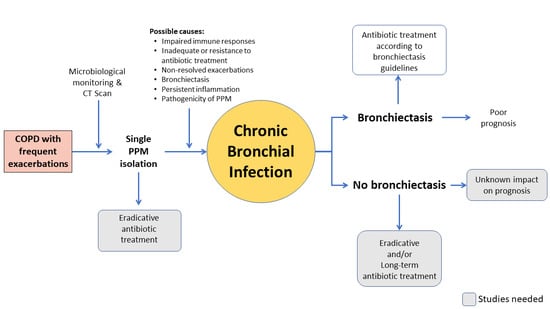
Current Challenges in Chronic Bronchial Infection in Patients with Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Bronchial Colonization versus Bronchial Infection
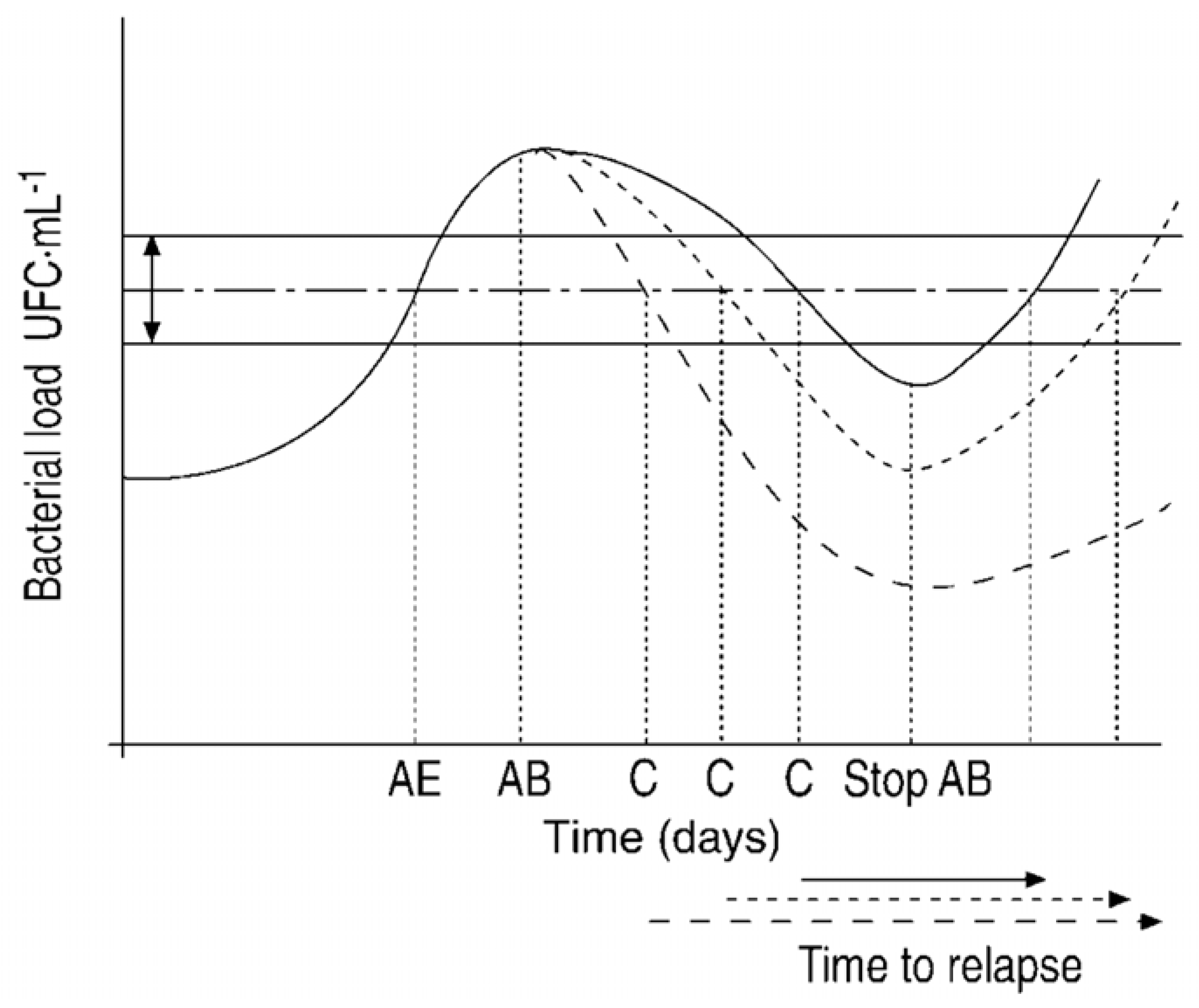
3. Exacerbations in the Context of Chronically Infected COPD Patients
4. P. aeruginosa in Patients with COPD
5. Macrolides in Chronically Infected COPD Patients
6. Inhaled Corticosteroids in Chronically Infected COPD Patients
7. Long-Term Systemic or Inhaled Antibiotic Treatment
7.1. Systemic Antibiotics Other than Macrolides
7.2. Inhaled Antibiotics
8. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Matkovic, Z.; Miravitlles, M. Chronic bronchial infection in copd. Is there an infective phenotype? Respir. Med. 2013, 107, 10–22. [Google Scholar] [PubMed]
- Martinez-Garcia, M.A.; Miravitlles, M. Bronchiectasis in copd patients: More than a comorbidity? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1401–1411. [Google Scholar]
- Miravitlles, M.; Anzueto, A. Antibiotics for acute and chronic respiratory infection in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Marin, A.; Serra-Batlles, J.; de la Rosa, D.; Solanes, I.; Pomares, X.; Lopez-Sanchez, M.; Munoz-Esquerre, M.; Miravitlles, M. Treatment of patients with copd and recurrent exacerbations: The role of infection and inflammation. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 515–525. [Google Scholar]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Yatera, K.; Noguchi, S.; Mukae, H. The microbiome in the lower respiratory tract. Respir. Investig. 2018, 56, 432–439. [Google Scholar]
- Sherrard, L.J.; Bell, S.C.; Tunney, M.M. The role of anaerobic bacteria in the cystic fibrosis airway. Curr. Opin. Pulm. Med. 2016, 22, 637–643. [Google Scholar]
- Sethi, S.; Maloney, J.; Grove, L.; Wrona, C.; Berenson, C.S. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 173, 991–998. [Google Scholar] [CrossRef]
- Canton, R.; Cobos, N.; de Gracia, J.; Baquero, F.; Honorato, J.; Gartner, S.; Alvarez, A.; Salcedo, A.; Oliver, A.; Garcia-Quetglas, E.; et al. Antimicrobial therapy for pulmonary pathogenic colonisation and infection by pseudomonas aeruginosa in cystic fibrosis patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2005, 11, 690–703. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Canton, R.; Torres, A.; Dimakou, K.; et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Maiz, L.; Olveira, C.; Giron, R.M.; de la Rosa, D.; Blanco, M.; Canton, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch. Bronconeumol. 2018, 54, 79–87. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa Carrillo, D.; López-Campos, J.L.; Olveira Fuster, C.; Alcázar Navarrete, B.; Máiz Carro, L.; Calle Rubio, M.; Cantón Moreno, R.; García-Rivero, J.L.; Martínez-García, M.A. Consensus document on the diagnosis and treatment of chronic bronchial infection in chronic obstructive pulmonary disease. Arch. Bronconeumol. 2020, in press. [Google Scholar]
- Eklof, J.; Sorensen, R.; Ingebrigtsen, T.S.; Sivapalan, P.; Achir, I.; Boel, J.B.; Bangsborg, J.; Ostergaard, C.; Dessau, R.B.; Jensen, U.S.; et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: An observational cohort study of 22 053 patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 227–234. [Google Scholar] [CrossRef]
- Rogers, G.B.; van der Gast, C.J.; Cuthbertson, L.; Thomson, S.K.; Bruce, K.D.; Martin, M.L.; Serisier, D.J. Clinical measures of disease in adult non-cf bronchiectasis correlate with airway microbiota composition. Thorax 2013, 68, 731–737. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Miller, B.E.; Tal-Singer, R.; Van Horn, S.; Tomsho, L.; Mackay, A.; Allinson, J.P.; Webb, A.J.; Brookes, A.J.; et al. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: An analysis of the copdmap study. Thorax 2018, 73, 331–338. [Google Scholar] [CrossRef]
- Anzueto, A.; Miravitlles, M. Chronic obstructive pulmonary disease exacerbations: A need for action. Am. J. Med. 2018, 131, 15–22. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Mayhew, D.; Devos, N.; Lambert, C.; Brown, J.R.; Clarke, S.C.; Kim, V.L.; Magid-Slav, M.; Miller, B.E.; Ostridge, K.K.; Patel, R.; et al. Longitudinal profiling of the lung microbiome in the aeris study demonstrates repeatability of bacterial and eosinophilic copd exacerbations. Thorax 2018, 73, 422–430. [Google Scholar] [CrossRef]
- Miravitlles, M.; Kruesmann, F.; Haverstock, D.; Perroncel, R.; Choudhri, S.H.; Arvis, P. Sputum colour and bacteria in chronic bronchitis exacerbations: A pooled analysis. Eur. Respir. J. 2012, 39, 1354–1360. [Google Scholar] [CrossRef]
