H.; Taylor, W.F.; Ivins, J.C.; Pritchard, D.J.; Soule, E.H. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer 1978, 41, 948–956. [Google Scholar] [CrossRef]
- Manca, G.; Romanini, A.; Pellegrino, D.; Borso, E.; Rondini, M.; Orlandini, C.; Zucchi, V.; Pasqualetti, F.; Mariani, G. Optimal detection of sentinel lymph node metastases by intraoperative radioactive threshold and molecular analysis in patients with melanoma. J. Nucl. Med. 2008, 49, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Essner, R.; Giuliano, A.E. Long-term clinical impact of sentinel lymph node biopsy in breast cancer and cutaneous melanoma. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 95–104. [Google Scholar] [PubMed]
- Wong, S.L.; Faries, M.B.; Kennedy, E.B.; Agarwala, S.S.; Akhurst, T.J.; Ariyan, C.; Balch, C.M.; Berman, B.S.; Cochran, A.; Delman, K.A.; et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American society of clinical oncology and society of surgical oncology clinical practice guideline update. J. Clin. Oncol. 2018, 36, 399–413. [Google Scholar] [CrossRef]
- Manca, G.; Rubello, D.; Romanini, A.; Mariani, G. False-negative sentinel lymph node biopsy in melanoma patients. Nucl. Med. Commun. 2014, 35, 989–994. [Google Scholar] [CrossRef]
- Testori, A.; De Salvo, G.L.; Montesco, M.C.; Trifiro, G.; Mocellin, S.; Landi, G.; Macripo, G.; Carcoforo, P.; Ricotti, G.; Giudice, G.; et al. Clinical considerations on sentinel node biopsy in melanoma from an italian multicentric study on 1313 patients (solism-imi). Ann. Surg. Oncol. 2009, 16, 2018–2027. [Google Scholar] [CrossRef]
- Nowecki, Z.I.; Rutkowski, P.; Nasierowska-Guttmejer, A.; Ruka, W. Survival analysis and clinicopathological factors associated with false-negative sentinel lymph node biopsy findings in patients with cutaneous melanoma. Ann. Surg. Oncol. 2006, 13, 1655–1663. [Google Scholar] [CrossRef]
- Cascinelli, N.; Bombardieri, E.; Bufalino, R.; Camerini, T.; Carbone, A.; Clemente, C.; Lenisa, L.; Mascheroni, L.; Maurichi, A.; Pennacchioli, E.; et al. Sentinel and nonsentinel node status in stage ib and ii melanoma patients: Two-step prognostic indicators of survival. J. Clin. Oncol. 2006, 24, 4464–4471. [Google Scholar] [CrossRef]
- Leong, S.P.; Tseng, W.W. Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: Clinical significance and biologic implications. CA Cancer J. Clin. 2014, 64, 195–206. [Google Scholar] [CrossRef]
- Salvianti, F.; Massi, D.; De Giorgi, V.; Gori, A.; Pazzagli, M.; Pinzani, P. Evaluation of the liquid biopsy for the detection of brafv600e mutation in metastatic melanoma patients. Cancer Biomark. 2019, 26, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Hoon, D.S. Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients. Mol. Oncol. 