Interaction between the Number of Chemotherapy Cycles and Brachytherapy Dose/Volume Parameters in Locally Advanced Cervical Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Treatments
2.3. Follow-Up and Statistical Analysis
3. Results
3.1. Patients, Tumors and Treatments
3.2. Patterns of Relapse
3.3. Survival and Disease Control Analysis
3.4. Subgroup Analysis and Interaction with Brachytherapy Parameters
3.5. Propensity Score Matching.
3.6. Radiation-Induced Toxicities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Thomas, G. Improved treatment for cervical cancer–concurrent chemotherapy and radiotherapy. N. Engl. J. Med. 1999, 340, 1198–2000. [Google Scholar] [CrossRef] [PubMed]
- Pearcey, R.; Brundage, M.; Drouin, P.; Jeffrey, J.; Johnston, D.; Lukka, H.; MacLean, G.; Souhami, L.; Stuart, G.; Tu, D. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J. Clin. Oncol. 2002, 20, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S.Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: Individual patient data meta-analysis. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef]
- Rydzewska, L.; Tierney, J.; Vale, C.L.; Symonds, P.R. Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Cochrane Database Syst. Rev. 2010, 1, CD008285. [Google Scholar]
- Dimopoulos, J.C.; Potter, R.; Lang, S.; Fidarova, E.; Georg, P.; Dörr, W.; Kirisits, C. Dose–effect relationship for local control of cervical cancer by magnetic resonance image guided brachytherapy. Radiother. Oncol. 2009, 93, 311–315. [Google Scholar] [CrossRef]
- Lindegaard, J.C.; Fokdal, L.U.; Nielsen, S.K.; Juul-Christensen, J.; Tanderup, K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol. 2013, 52, 1510–1519. [Google Scholar] [CrossRef]
- Nomden, C.N.; de Leeuw, A.A.; Roesink, J.M.; Tersteeg, R.J.; Moerland, M.A.; Witteveen, P.O.; Schreuder, H.W.; van Dorst, E.B.; Jürgenliemk-Schulz, I.M. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: A single institution experience. Radiother. Oncol. 2013, 107, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Magné, N.; Dumas, I.; Messai, T.; Vicenzi, L.; Gillion, N.; Morice, P.; Haie-Meder, C. Physics and clinical contribution with MRI-based Pulsed dose rate brachytherapy in patients with cervix carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.P.; Franckena, M.; Kirchheiner, K.; Sturdza, A.; Georg, P.; Dörr, W.; Pötter, R. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecol. Oncol. 2014, 133, 256–262. [Google Scholar] [CrossRef]
- Magné, N.; Chargari, C.; SanFilippo, N.; Messai, T.; Gerbaulet, A.; Haie-Meder, C. Technical aspects and perspectives of the vaginal mold applicator for brachytherapy of gynecological malignancies. Brachytherapy 2009, 9, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Liu, M.; Rogers, S.; Klingbiel, D.; Siebenhüner, A.; Singh, S.; Bodis, S. Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2017, 145, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Charra-Brunaud, C.; Harter, V.; Delannes, M.; Haie-Meder, C.; Quetin, P.; Kerr, C.; Castelain, B.; Thomas, L.; Peiffert, D. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother. Oncol. 2012, 103, 305–313. [Google Scholar] [CrossRef]
- Mazeron, R.; Castelnau-Marchand, P.; Dumas, I.; del Campo, E.R.; Kom, L.K.; Martinetti, F.; Farha, G.; Tailleur, A.; Morice, P.; Chargari, C.; et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother. Oncol. 2015, 114, 257–263. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Available online: https://www.embracestudy.dk/ (accessed on 27 May 2020).