- Chang, C.; Zhu, H.; Shen, N.; Chen, Y.; He, B.; Zhao, J.; Yao, W. Bacterial infection, airway and systemic inflammation and clinical outcomes before and after treatment of aecopd, a longitudinal and cross-sectional study. COPD 2015, 12, 19–30. [Google Scholar] [CrossRef]
- Wilson, R.; Anzueto, A.; Miravitlles, M.; Arvis, P.; Alder, J.; Haverstock, D.; Trajanovic, M.; Sethi, S. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of copd: Maestral results. Eur. Respir. J. 2012, 40, 17–27. [Google Scholar]
- Bafadhel, M.; Haldar, K.; Barker, B.; Patel, H.; Mistry, V.; Barer, M.R.; Pavord, I.D.; Brightling, C.E. Airway bacteria measured by quantitative polymerase chain reaction and culture in patients with stable copd: Relationship with neutrophilic airway inflammation, exacerbation frequency, and lung function. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1075–1083. [Google Scholar]
- Miravitlles, M. Exacerbations of chronic obstructive pulmonary disease: When are bacteria important? Eur. Respir. J. Suppl. 2002, 36, 9s–19s. [Google Scholar] [CrossRef]
- Sethi, S.; Wrona, C.; Grant, B.J.; Murphy, T.F. Strain-specific immune response to haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 169, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Oliver, B.G.; Lim, S.; Wark, P.; Laza-Stanca, V.; King, N.; Black, J.L.; Burgess, J.K.; Roth, M.; Johnston, S.L. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax 2008, 63, 519–525. [Google Scholar] [PubMed]
- Mallia, P.; Message, S.D.; Gielen, V.; Contoli, M.; Gray, K.; Kebadze, T.; Aniscenko, J.; Laza-Stanca, V.; Edwards, M.R.; Slater, L.; et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 2011, 183, 734–742. [Google Scholar] [CrossRef] [PubMed]
- George, S.N.; Garcha, D.S.; Mackay, A.J.; Patel, A.R.; Singh, R.; Sapsford, R.J.; Donaldson, G.C.; Wedzicha, J.A. Human rhinovirus infection during naturally occurring copd exacerbations. Eur. Respir. J. 2014, 44, 87–96. [Google Scholar] [CrossRef]
- Lode, H.; Eller, J.; Linnhoff, A.; Ioanas, M.; Evaluation of Therapy-Free Interval in COPD Patients Study Group. Levofloxacin versus clarithromycin in copd exacerbation: Focus on exacerbation-free interval. Eur. Respir. J. 2004, 24, 947–953. [Google Scholar] [CrossRef]
- Sethi, S.; Jones, P.W.; Theron, M.S.; Miravitlles, M.; Rubinstein, E.; Wedzicha, J.A.; Wilson, R.; PULSE Study Group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: A randomized controlled trial. Respir. Res. 2010, 11, 10. [Google Scholar] [CrossRef]
- Vermeersch, K.; Gabrovska, M.; Aumann, J.; Demedts, I.K.; Corhay, J.L.; Marchand, E.; Slabbynck, H.; Haenebalcke, C.; Haerens, M.; Hanon, S.; et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalization (bace). A multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2019, 200, 857–868. [Google Scholar] [CrossRef]
- Miravitlles, M.; Marin, A.; Monso, E.; Vila, S.; de la Roza, C.; Hervas, R.; Esquinas, C.; Garcia, M.; Millares, L.; Morera, J.; et al. Colour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary disease. Respir. Res. 2010, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; de la Rosa-Carrillo, D.; Soler-Cataluna, J.J.; Catalan-Serra, P.; Ballester, M.; Roca Vanaclocha, Y.; Agramunt, M.; Ballestin, J.; Garcia-Ortega, A.; Oscullo, G.; et al. Bronchial infection and temporal evolution of bronchiectasis in patients with chronic obstructive pulmonary disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020. [Google Scholar] [CrossRef] [PubMed]
- Canton, R.; Fernandez Olmos, A.; de la Pedrosa, E.G.; del Campo, R.; Antonia Meseguer, M. Chronic bronchial infection: The problem of pseudomonas aeruginosa. Arch. Bronconeumol. 2011, 47 (Suppl. 6), 8–13. [Google Scholar] [CrossRef]
- Montero, M.; Horcajada, J.P.; Sorli, L.; Alvarez-Lerma, F.; Grau, S.; Riu, M.; Sala, M.; Knobel, H. Effectiveness and safety of colistin for the treatment of multidrug-resistant pseudomonas aeruginosa infections. Infection 2009, 37, 461–465. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Maiz, L.; Olveira, C.; Giron, R.M.; de la Rosa, D.; Blanco, M.; Canton, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch. Bronconeumol. 2018, 54, 88–98. [Google Scholar]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, S.J.; Floto, A.R.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British thoracic society guideline for bronchiectasis in adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef]