2016, 10, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Loeian, M.S.; Mehdi Aghaei, S.; Farhadi, F.; Rai, V.; Yang, H.W.; Johnson, M.D.; Aqil, F.; Mandadi, M.; Rai, S.N.; Panchapakesan, B. Liquid biopsy using the nanotube-ctc-chip: Capture of invasive ctcs with high purity using preferential adherence in breast cancer patients. Lab Chip 2019, 19, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Trainor, P.J.; Lambert, C.; Kloecker, G.; Wickstrom, E.; Rai, S.N.; Panchapakesan, B. Static micro-array isolation, dynamic time series classification, capture and enumeration of spiked breast cancer cells in blood: The nanotube-ctc chip. Nanotechnology 2016, 27, 44lt03. [Google Scholar] [CrossRef]
- Hoshimoto, S.; Shingai, T.; Morton, D.L.; Kuo, C.; Faries, M.B.; Chong, K.; Elashoff, D.; Wang, H.J.; Elashoff, R.M.; Hoon, D.S. Association between circulating tumor cells and prognosis in patients with stage iii melanoma with sentinel lymph node metastasis in a phase iii international multicenter trial. J. Clin. Oncol. 2012, 30, 3819–3826. [Google Scholar] [CrossRef]
- Nakagawa, T.; Martinez, S.R.; Goto, Y.; Koyanagi, K.; Kitago, M.; Shingai, T.; Elashoff, D.A.; Ye, X.; Singer, F.R.; Giuliano, A.E.; et al. Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clin. Cancer Res. 2007, 13, 4105–4110. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, H.; Choi, J.-W.; Oh, H.E.; Kim, Y.-S. Liquid biopsy prediction of axillary lymph node metastasis, cancer recurrence, and patient survival in breast cancer: A meta-analysis. Medicine 2018, 97, e12862. [Google Scholar] [CrossRef]
- Mariani, G.; Erba, P.; Manca, G.; Villa, G.; Gipponi, M.; Boni, G.; Buffoni, F.; Suriano, S.; Castagnola, F.; Bartolomei, M.; et al. Radioguided sentinel lymph node biopsy in patients with malignant cutaneous melanoma: The nuclear medicine contribution. J. Surg. Oncol. 2004, 85, 141–151. [Google Scholar] [CrossRef]
- Perissinotti, A.; Rietbergen, D.D.; Vidal-Sicart, S.; Riera, A.A.; Olmos, R.A.V. Melanoma & nuclear medicine: New insights & advances. Melanoma Manag. 2018, 5, MMT06. [Google Scholar]
- Quartuccio, N.; Siracusa, M.; Pappalardo, M.; Arnone, A.; Arnone, G. Sentinel node identification in melanoma: Current clinical impact, new emerging spect radiotracers and technological advancements. An update of the last decade. Curr. Radiopharm. 2019, 13, 32–41. [Google Scholar] [CrossRef]
- Pappalardo, M.; Cheng, M.H. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J. Surg. Oncol. 2020, 121, 37–47. [Google Scholar] [CrossRef]
- Pappalardo, M.; Lin, C.; Ho, O.A.; Kuo, C.F.; Lin, C.Y.; Cheng, M.H. Staging and clinical correlations of lymphoscintigraphy for unilateral gynecological cancer-related lymphedema. J. Surg. Oncol. 2019, 121, 422–434. [Google Scholar] [CrossRef]
- Bluemel, C.; Herrmann, K.; Giammarile, F.; Nieweg, O.E.; Dubreuil, J.; Testori, A.; Audisio, R.A.; Zoras, O.; Lassmann, M.; Chakera, A.H.; et al. Eanm practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1750–1766. [Google Scholar] [CrossRef]