- Chargari, C.; Mazeron, R.; Escande, A.; Maroun, P.; Dumas, I.; Martinetti, F.; Tafo-Guemnie, A.; Deutsch, E.; Morice, P.; Haie-Meder, C. Image-guided adaptive brachytherapy in cervical cancer: Patterns of relapse by brachytherapy planning parameters. Brachytherapy 2016, 15, 456–462. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The concurrent chemoradiation paradigm--general principles. Nat. Clin. Pract. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Escande, A.; Haie-Meder, C.; Maroun, P.; Gouy, S.; Mazeron, R.; Leroy, T.; Bentivegna, E.; Morice, P.; Deutsch, E.; Chargari, C. Neutrophilia in locally advanced cervical cancer: A novel biomarker for image-guided adaptive brachytherapy? Oncotarget 2016, 7, 74886–74994. [Google Scholar] [CrossRef]
- Mazeron, R.; Castelnau-Marchand, P.; Escande, A.; Del Rivin Campo, E.; Maroun, P.; Lefkopoulos, D.; Chargari, C.; Haie-Meder, C. Tumor dose-volume response in image-guided adaptive brachytherapy for cervical cancer: A meta-regression analysis. Brachytherapy 2016, 15, 537–542. [Google Scholar] [CrossRef]
- Brizel, D.M.; Esclamado, R. Concurrent chemoradiotherapy for locally advanced nonmetastatic, squamous cell carcinoma of the head and neck: Consensus, controversy, and conundrum. J. Clin. Oncol. 2006, 24, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; De RIdder, M.; Navran, A.; Klop, W.M.; de Boer, J.P.; Tesselaar, M.E. The impact of cumulative dose of cisplatin on outcome of patients with head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2017, 274, 3757–3765. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01414608 (accessed on 27 May 2020).
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guérin, F.; Dumas, I.; Bossi, A.; Morice, P.; Viswanathan, A.N.; et al. Brachytherapy: An overview for clinicians. CA Cancer J. Clin. 2019, 69, 386–401. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number (%) or Median (IQ) | p-Value | |
|---|---|---|---|
| 4 Cycles Delivered | 5 Cycles Delivered | ||
| Patients | |||
| Number of patients | 55 | 154 | |
| Age (years) | 47.0 (42.0–53.7) | 47.4 (39.8–53.5) | 0.690 |
| Tobacco use | 20/55 (36.4) | 51/154 (33.1) | 0.740 |
| PS | |||
| 0 | 23/55 (41.9) | 92/154 (59.7) | 0.055 |