- Elborn, J.S.; Bell, S.C.; Madge, S.L.; Burgel, P.R.; Castellani, C.; Conway, S.; De Rijcke, K.; Dembski, B.; Drevinek, P.; Heijerman, H.G.; et al. Report of the european respiratory society/european cystic fibrosis society task force on the care of adults with cystic fibrosis. Eur. Respir. J. 2016, 47, 420–428. [Google Scholar] [CrossRef]
- Leung, J.M.; Tiew, P.Y.; Mac Aogain, M.; Budden, K.F.; Yong, V.F.; Thomas, S.S.; Pethe, K.; Hansbro, P.M.; Chotirmall, S.H. The role of acute and chronic respiratory colonization and infections in the pathogenesis of copd. Respirology 2017, 22, 634–650. [Google Scholar] [CrossRef]
- Murphy, T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009, 15, 138–142. [Google Scholar] [CrossRef]
- Gallego, M.; Pomares, X.; Espasa, M.; Castaner, E.; Sole, M.; Suarez, D.; Monso, E.; Monton, C. Pseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: Characterization and risk factors. BMC Pulm. Med. 2014, 14, 103. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Faner, R.; Oscullo, G.; la Rosa-Carrillo, D.; Soler-Cataluna, J.J.; Ballester, M.; Agusti, A. Inhaled steroids, circulating eosinophils, chronic airway infection and pneumonia risk in chronic obstructive pulmonary disease: A network analysis. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Drannik, A.G.; Pouladi, M.A.; Robbins, C.S.; Goncharova, S.I.; Kianpour, S.; Stampfli, M.R. Impact of cigarette smoke on clearance and inflammation after pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2004, 170, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.M.; Ochs-Balcom, H.M.; Noyes, K.; Zhao, J.; Leung, W.Y.; Pu, C.Y.; Murphy, T.F.; Sethi, S. Impact of pseudomonas aeruginosa isolation on mortality and outcomes in an outpatient chronic obstructive pulmonary disease cohort. Open Forum Infect. Dis. 2020, 7, ofz546. [Google Scholar]
- Boutou, A.K.; Raste, Y.; Reid, J.; Alshafi, K.; Polkey, M.I.; Hopkinson, N.S. Does a single pseudomonas aeruginosa isolation predict copd mortality? Eur. Respir. J. 2014, 44, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Monso, E.; Soler, N.; Torres, F.; Angrill, J.; Riise, G.; Zalacain, R.; Morera, J.; Torres, A. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch. Intern. Med. 2005, 165, 891–897. [Google Scholar] [CrossRef]
- Murphy, T.F.; Brauer, A.L.; Eschberger, K.; Lobbins, P.; Grove, L.; Cai, X.; Sethi, S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 853–860. [Google Scholar] [CrossRef]
- Almagro, P.; Salvado, M.; Garcia-Vidal, C.; Rodriguez-Carballeira, M.; Cuchi, E.; Torres, J.; Heredia, J.L. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respir. Int. Rev. Thorac. Dis. 2012, 84, 36–43. [Google Scholar]
- Lode, H.; Allewelt, M.; Balk, S.; De Roux, A.; Mauch, H.; Niederman, M.; Schmidt-Ioanas, M. A prediction model for bacterial etiology in acute exacerbations of copd. Infection 2007, 35, 143–149. [Google Scholar]
- Montero, M.; Dominguez, M.; Orozco-Levi, M.; Salvado, M.; Knobel, H. Mortality of copd patients infected with multi-resistant pseudomonas aeruginosa: A case and control study. Infection 2009, 37, 16–19. [Google Scholar]
- Vitacca, M.; Marino, S.; Comini, L.; Fezzardi, L.; Paneroni, M. Bacterial colonization in copd patients admitted to a rehabilitation respiratory unit and impact on length of stay: A real-life study. COPD 2018, 15, 581–587. [Google Scholar]
- Martinez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. Chronic pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Almagro, P.; Romani, V.; Rodriguez-Carballeira, M.; Cuchi, E.; Canales, L.; Blasco, D.; Heredia, J.L.; Garau, J. Pseudomonas aeruginosa in patients hospitalised for copd exacerbation: A prospective study. Eur. Respir. J. 2009, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Troyano, A.; Suarez-Cuartin, G.; Peiro, M.; Barril, S.; Castillo, D.; Sanchez-Reus, F.; Plaza, V.; Restrepo, M.I.; Chalmers, J.D.; Sibila, O. Pseudomonas aeruginosa resistance patterns and clinical outcomes in hospitalized exacerbations of copd. Respirology 2016, 21, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Rakhimova, E.; Wiehlmann, L.; Brauer, A.L.; Sethi, S.; Murphy, T.F.; Tummler, B. Pseudomonas aeruginosa population biology in chronic obstructive pulmonary disease. J. Infect. Dis. 2009, 200, 1928–1935. [Google Scholar] [CrossRef]
- Halldorsson, S.; Gudjonsson, T.; Gottfredsson, M.; Singh, P.K.; Gudmundsson, G.H.; Baldursson, O. Azithromycin maintains airway epithelial integrity during pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 2010, 42, 62–68. [Google Scholar] [CrossRef]
- Cao, Y.; Xuan, S.; Wu, Y.; Yao, X. Effects of long-term macrolide therapy at low doses in stable copd. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1289–1298. [Google Scholar] [CrossRef]