- Hoogendam, J.P.; Veldhuis, W.B.; Hobbelink, M.G.; Verheijen, R.H.; van den Bosch, M.A.; Zweemer, R.P. 99mtc SPECT/CT versus planar lymphoscintigraphy for preoperative sentinel lymph node detection in cervical cancer: A systematic review and metaanalysis. J. Nucl. Med. 2015, 56, 675–680. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Int. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Benke, M.; Wocial, K.; Lewandowska, W.; Rutkowski, P.; Teterycz, P.; Jarek, P.; Dedecjus, M. Value of planar lymphoscintigraphy (pl) versus spect/ct in evaluation of sentinel lymph node in trunk melanoma—One center, large series retrospective study. Nucl. Med. Rev. Cent. East. Eur. 2018, 21, 79–84. [Google Scholar] [CrossRef]
- Bennie, G.; Vorster, M.; Buscombe, J.; Sathekge, M. The added value of a single-photon emission computed tomography-computed tomography in sentinel lymph node mapping in patients with breast cancer and malignant melanoma. World J. Nucl. Med. 2015, 14, 41–46. [Google Scholar]
- Borbon-Arce, M.; Brouwer, O.R.; van den Berg, N.S.; Matheron, H.; Klop, W.M.; Balm, A.J.; van Leeuwen, F.W.; Valdes-Olmos, R.A. An innovative multimodality approach for sentinel node mapping and biopsy in head and neck malignancies. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 274–279. [Google Scholar]
- Brouwer, O.R.; Klop, W.M.; Buckle, T.; Vermeeren, L.; van den Brekel, M.W.; Balm, A.J.; Nieweg, O.E.; Valdes Olmos, R.A.; van Leeuwen, F.W. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann. Surg. Oncol. 2012, 19, 1988–1994. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Lerman, H.; Lievshitz, G.; Khafif, A.; Fliss, D.M.; Schwartz, A.; Gur, E.; Skornick, Y.; Schneebaum, S. Lymphoscintigraphy for sentinel node mapping using a hybrid spect/ct system. J. Nucl. Med. 2003, 44, 1413–1420. [Google Scholar] [PubMed]
- Fairbairn, N.; Munson, C.; Khan, Z.A.; Butterworth, M. The role of hybrid SPECT/CT for lymphatic mapping in patients with melanoma. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Heffernan, A.; Ellmann, A.; Sado, H.; Huic, D.; Bal, C.; Parameswaran, R.; Giammarile, F.; Pruzzo, R.; Kostadinova, I.; Vorster, M.; et al. Results of a prospective multicenter international atomic energy agency sentinel node trial on the value of spect/ct over planar imaging in various malignancies. J. Nucl. Med. 2015, 56, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Klode, J.; Poeppel, T.; Boy, C.; Mueller, S.; Schadendorf, D.; Korber, A.; Stoffels, I.; Dissemond, J. Advantages of preoperative hybrid spect/ct in detection of sentinel lymph nodes in cutaneous head and neck malignancies. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1213–1221. [Google Scholar] [CrossRef]
- Kraft, O.; Havel, M. Localisation of sentinel lymph nodes in patients with melanomas by planar lymphoscintigraphic and hybrid spect/ct imaging. Nucl. Med. Rev. Cent. East. Eur. 2012, 15, 101–107. [Google Scholar]
- Kraft, O.; Havel, M. Detection of sentinel lymph nodes by spect/ct and planar scintigraphy: The influence of age, gender and bmi. J. Biomed. Graph. Comput. 2012, 2, 11. [Google Scholar] [CrossRef][Green Version]