| 1 | 28/55 (50.9) | 57/154 (37.0) | |
| 2 | 4/55 (7.3) | 5/154 (3.2) | |
| Tumors | |||
| SCC | 48/55 (87.3) | 124/154 (80.5) | 0.308 |
| Poor differentiation | 16/55 (29.1) | 30/154 (19.5) | 0.184 |
| FIGO stage | |||
| IB1 | 0/55 (0) | 4/154 (2.6) | 0.011 * |
| IB2 | 17/55 (30.9) | 42/154 (27.3) | |
| IIA | 0/55 (0) | 11/154 (7.1) | |
| IIB | 21/55 (38.2) | 72/154 (46.8) | |
| IIIA | 0/55 (0) | 4/154 (2.6) | |
| IIIB | 9/55 (16.4) | 6/154 (3.9) | |
| IVA | 3/55 (5.5) | 3/154 (1.9) | |
| IVB | 5/55 (9.1) | 12/154 (7.8) | |
| Pelvic nodal metastases | 23/55 (41.8) | 60/154 (40) | 0.640 |
| Neutrophilia at diagnosis | 13/43 (30.2) | 29/132 (22.0) | 0.306 |
| Treatments | |||
| Cycle during IGABT | 21/55 (38.2) | 80/154 (51.9) | 0.086 |
| OTT (days) | 47 (43–52) | 48 (44–52) | 0.164 |
| CTVHR volume (cm3) | 26.6 (18.5–40.9) | 21.4 (16.3–28.1) | 0.014 * |
| D90CTVIR (Gy EQD2) | 67.2 (60.8–70.0) | 68.5 (66.2–72.0) | 0.006 * |
| D90CTVHR (Gy EQD2) | 78.7 (74.0–86.1) | 82.8 (74.9–90.4) | 0.014 * |
| TRAK | 1.80 (1.58–1.96) | 1.74 (1.57–1.93) | 0.328 |
| 2 fractions used | 12/55 (21.1) | 15/154 (9.7) | 0.031 * |
| Number of Cycles Delivered | 4 Cycles (n = 55) | 5 Cycles (n = 154) | p-Value |
| Death (%) | 24 (43.6) | 27 (17.5) | 0.000 * |
| Relapse (%) | 28 (50.9) | 38 (24.7) | 0.000 * |
| Local relapse (%) | 12 (21.8) | 12 (7.8) | 0.012 * |
| Regional relapse (%) | 15 (27.3) | 22 (14.3) | 0.039 * |
| Loco-regionale relapse (%) | 20 (36.4) | 28 (18.2) | 0.009 * |
| Metastasis relapse (%) | 18 (32.7) | 17 (11.0) | 0.001 * |
| Number of Cycles Delivered | 4 Cycles | 5 Cycles | HR (95% CI) |
| 3-y OS (SE) | 62.0% (6.7) | 87.8% (2.7) | 0.340 (0.196–0.589) * |
| 3-y PFS (SE) | 53.4% (6.8) | 78.8% (3.3) | 0.388 (0.242–0.624) * |
| 3-y LFS (SE) | 58.9% (6.8) | 84.4% (3.0) | 0.345 (0.202–0.589) * |
| 3-y RFS (SE) | 59.2% (6.8) | 80.5% (3.3) | 0.405 (0.241–0.682) * |
| 3-y LFRFS (SE) | 55.9% (6.8) | 80.0% (3.3) | 0.389 (0.237–0.637) * |
| 3-y DMFS (SE) | 56.3% (6.9) | 81.9% (3.2) | 0.346 (0.208–0.573) * |
| 3-y LC (SE) | 77.2% (5.8) | 93.9% (2.0) | 0.306 (0.137–0.681) * |
| 3-y LRC (SE) | 62.8% (6.6) | 84.6% (3.0) | 0.429 (0.242–0.762) * |
| 3-y DMC (SE) | 69.1% (6.7) | 90.5% (2.4) | 0.280 (0.144–0.545) * |