- Henkle, E.; Curtis, J.R.; Chen, L.; Chan, B.; Aksamit, T.R.; Daley, C.L.; Griffith, D.E.; Winthrop, K.L. Comparative risks of chronic inhaled corticosteroids and macrolides for bronchiectasis. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Uzun, S.; Djamin, R.S.; Kluytmans, J.A.; Mulder, P.G.; van’t Veer, N.E.; Ermens, A.A.; Pelle, A.J.; Hoogsteden, H.C.; Aerts, J.G.; van der Eerden, M.M. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (columbus): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2014, 2, 361–368. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, L.; Li, C.; Chen, P.; Chen, Y. Long-term macrolide treatment for the prevention of acute exacerbations in copd: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3813–3829. [Google Scholar] [CrossRef]
- Miravitlles, M.; Anzueto, A. Antibiotic prophylaxis in copd: Why, when, and for whom? Pulm. Pharmacol. Ther. 2015, 32, 119–123. [Google Scholar] [CrossRef]
- Wedzicha, J.A.E.C.-C.; Miravitlles, M.; Hurst, J.R.; Calverley, P.M.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Management of copd exacerbations: A european respiratory society/american thoracic society guideline. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yanai, M.; Yamaya, M.; Satoh-Nakagawa, T.; Sekizawa, K.; Ishida, S.; Sasaki, H. Erythromycin and common cold in copd. Chest 2001, 120, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.; Wilkinson, T.M.; Hurst, J.R.; Perera, W.R.; Sapsford, R.J.; Wedzicha, J.A. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am. J. Respir. Crit. Care Med. 2008, 178, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of copd. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Svanstrom, H.; Pasternak, B.; Hviid, A. Cardiovascular risks with azithromycin. N. Engl. J. Med. 2013, 369, 580–581. [Google Scholar] [PubMed]
- Mosholder, A.D.; Mathew, J.; Alexander, J.J.; Smith, H.; Nambiar, S. Cardiovascular risks with azithromycin and other antibacterial drugs. N. Engl. J. Med. 2013, 368, 1665–1668. [Google Scholar] [CrossRef]
- Miravitlles, M.; Soler-Cataluna, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosio, B.G.; Casanova, C.; Antonio Riesco, J.; et al. Spanish guidelines for management of chronic obstructive pulmonary disease (gesepoc) 2017. Pharmacological treatment of stable phase. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef]
- Pomares, X.; Monton, C.; Espasa, M.; Casabon, J.; Monso, E.; Gallego, M. Long-term azithromycin therapy in patients with severe copd and repeated exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 449–456. [Google Scholar] [CrossRef]
- He, Z.Y.; Ou, L.M.; Zhang, J.Q.; Bai, J.; Liu, G.N.; Li, M.H.; Deng, J.M.; MacNee, W.; Zhong, X.N. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respir. Int. Rev. Thorac. Dis. 2010, 80, 445–452. [Google Scholar] [CrossRef]
- Blasi, F.; Bonardi, D.; Aliberti, S.; Tarsia, P.; Confalonieri, M.; Amir, O.; Carone, M.; Di Marco, F.; Centanni, S.; Guffanti, E. Long-term azithromycin use in patients with chronic obstructive pulmonary disease and tracheostomy. Pulm. Pharmacol. Ther. 2010, 23, 200–207. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Strange, C. Inhaled corticosteroids for chronic obstructive pulmonary disease: What is their role in therapy? Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2587–2601. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The gold science committee report 2019. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Cascini, S.; Kirchmayer, U.; Belleudi, V.; Bauleo, L.; Pistelli, R.; Di Martino, M.; Formoso, G.; Davoli, M.; Agabiti, N. Inhaled corticosteroid use in chronic obstructive pulmonary disease and risk of pneumonia: A nested case-control population-based study in lazio (italy)-the outpul study. COPD 2017, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Glanville, N.; Girkin, J.L.; Ching, Y.M.; Marcellini, A.; Porter, J.D.; Toussaint, M.; Walton, R.P.; Finney, L.J.; Aniscenko, J.; et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in copd exacerbations. Nat. Commun. 2018, 9, 2229. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Glanville, N.; Cuthbertson, L.; Bartlett, N.W.; Finney, L.J.; Turek, E.; Bakhsoliani, E.; Calderazzo, M.A.; Trujillo-Torralbo, M.B.; Footitt, J.; et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Pauletti, A.; Rossi, M.R.; Spanevello, A.; Casolari, P.; Marcellini, A.; Forini, G.; Gnesini, G.; Marku, B.; Barnes, N.; et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in copd. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Du, Y.; Chen, H.; Jiang, D.; Xu, Z. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2019, 77, 105950. [Google Scholar] [CrossRef]