- López-Rodríguez, E.; García-Gómez, F.J.; Álvarez-Pérez, R.M.; Martínez-Castillo, R.; Borrego-Dorado, I.; Fernández-Ortega, P.; Zulueta-Dorado, T. Role of spect-ct in sentinel lymph node biopsy in patients diagnosed with head and neck melanoma. Rev. Esp. Med. Nucl. Imagen Mol. 2016, 35, 22–28. [Google Scholar] [CrossRef]
- Martínez Castillo, R.; Fernández López, R.; Acevedo Bañez, I.; Álvarez Pérez, R.M.; García Solis, D.; Vázquez Albertino, R.; Fernández Ortega, P. Utilidad de la tomografía de emisión de fotón único-tomografía computarizada en la biopsia selectiva del ganglio centinela en pacientes con melanoma. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 129–135. [Google Scholar] [CrossRef]
- Mucientes Rasilla, J.; Cardona Arboniés, J.; Delgado Bolton, R.; Izarduy Pereyra, L.; Salazar Andía, G.; Prieto Soriano, A.; Anula Fernández, R.; Mayol Martínez, J.; Lapeña Gutiérrez, L.; González Maté, A.; et al. Spect-tac en la localización del ganglio centinela en pacientes con melanoma. Rev. Esp. Med. Nucl. Imagen Mol. 2009, 28, 229–234. [Google Scholar] [CrossRef]
- Nielsen, K.R.; Chakera, A.H.; Hesse, B.; Scolyer, R.A.; Stretch, J.F.; Thompson, J.F.; Nielsen, M.B.; Uren, R.F.; Oturai, P.S. The diagnostic value of adding dynamic scintigraphy to standard delayed planar imaging for sentinel node identification in melanoma patients. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1999–2004. [Google Scholar] [CrossRef]
- Tew, K.; Farlow, D. Utility of hybrid spect/ct in primary melanoma lymphoscintigraphy: A retrospective case series. J. Med. Imaging Radiat. Oncol. 2017, 61, 204–211. [Google Scholar] [CrossRef]
- Trinh, B.B.; Chapman, B.C.; Gleisner, A.; Kwak, J.J.; Morgan, R.; McCarter, M.D.; Gajdos, C.; Kounalakis, N. Spect/ct adds distinct lymph node basins and influences radiologic findings and surgical approach for sentinel lymph node biopsy in head and neck melanoma. Ann. Surg. Oncol. 2018, 25, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, I.M.; Valdes Olmos, R.A.; Kroon, B.B.; Wouters, M.W.; van den Brekel, M.W.; Vogel, W.V.; Hoefnagel, C.A.; Nieweg, O.E. The yield of spect/ct for anatomical lymphatic mapping in patients with melanoma. Ann. Surg. Oncol. 2009, 16, 1537–1542. [Google Scholar] [CrossRef]
- Veenstra, H.J.; Vermeeren, L.; Olmos, R.A.; Nieweg, O.E. The additional value of lymphatic mapping with routine spect/ct in unselected patients with clinically localized melanoma. Ann. Surg. Oncol. 2012, 19, 1018–1023. [Google Scholar] [CrossRef]
- Vermeeren, L.; Valdes Olmos, R.A.; Klop, W.M.; van der Ploeg, I.M.; Nieweg, O.E.; Balm, A.J.; van den Brekel, M.W. Spect/ct for sentinel lymph node mapping in head and neck melanoma. Head Neck 2011, 33, 1–6. [Google Scholar] [CrossRef]
- Zender, C.; Guo, T.; Weng, C.; Faulhaber, P.; Rezaee, R. Utility of spect/ct for periparotid sentinel lymph node mapping in the surgical management of head and neck melanoma. Am. J. Otolaryngol. 2014, 35, 12–18. [Google Scholar] [CrossRef]