| Toxicity | 4 Cycles | 5 Cycles | p-Value |
| Grade 2+ late toxicities (%) | 23 (41.8) | 77 (50.0) | 0.346 |
| Grade 3+ late toxicities (%) | 2 (3.6) | 9 (5.8) | 0.731 |
| FIGO < IIIA | FIGO ≥ IIIA | N0 | N1 | Neutrophils ≤ 7500/mm3 | |
| OS | 0.278 (0.142–0.546) * | 0.704 (0.271–10.827) | 0.373 (0.169–0.823)* | 0.329 (0.152–0.710) * | 0.285 (0.126–0.646) * |
| PFS | 0.344 (0.19–0.617) * | 0.682 (0.310–10.498) | 0.383 (0.192–0.765)* | 0.412 (0.215–0.791) * | 0.415 (0.214–0.808) |
| LFS | 0.319 (0.165–0.616) * | 0.562 (0.223–10.417) | 0.389 (0.184–0.824)* | 0.307 (0.142–0.663) * | 0.298 (0.133–0.660) |
| LRFS | 0.343 (0.186–0.343) * | 0.703 (0.303–10.630) | 0.382 (0.190–0.768)* | 0.408 (0.203–0.822) | 0 |
| DMFS | 0.344 (0.180–0.655) * | 0.542 (0.237–10.236) | 0.327 (0.155–0.689)* | 0.386 (0.194–0.770) * | 0.369 (0.176–0.774) |
| LC | 0.340 (0.127–0.914) * | 0.346 (0.082–10.452) | 0.340 (0.127–0.914)* | 0.264 (0.080–0.865) * | 0.301 (0.091–988) |
| LRC | 0.439 (0.214–0.898) * | 0.582 (0.218–10.554) | 0.388 (0.170–0.886)* | 0.475 (0.213–10.059) | 0.482 (0.215–0.10.083) |
| DMC | 0.286 (0.110–0.742) * | 0.478 (0.187–10.223) | 0.193 (0.069–0.545)* | 0.401 (0.165–0.974) | |
| CTVHR ≤ 25cm3 | CTVHR > 25cm3 | D90 CTVHR ≤ 80GyEQD2 | D90 CTVHR > 80GyEQD2 | Neutrophils > 7500/mm3 | |
| OS | 0.569 (0.219–10.482) | 0.304 (0.149–0.622) * | 0.360 (0.179–0.723) * | 0.399 (0.157–10.014) | 0.464 (0.172–10.251) |
| PFS | 0.598 (0.263–10.359) | 0.369 (0.201–0.678) * | 0.411 (0.222–0.761) * | 0.431 (0.200–0.927) * | 0.481 (0.193–0.1198) |
| LFS | 0.482 (0.195–10.195) | 0.339 (0.171–0.674) * | 0.402 (0.205–0.789) * | 0.341 (0.139–0.834) * | 0.536 (0.203–10.412) |
| LRFS | 0.664 (0.279–10.579) | 0.346 (0.182–0.658) * | 0.433 (0.231–0.813) * | 0.415 (0.183–0.939) * | 0.489 (0.193–10.242) |
| DMFS | 0.583 (0.242–10.407) | 0.310 (0.161–0.597) * | 0.348 (0.183–0.662) * | 0.437 (0.185–10.031) | 0.442 (0.174–10.124) |
| LC | 0.365 (0.103–10.295) | 0.325 ( 0.112–0.938) * | 0.379 (0.137–10.048) | 0.289 (0.078–10.076) | 0.769 (0.140–40.224) |
| LRC | 0.611 (0.237–10.574) | 0.406 (190–864) * | 0.488 (0.232–10.028) | 0.434 (0.173–10.088) | 0.640 (0.209–10.959) |