- Brode, S.K.; Campitelli, M.A.; Kwong, J.C.; Lu, H.; Marchand-Austin, A.; Gershon, A.S.; Jamieson, F.B.; Marras, T.K. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur. Respir. J. 2017, 50, 1700037. [Google Scholar] [CrossRef]
- Singanayagam, A.; Johnston, S.L. Long-term impact of inhaled corticosteroid use in asthma and chronic obstructive pulmonary disease (copd): Review of mechanisms that underlie risks. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Calverley, P.M.A.; Stockley, R.A.; Seemungal, T.A.R.; Hagan, G.; Willits, L.R.; Riley, J.H.; Wedzicha, J.A. Investigating New Standards for Prophylaxis in Reduction of Exacerbations, I. Reported pneumonia in patients with copd: Findings from the inspire study. Chest 2011, 139, 505–512. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Soler-Cataluna, J.J.; Miravitlles, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: Future challenges. Arch. Bronconeumol. 2020, 56, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Bafadhel, M.; Brightling, C.E.; Sciurba, F.C.; Curtis, J.L.; Martinez, F.J.; Pasquale, C.B.; Merrill, D.D.; Metzdorf, N.; Petruzzelli, S.; et al. Blood eosinophil counts in clinical trials for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.L.; Morena, D.; Gonzalez, Y.; Paredero, J.M.; Perez, B.; Graziani, D.; Gutierrez, M.; Rodriguez, J.M. Clinical management of copd in a real-world setting. A big data analysis. Arch. Bronconeumol. 2020. [Google Scholar]
- Hnin, K.; Nguyen, C.; Carson, K.V.; Evans, D.J.; Greenstone, M.; Smith, B.J. Prolonged antibiotics for non-cystic fibrosis bronchiectasis in children and adults. Cochrane Database Syst. Rev. 2015, CD001392. [Google Scholar]
- Staykova, T.; Black, P.N.; Chacko, E.E.; Poole, P. Prophylactic antibiotic therapy for chronic bronchitis. Cochrane Database Syst. Rev. 2003, CD004105. [Google Scholar]
- Herath, S.C.; Normansell, R.; Maisey, S.; Poole, P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (copd). Cochrane Database Syst. Rev. 2018, 10, CD009764. [Google Scholar] [CrossRef]
- Wilson, R.; Sethi, S.; Anzueto, A.; Miravitlles, M. Antibiotics for treatment and prevention of exacerbations of chronic obstructive pulmonary disease. J. Infect. 2013, 67, 497–515. [Google Scholar] [CrossRef]
- Brill, S.E.; Law, M.; El-Emir, E.; Allinson, J.P.; James, P.; Maddox, V.; Donaldson, G.C.; McHugh, T.D.; Cookson, W.O.; Moffatt, M.F.; et al. Effects of different antibiotic classes on airway bacteria in stable copd using culture and molecular techniques: A randomised controlled trial. Thorax 2015, 70, 930–938. [Google Scholar] [CrossRef]
- Pettigrew, M.M.; Tsuji, B.T.; Gent, J.F.; Kong, Y.; Holden, P.N.; Sethi, S.; Murphy, T.F. Effect of fluoroquinolones and macrolides on eradication and resistance of haemophilus influenzae in chronic obstructive pulmonary disease. Antimicrob. Agents Chemother. 2016, 60, 4151–4158. [Google Scholar] [CrossRef]
- Moyes, E.N.; Kershaw, R.A. Long-continued treatment with tetracycline and prednisolone in chronic bronchitis; a controlled trial. Lancet 1957, 273, 1187–1191. [Google Scholar]
- Murdoch, J.M.; Leckie, W.J.; Downie, J.; Swain, R.H.; Gould, J.C. An evaluation of continuous antibiotic therapy in chronic bronchitis. Br. Med. J. 1959, 2, 1277–1285. [Google Scholar] [PubMed]
- Francis, R.S.; Spicer, C.C. Chemotherapy in chronic bronchitis. Influence of daily penicillin and tetracycline on exacerbations and their cost. Br. Med. J. 1960, 1, 297–303. [Google Scholar] [PubMed]
- Pridie, R.B.; Datta, N.; Massey, D.G.; Poole, G.W.; Schneeweiss, J.; Stradling, P. A trial of continuous winter chemotherapy in chronic bronchitis. Lancet 1960, 2, 723–727. [Google Scholar] [CrossRef]
- Value of chemoprophylaxis and chemotherapy in early chronic bronchitis. A report to the medical research council by their working party on trials of chemotherapy in early chronic bronchitis. Br. Med. J. 1966, 1, 1317–1322. [CrossRef] [PubMed][Green Version]
- Goslings, W.R.; Djajadiningrat, R.J.; Bergstein, P.G.; Holle, P. Continuous suppressive antimicrobial treatment in chronic infected bronchitis during the winter months. Dis. Chest 1967, 52, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.N.; McNeill, R.S.; Smith, D.H.; Dempster, M.B.; Nairn, J.R.; Purvis, M.S.; Watson, J.M.; Ward, F.G. Five-year winter chemoprophylaxis for chronic bronchitis. Br. Med. J. 1969, 4, 265–269. [Google Scholar] [CrossRef]
- Liippo, K.; Pelliniemi, T.T.; Lehto, H. Trimethoprim prophylaxis of acute exacerbations in chronic obstructive pulmonary diseases. Acta Med. Scand. 1987, 221, 455–459. [Google Scholar] [CrossRef]