- Manca, G.; Rubello, D.; Romanini, A.; Boni, G.; Chiacchio, S.; Tredici, M.; Mazzarri, S.; Duce, V.; Colletti, P.M.; Volterrani, D.; et al. Sentinel lymph node mapping in melanoma: The issue of false-negative findings. Clin. Nucl. Med. 2014, 39, e346–e354. [Google Scholar] [CrossRef]
- Stoffels, I.; Boy, C.; Poppel, T.; Kuhn, J.; Klotgen, K.; Dissemond, J.; Schadendorf, D.; Klode, J. Association between sentinel lymph node excision with or without preoperative spect/ct and metastatic node detection and disease-free survival in melanoma. JAMA 2012, 308, 1007–1014. [Google Scholar] [CrossRef]
- Manca, G.; Mazzarri, S.; Rubello, D.; Tardelli, E.; Delgado-Bolton, R.C.; Giammarile, F.; Roncella, M.; Volterrani, D.; Colletti, P.M. Radioguided occult lesion localization: Technical procedures and clinical applications. Clin. Nucl. Med. 2017, 42, e498–e503. [Google Scholar] [CrossRef]
- Hasegawa, B.H.; Wong, K.H.; Iwata, K.; Barber, W.C.; Hwang, A.B.; Sakdinawat, A.E.; Ramaswamy, M.; Price, D.C.; Hawkins, R.A. Dual-modality imaging of cancer with spect/ct. Technol. Cancer Res. Treat. 2002, 1, 449–458. [Google Scholar] [CrossRef]
- Garau, L.M.; Rubello, D.; Morganti, R.; Boni, G.; Volterrani, D.; Colletti, P.M.; Manca, G. Sentinel lymph node biopsy in small papillary thyroid cancer: A meta-analysis. Clin. Nucl. Med. 2019, 44, 107–118. [Google Scholar] [CrossRef]
- Tardelli, E.; Mazzarri, S.; Rubello, D.; Gennaro, M.; Fantechi, L.; Duce, V.; Romanini, A.; Chondrogiannis, S.; Volterrani, D.; Colletti, P.M.; et al. Sentinel lymph node biopsy in cutaneous melanoma: Standard and new technical procedures and clinical advances. A systematic review of the literature. Clin. Nucl. Med. 2016, 41, e498–e507. [Google Scholar] [CrossRef]
- Duce, V.; Manca, G.; Mazzarri, S.; Lorenzetti, F.; Colletti, P.M.; Rubello, D.; Volterrani, D. Sentinel node mapping in melanoma of the back: Spect/ct helps discriminate “true” and “false” in-transit lymph nodes. Clin. Nucl. Med. 2016, 41, e66–e67. [Google Scholar] [CrossRef]
- Stoffels, I.; Muller, M.; Geisel, M.H.; Leyh, J.; Poppel, T.; Schadendorf, D.; Klode, J. Cost-effectiveness of preoperative spect/ct combined with lymphoscintigraphy vs. Lymphoscintigraphy for sentinel lymph node excision in patients with cutaneous malignant melanoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1723–1731. [Google Scholar] [CrossRef]
- Valdes Olmos, R.A.; Vidal-Sicart, S.; Manca, G.; Mariani, G.; Leon-Ramirez, L.F.; Rubello, D.; Giammarile, F. Advances in radioguided surgery in oncology. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 247–270. [Google Scholar]


| Authors | Year | Country | Tracer | Tracer Activity (MBq) | Tracer Injections | Time Interval (Tracer Injection–SPECT/CT) in min | Anatomical Region | BMI (Mean ± SD) | Breslow Thickness in mm | Number of Patients | M | F | Age (Mean ± SD/Median; Range) in Years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benke | 2018 | Poland | 99mTc-Nanocoll | 5–20 | 2–6 | 60–180 | Trunk | NR | median: 2.0 ± 3.13 | 255 | 160 | 95 | median: 61 (17–88) |