| DMC | 0.429 (0.143–10.280) | 0.260 (0.107–0.634) * | 0.303 (0.132–0.697) * | 0.330 (0.105–10.040) | 0.302 (0.091–10.128) |
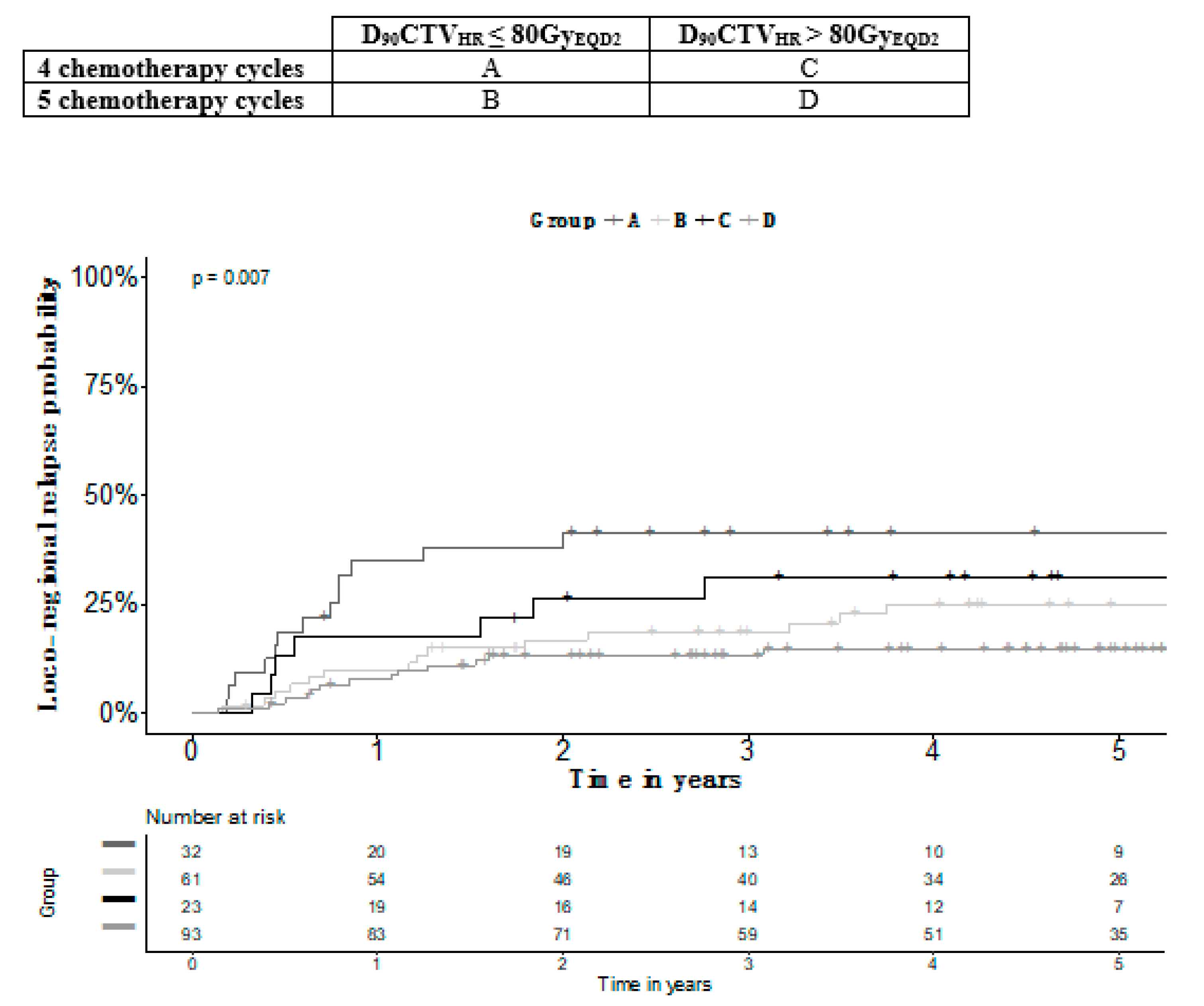
| Number of Cycles | 4 | 5 | 4 | 5 | Overall p-Value | B versus C p-Value |
|---|---|---|---|---|---|---|
| D90CTVHR | ≤80GyEQD2 | ≤80GyEQD2 | >80GyEQD2 | >80 GyEQD2 | ||
| Number of Patients | 32 | 61 | 23 | 93 | ||
| OS (SE) | 54.1% (9.1) | 83.7% (4.7) | 68.5% (9.9) | 89.2% (3.4) | 0.000 | 0.408 |
| PFS (SE) | 48.7% (9.0) | 72.3% (5.6) | 60.1% (10.4) | 83.1% (4.0) | 0.000 | 0.609 |
| LRFS (SE) | 49.6% (8.9) | 72.3% (5.7) | 64.7% (10.1) | 85.4% (3.7) | 0.000 | 0.711 |
| LRC (SE) | 58.6% (8.8) | 81.4 (5.2) | 69.7% (9.8) | 86.7% (3.5) | 0.007 | 0.752 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escande, A.; Khettab, M.; Bockel, S.; Dumas, I.; Schernberg, A.; Gouy, S.; Morice, P.; Pautier, P.; Deutsch, E.; Haie-Meder, C.; et al. Interaction between the Number of Chemotherapy Cycles and Brachytherapy Dose/Volume Parameters in Locally Advanced Cervical Cancer Patients. J. Clin. Med. 2020, 9, 1653. https://doi.org/10.3390/jcm9061653
Escande A, Khettab M, Bockel S, Dumas I, Schernberg A, Gouy S, Morice P, Pautier P, Deutsch E, Haie-Meder C, et al. Interaction between the Number of Chemotherapy Cycles and Brachytherapy Dose/Volume Parameters in Locally Advanced Cervical Cancer Patients. Journal of Clinical Medicine. 2020; 9(6):1653. https://doi.org/10.3390/jcm9061653
Chicago/Turabian StyleEscande, Alexandre, Mohamed Khettab, Sophie Bockel, Isabelle Dumas, Antoine Schernberg, Sebastien Gouy, Philippe Morice, Patricia Pautier, Eric Deutsch, Christine Haie-Meder, and et al. 2020. "Interaction between the Number of Chemotherapy Cycles and Brachytherapy Dose/Volume Parameters in Locally Advanced Cervical Cancer Patients" Journal of Clinical Medicine 9, no. 6: 1653. https://doi.org/10.3390/jcm9061653
APA StyleEscande, A., Khettab, M., Bockel, S., Dumas, I., Schernberg, A., Gouy, S., Morice, P., Pautier, P., Deutsch, E., Haie-Meder, C., & Chargari, C. (2020). Interaction between the Number of Chemotherapy Cycles and Brachytherapy Dose/Volume Parameters in Locally Advanced Cervical Cancer Patients. Journal of Clinical Medicine, 9(6), 1653. https://doi.org/10.3390/jcm9061653