- Maiz, L.; Giron, R.M.; Olveira, C.; Quintana, E.; Lamas, A.; Pastor, D.; Canton, R.; Mensa, J. Inhaled antibiotics for the treatment of chronic bronchopulmonary pseudomonas aeruginosa infection in cystic fibrosis: Systematic review of randomised controlled trials. Expert Opin. Pharmacother. 2013, 14, 1135–1149. [Google Scholar]
- Athanazio, R.; da Costa, J.C.; de la Rosa Carrillo, D.; Martinez-Garcia, M.A. Current and future pharmacotherapy options for non-cystic fibrosis bronchiectasis. Expert Rev. Respir. Med. 2018, 12, 569–584. [Google Scholar] [CrossRef]
- Dal Negro, R.; Micheletto, C.; Tognella, S.; Visconti, M.; Turati, C. Tobramycin nebulizer solution in severe copd patients colonized with pseudomonas aeruginosa: Effects on bronchial inflammation. Adv. Ther. 2008, 25, 1019–1030. [Google Scholar]
- Nijdam, L.C.; Assink, M.D.; Kuijvenhoven, J.C.; de Saegher, M.E.; van der Valk, P.D.; van der Palen, J.; Brusse-Keizer, M.G.; Movig, K.L. Safety and tolerability of nebulized amoxicillin-clavulanic acid in patients with copd (stonac 1 and stonac 2). COPD 2016, 13, 448–454. [Google Scholar] [PubMed]
- Bruguera-Avila, N.; Marin, A.; Garcia-Olive, I.; Radua, J.; Prat, C.; Gil, M.; Ruiz-Manzano, J. Effectiveness of treatment with nebulized colistin in patients with copd. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2909–2915. [Google Scholar] [CrossRef]
- Monton, C.; Prina, E.; Pomares, X.; Cugat, J.R.; Casabella, A.; Oliva, J.C.; Gallego, M.; Monso, E. Nebulized colistin and continuous cyclic azithromycin in severe copd patients with chronic bronchial infection due to pseudomonas aeruginosa: A retrospective cohort study. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Papaioannou, O.; Kaponi, M.; Kozanidou, A.; Hillas, G.; Stavropoulou, E.; Bouros, D.; Dimakou, K. Low penetrance of antibiotics in the epithelial lining fluid. The role of inhaled antibiotics in patients with bronchiectasis. Pulm. Pharmacol. Ther. 2020, 60, 101885. [Google Scholar] [CrossRef]
- Khaddaj-Mallat, R.; Morin, C.; Rousseau, É. Novel n-3 pufa monoacylglycerides of pharmacological and medicinal interest: Anti-inflammatory and anti-proliferative effects. Eur. J. Pharmacol. 2016, 792, 70–77. [Google Scholar] [CrossRef]
- Morin, C.; Cantin, A.M.; Vézina, F.A.; Fortin, S. The efficacy of mag-dha for correcting aa/dha imbalance of cystic fibrosis patients. Mar. Drugs 2018, 16, 184. [Google Scholar]
- Khaddaj-Mallat, R.; Sirois, C.; Sirois, M.; Rizcallah, E.; Morin, C.; Rousseau, É. Reversal of il-13-induced inflammation and ca(2+) sensitivity by resolvin and mag-dha in association with asa in human bronchi. Prostaglandins Other Lipid Mediat. 2015, 121, 145–154. [Google Scholar]

| Potentially Pathogenic Microorganisms | Non-Potentially Pathogenic Microorganisms |
|---|---|
|
|
| Clinical impact of CBI in COPD |
|---|
| Patients with CBI suffer from more frequent exacerbations. |
| Patients with CBI suffer from more severe exacerbations. |
| Incomplete eradication after antibiotic treatment of exacerbations may result in CBI. |
| PPM identified during CBI are the same ones that produce exacerbations in most cases. |
| Both CBI and bacterial exacerbations may be identified by the production of muco-purulent or purulent sputum. |
| Long-term antibiotic treatment of CBI can reduce the frequency of exacerbations. |
| The presence of CBI and hospital admissions for exacerbations are associated with the development of bronchiectasis in COPD. |
| Study | Design and Treatment Groups | Patient Population | Results |
|---|---|---|---|
| Suzuki et al, 2001 [62] | Randomized, non-blinded study of 109 patients, 55 treated with erythromycin 200–400 mg/day and 54 in the control group for 1 year | Mean age 70 years, mean FEV1 between 1.3 to 1.47 L | Reduction in common colds and exacerbations in the antibiotic group. |
| Seemungal et al, 2008 [63] | Randomized, double-blind, placebo-controlled study. 109 patients, 53 treated with erythromycin 250 b.i.d and 56 with placebo for 1 year | Mean age 67.2 years, mean FEV1(%) = 50% | 35% reduction of exacerbation frequency with antibiotic (p = 0.006). Median time to first exacerbation was 271 vs. 89 days in the placebo arm (p = 0.02). Reduction in duration of the exacerbation with macrolide. |
| Pomares et al, 2011 [68] | Retrospective study of 24 COPD patients treated with azithromycin 500 mg three times per week for 1 year | Mean age 70.9 years, mean FEV1(%) = 32.2%, a mean of 3.3 hospitalization and 7 exacerbations the previous year | A 58.9% reduction in exacerbations and 61.2% reduction in hospitalizations compared with previous year without macrolides. |