| Bennie | 2015 | South Africa | 99mTc-Nanocoll. 99mTc-Sentiscint | NR | 4 | 60 | Trunk, upper limb, lower limb | 28.5 (n = 23) | NR | 42 | 22 | 20 | mean: M: 50. F:52 |
| Borbón-Arce | 2014 | Spain | 99mTc-Nanocoll | Median: 85 (66–158) | 3–4 | 120 | Head and neck | NR | mean: 2.7 (1.0–6.0); median: 2.0 | 16 | 9 | 7 | mean: 58 (41–77) |
| Brouwer | 2012 | The Netherlands | ICG–99mTc-nanocolloid | 70 | 4 | 120 | Head and neck | NR | 2.7 | 11 | 5 | 6 | mean: 54 (32–75) |
| Even-Sapir | 2003 | Israel | 99mTc-rhenium colloid | 74 | 4 | NR | Trunk, head and neck, upper limb, lower limb, penis | NR | NR | 15 * | 12 | 3 | mean: 57.6 (24–81) |
| Fairbairn | 2013 | Scotland | 99mTc-Nanocoll | 20 or 40 | 2 or 4 | 60 | Trunk, head and neck, upper limb, lower limb | NR | mean: 2.03 ± 2.26 (0.51–12); median: 1.4 | 32 | 12 | 20 | 55 ± 13.66 (17–77) |
| Jimenez-Heffernan | 2015 | Spain | 99mTc-Nanocoll | Mean: 50 ± 27.4 | 1–6 | NR | All regions | NR | 0.75–4 | 262 | 117 | 145 | 53.9 ± 15.2 |
| Klode # | 2011 | Germany | 99mTc-Nanocoll | 16 or 80 | 4 | 120 | Head and neck | NR | 2.26 (1–7.5); median: 1.7 | 34 | NR | NR | NR |
| Kraft # | 2012 | Czech Republic | 99mTc-Nanocis. 99mTc-Nanocoll, 99mTc-SentiScint, 99mTc-NanoAlbumon | 100 | 4 | NR | Trunk, head and neck, upper limb, lower limb | 29.4 ± 12.5 | NR | 113 | 59 | 54 | mean: 57.6 (11–87) |
| Kraft | 2012 | Czech Republic | 99mTc-Nanocis, 99mTc-Nanocoll, 99mTc-SentiScint, 99mTc-NanoAlbumon. | 100 | 4 | NR | All regions | 28.4 ± 5.1 | NR | 161 | 87 | 74 | 57.1 ± 14.8 |
| Lopez-Rodriguez | 2016 | Spain | 99mTc-Nanocoll | 74 | At least 4 | NR | Head and neck | NR | mean: 2.96 (1–6) | 22 | 13 | 9 | mean: 55 (24–83) |
| Martinez Castillo | 2014 | Spain | 99mTc-Nanocoll | 74 | 4 | NR | Trunk, head and neck, upper limb, lower limb | NR | NR | 63 | 32 | 31 | mean: 55 (25–88) |
| Mucientes Rasilla | 2009 | Spain | 99mTc-nanocolloids (not specificied) | 74 | 4 | NR | Trunk, head and neck, upper limb, lower limb | NR | mean: 1.75 ± 1.15 (0.47–4.45) | 18 | 8 | 10 | 57.1 ± 20.1 (14–83) |
| Nielsen | 2011 | Denmark | 99mTc-antimony sulphide colloid | 40–80 | 1–2 | NR | Trunk, head and neck, upper limb, lower limb | NR | 1–4 | 307 | 177 | 130 | 60 ± 16.9 |
| Tew | 2017 | Australia | 99mTc-antimony sulphide colloid | 8 or 26 MBq per injection | up to 4 | NR | Trunk, head and neck, upper limb, lower limb | NR | NR | 86 | 53 | 33 | mean: 58.8 (22–84) |
| Trinh # | 2018 | USA | 99mTc-filtered sulphur colloid | Mean: 37 ± 10% | NR | NR | Head and neck | NR | NR | 118 | 87 | 31 | 58.9 ± 16.7; median: 61 (16–91 |
| van der Ploeg | 2009 | The Netherlands | 99mTc-Nanocoll | 80 | 4 | 120 | Trunk, head and neck, upper limb, lower limb | NR | NR | 85 | NR | NR | mean: 54 |
| Veenstra | 2012 | The Netherlands | 99mTc-Nanocoll | 69.8 (mean) | NR | 120 | Trunk, head and neck, upper limb, lower limb | NR | at least 1; or less if Clark level = 4 | 35 | 14 | 21 | mean: 60 |
| Vermeeren | 2011 | The Netherlands | 99mTc-Nanocoll | 71 (mean) | 4 | 120 | Head and neck | NR | mean: 2.9 (0.8–7.8); median: 2.2 | 38 | 30 | 8 | mean: 53 (24–86) |