| He et al, 2010 [69] | Randomized, double-blind, placebo-controlled study of 36 patients, 18 treated with erythromycin 125 mg t.i.d and 18 with placebo for 6 months. | Mean age 69 years. Mean FEV1(%) = 43% | Reduction in total numbers of sputum cells and neutrophil elastase. A reduction of 44% in relative risk of exacerbation with the antibiotic. Delayed time to first exacerbation with macrolide. |
| Blasi et al, 2010 [70] | Open label, randomized, uncontrolled trial of 22 patients with COPD and tracheostomy, 11 treated with azithromycin 500 mg 3 days a week for 6 months and 11 in standard care group | Mean age 72 and 73 years. No lung function available. 91% and 73% were colonized. | Longer time to the first exacerbation with the macrolide. Estimated hazard ratio for first exacerbation associated with standard care 5.41 (95% CI: 1.67–17.5). Reduction in hospitalization with azithromycin. |
| Albert et al, 2011 [64] | Randomized, double-blind, placebo-controlled trial of 1142 patients. 570 assigned to azithromycin 250 mg daily and 572 to placebo for a year. | Mean age 66 years. Mean FEV1(%) 39-40%, up to 50% required hospital visit for exacerbation the previous year. | Reduction in risk of exacerbation with azithromycin (p <0.001). Median time to first exacerbation prolonged from 174 with placebo to 266 with macrolide. Hazard ratio for time to the first exacerbation was 0.71 (95% CI: 0.61 to 0.83; p <0.001) |
| Uzun et al., 2014 [58] | Randomized, double-blind, placebo-controlled study of 92 patients, 47 assigned to azithromycin 500 mg 3 times a week for 12 months and 45 to placebo | Mean age 65 years, mean FEV1(%) = 45%, at least 3 exacerbations the previous year. Patients with bronchiectasis in CT were excluded. | Reduction of exacerbation of 42% with azithromycin (reduction risk 0.58, 95% CI: 0.42–0.79; p = 0.001). Median time to first exacerbation was 59 days with placebo and 130 with azithromycin (p = 0.001). |
| Vermeersch et al., 2019 [30] | Randomized, double-blind, placebo-controlled trial of 301 patients admitted for an exacerbation of COPD. 147 assigned to azithromycin 500 mg 3 for days and 250 mg every 2 days for 3 months and 154 assigned to placebo | Mean age 67 years, mean FEV1(%) = 37%, at least 1 exacerbation the previous year. | There was no change in the treatment success. Azithromycin decreased treatment failure: 49% azithromycin and 60% placebo (HR 0.73; 95% CI: 0.53–1.01; p = 0.052). Clinical benefits were lost 6 months after withdrawal. |
| Study and Design | Study Population | Main Results | Other Results |
|---|---|---|---|
| Sethi et al. (2010) [29] Double-blind, randomized, placebo-controlled trial Moxifloxacin 400 mg once daily for 5 days, every 8 weeks for a total of six courses vs placebo 1157 patients 48-week treatment period Further 24-week follow-up | Stable COPD patients with chronic bronchitis and at least two exacerbations in the 12 months prior to enrolment. Exclusions: tendon disease, arrhythmias, hepatic impairment, other respiratory disease, chronic colonization of pathogenic organisms resistant to moxifloxacin, systemic or inhaled antibiotic therapy during the 6 weeks prior to screening, need for home ventilatory support for COPD. | Reduced odds of exacerbation:
|
|
| Brill et al. (2015) [84] Single-blind, randomized, placebo-controlled trial 4 treatment groups -Moxifloxacin 400 mg daily for 5 days every 4 weeks -Doxycycline 100 mg/day -Azithromycin 250 mg 3 times a week - Placebo 99 patients 13-weeks treatment period | Stable COPD patients with chronic bronchitis. Exclusions: Other clinically significant respiratory disease, COPD exacerbation in the 4 weeks preceding screening or before randomization, hepatic or renal impairment, evidence of tuberculosis, uncontrolled hypertension, prolonged Q-T interval, long term antibiotics for any reason. |
| More treatment-related adverse events with moxifloxacin. Mean inhibitory concentrations increased x3 times over placebo in all treatment arms. |
| Pettigrew (2016) [85] Retrospective study Fluorquinolones vs. macrolides 77 patients 15 years follow-up | COPD patients with chronic bronchitis and at least 1 H. influenzae isolation. Exclusions: asthma, bronchiectasis, inability to comply with a schedule of monthly clinical visits, immunosuppressive or other life-threatening disorders. |
|
|
| Study Type | Study Population | Antibiotic Studied and Doses | n/Treatment Duration | Main Results | Other Results | Antibiotic Resistance |
|---|---|---|---|---|---|---|
| Dal Negro (2008) [100] Single arm prospective intervention study | Severe COPD patients chronically colonized with P. aeruginosa resistant to oral/intravenous specific antibiotics. Exclusions: asthma; bronchiectasis; pregnancy or lactation; pneumonia; lung malignancy; immunosuppression; liver or renal insufficiency; cardiac failure; use of antibiotics in the previous 4 weeks; other infections. | Tobramycin Nebulizer Solution (TNS) TNS 300 mg/12 h | 13 patients, 14 days further 6 months follow-up |
|
|
|
| Nijdam (2016) [101] Two phase 1 single-arm prospective intervention studies | COPD patients, able to produce sputum. STONAC 1: stable COPD outpatients; STONAC 2: patients hospitalized for exacerbation. Exclusions: allergy to penicillin, amoxicillin or clavulanic acid, current pneumonia, and FEV1 post bronchodilator < 1.2 L (STONAC 1 only), systemic use of amoxicillin (STONAC 2 only). | Amoxicillin/clavulanic acid 1000 mg/200 mg powder for solution for injection (registered for intravenous administration) STONAC 1: ascending doses, up to 300:60 mg of amoxicillin-clavulanic acid. STONAC 2: amoxicillin clavulanic acid 200:40 mg, twice daily during hospitalization (with a maximum of 7 days). | STONAC 1: 8 patients. Each patient received 4 doses with at least 7 days between each dose. STONAC 2: 8 patients. |
|
|
|
| Bruguera (2017) [102] Retrospective study | Severe COPD patients (FEV1 ≤ 50%) with chronic or intermittent colonization by P. aeruginosa who initiated treatment with nebulized colistin between 2010 and 2014. Exclusions: asthma, malignancy, unstable heart disease, main diagnosis of bronchiectasis. | Colistimethate sodium Colistimethate sodium 1 million IU/12 h administered through the I-neb adaptive aerosol delivery device. | 36 patients, 5-year review. Comparison between the year prior to and the year after starting the treatment. |
|
|
|
| Montón (2019) [103] Retrospective study | Severe COPD patients with chronic bronchial infection by P. aeruginosa who initiated a treatment with nebulized colistin between 2005 and 2015, in combination with long-term oral azithromycin. Exclusions: main diagnosis of bronchiectasis. | Colistimethate sodium + azithromycin -Colistimethate sodium with jet nebulizer (1–2 million IU/12 h) OR with I-neb adaptive aerosol delivery device (0.5–1 million IU/12 h). -Azithromycin 500 mg three times/week. | 53 patients (32 in final analysis)10-year review. Comparison between the two years prior to and the two years after starting the treatment. |
|
|
|
| Dose, Posology | Administration Time | Inhalation System | |
|---|---|---|---|
| Aztreonam lysine, solution for nebulization | 75 mg, 3 times a day, on/off * | 2–3 min | e-Flow® (Altera) |
| Colistimethate, dry powder for inhalation | 1,662,500 de UI, twice a day, continuous treatment | 1–2 min | Turbospin® |
| Colistimethate, solution for nebulization | 1–2 million IU, twice a day, continuous | Variable, depending on nebulizer | e-Flow®, Pari LC plus® |
| 0.5–1 million IU, twice a day, continuous | 3–6 min | I-neb AAD® | |
| Tobramycin, dry powder for inhalation | 112 mg, twice a day, on/off * | ~ 6 min | T-326 Inhalator |
| Tobramycin, solution for nebulization | 300 mg/5 mL, twice a day, on/off * | Variable, depending on nebulizer | e-Flow®, Pari LC plus® |
| 300 mg/4 mL, twice a day, on/off * | Variable, depending on nebulizer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Campos, J.L.; Miravitlles, M.; de la Rosa Carrillo, D.; Cantón, R.; Soler-Cataluña, J.J.; Martinez-Garcia, M.A. Current Challenges in Chronic Bronchial Infection in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 1639. https://doi.org/10.3390/jcm9061639
Lopez-Campos JL, Miravitlles M, de la Rosa Carrillo D, Cantón R, Soler-Cataluña JJ, Martinez-Garcia MA. Current Challenges in Chronic Bronchial Infection in Patients with Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2020; 9(6):1639. https://doi.org/10.3390/jcm9061639
Chicago/Turabian StyleLopez-Campos, José Luis, Marc Miravitlles, David de la Rosa Carrillo, Rafael Cantón, Juan Jose Soler-Cataluña, and Miguel Angel Martinez-Garcia. 2020. "Current Challenges in Chronic Bronchial Infection in Patients with Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 9, no. 6: 1639. https://doi.org/10.3390/jcm9061639
APA StyleLopez-Campos, J. L., Miravitlles, M., de la Rosa Carrillo, D., Cantón, R., Soler-Cataluña, J. J., & Martinez-Garcia, M. A. (2020). Current Challenges in Chronic Bronchial Infection in Patients with Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 9(6), 1639. https://doi.org/10.3390/jcm9061639