| Zender | 2014 | USA | 99mTc-microfiltered sulfur colloid | 18–37 | NR | NR | Head and neck | NR | mean: 2.68 (1.13–7.0) | 14 | 9 | 5 | mean: 65.43 (31–89) |
| Authors | Number of Patients | DR of SPECT/CT (%) | DR of PL (%) | Number of SLNs Detected by SPECT/CT | Number of SLNs Detected by PL | % of Patients with Additional SLNs in SPECT/CT | % of Patients with Change in Surgical Plan Based on SPECT/CT Findings |
|---|---|---|---|---|---|---|---|
| Benke | 255 | 99.21 | 92.54 | 497 | 419 | 15.69 | NR |
| Bennie | 42 | 100 | 90.48 | NR | NR | 9.52 | 40.48 |
| Borbón-Arce | 16 | 100 | 100 | 66 | 55 | NR | NR |
| Brouwer | 11 | 100 | 100 | 27 | 27 | 0.00 | NR |
| Even-Sapir | 15 * | 100 | 93.33 | NR | NR | NR | 35.71 |
| Fairbairn | 32 | 96.88 | 96.88 | 67 | 65 | 18.75 | 37.50 |
| Jimenez-Heffernan | 262 | 99.24 | 98.85 | 602 | 532 | 20.20 | 37 |
| Klode | 34 * | NR | NR | NR | NR | NR | 26.47 |
| Kraft | 113 | NA | NA | NA | NA | 43.36 | NR |
| Kraft | 161 | 92.50 | 85.70 | 487 | 351 | NR | NR |
| Lopez-Rodriguez | 22 | 95.45 | 90.90 | NR | NR | 27.27 | 63.63 |
| Martinez Castillo | 63 | 100 | 98.41 | 266 | 222 | 42.86 | 21.20 |
| Mucientes Rasilla | 18 | 100 | 88.88 | 31 | 27 | 16.67 | 22.22 |
| Nielsen | 307 | 100 | 99.67 | 709 ** | 692 ** | NR | NR |
| Tew | 86 | 94.20 | 95.35 | 143 | 144 | 0.00 | 39.00 |
| Trinh | 118 | NR | NR | 268 | 234 | NR | NR |
| van der Ploeg | 85 | 100 | 98.82 | 226 | 214 | 8.24 | 35.29 |
| Veenstra | 35 | 100 | 100 | 77 | 69 | 20.00 | 31.42 |
| Vermeeren | 38 | 100 | 100 | 100 | 94 | 15.79 | 55 *** |
| Zender | 14 | 92.86 | 85.71 | 21 | 17 | 28.57 | 57.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, N.; Garau, L.M.; Arnone, A.; Pappalardo, M.; Rubello, D.; Arnone, G.; Manca, G. Comparison of 99mTc-Labeled Colloid SPECT/CT and Planar Lymphoscintigraphy in Sentinel Lymph Node Detection in Patients with Melanoma: A Meta-Analysis. J. Clin. Med. 2020, 9, 1680. https://doi.org/10.3390/jcm9061680
Quartuccio N, Garau LM, Arnone A, Pappalardo M, Rubello D, Arnone G, Manca G. Comparison of 99mTc-Labeled Colloid SPECT/CT and Planar Lymphoscintigraphy in Sentinel Lymph Node Detection in Patients with Melanoma: A Meta-Analysis. Journal of Clinical Medicine. 2020; 9(6):1680. https://doi.org/10.3390/jcm9061680
Chicago/Turabian StyleQuartuccio, Natale, Ludovico Maria Garau, Annachiara Arnone, Marco Pappalardo, Domenico Rubello, Gaspare Arnone, and Gianpiero Manca. 2020. "Comparison of 99mTc-Labeled Colloid SPECT/CT and Planar Lymphoscintigraphy in Sentinel Lymph Node Detection in Patients with Melanoma: A Meta-Analysis" Journal of Clinical Medicine 9, no. 6: 1680. https://doi.org/10.3390/jcm9061680
APA StyleQuartuccio, N., Garau, L. M., Arnone, A., Pappalardo, M., Rubello, D., Arnone, G., & Manca, G. (2020). Comparison of 99mTc-Labeled Colloid SPECT/CT and Planar Lymphoscintigraphy in Sentinel Lymph Node Detection in Patients with Melanoma: A Meta-Analysis. Journal of Clinical Medicine, 9(6), 1680. https://doi.org/10.3390/jcm9061680







