Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits
Abstract
1. Introduction
1.1. Utilisation of Science
1.2. The Evolution of Medicine
1.3. The Evolution of Translational Medicine
1.3.1. One-Way Concept
1.3.2. Two-Way Concept
1.3.3. One-Way Multiple Steps Concept
2. Methods
2.1. Initiation and Work Packages
2.2. Chronological Order of the Work
3. Results
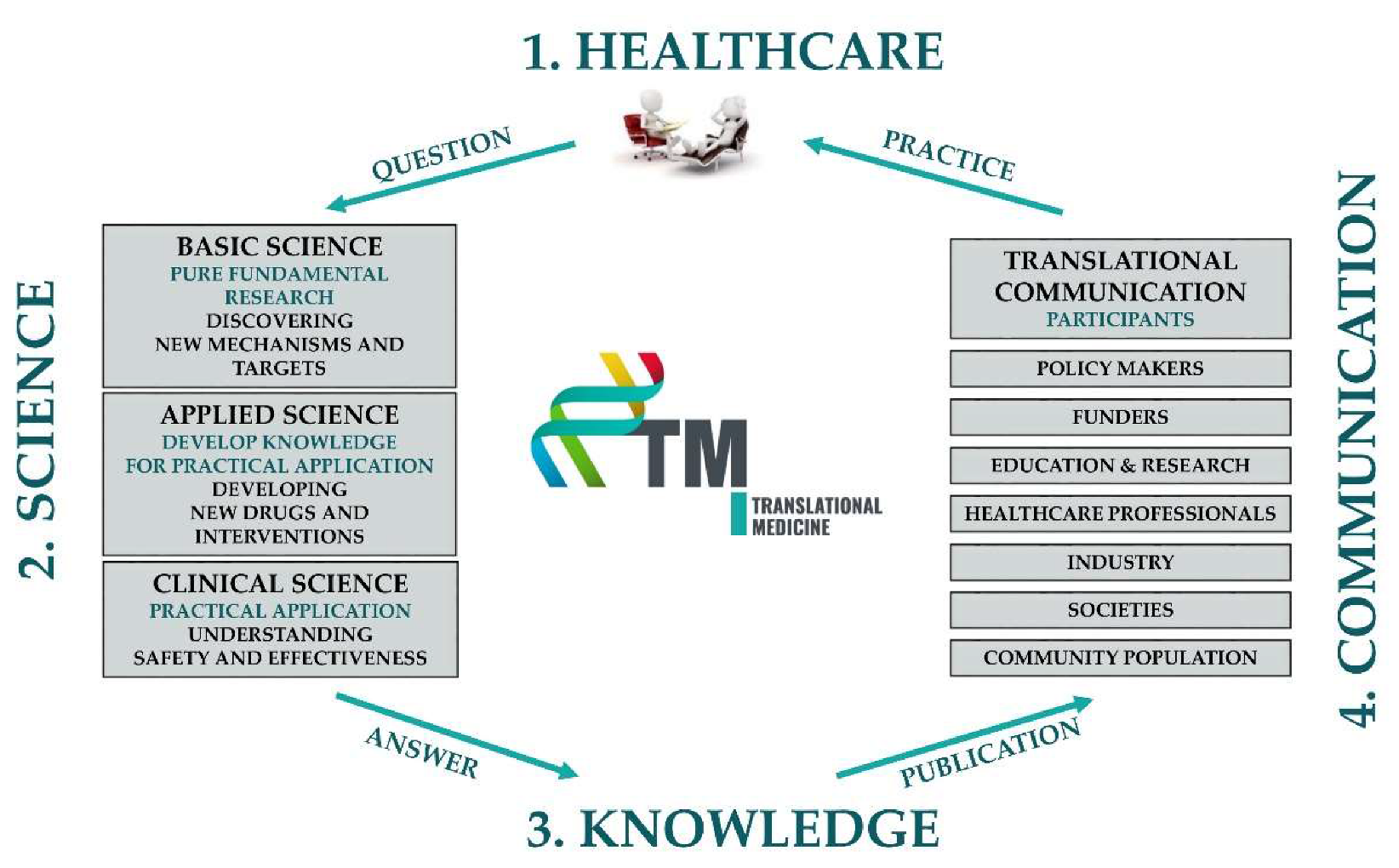
3.1. The TM Cycle
3.1.1. TM Healthcare
Definition of TM Healthcare
Multidisciplinary Care
Patient Care in Specialised, High Volume Centres (Tertiary Care)
Involvement in Healthcare Data Collection
Involvement in Biological Sample Collections
Financing TM Healthcare
Levels of Progressivity in TM Healthcare
Benefits of TM Healthcare
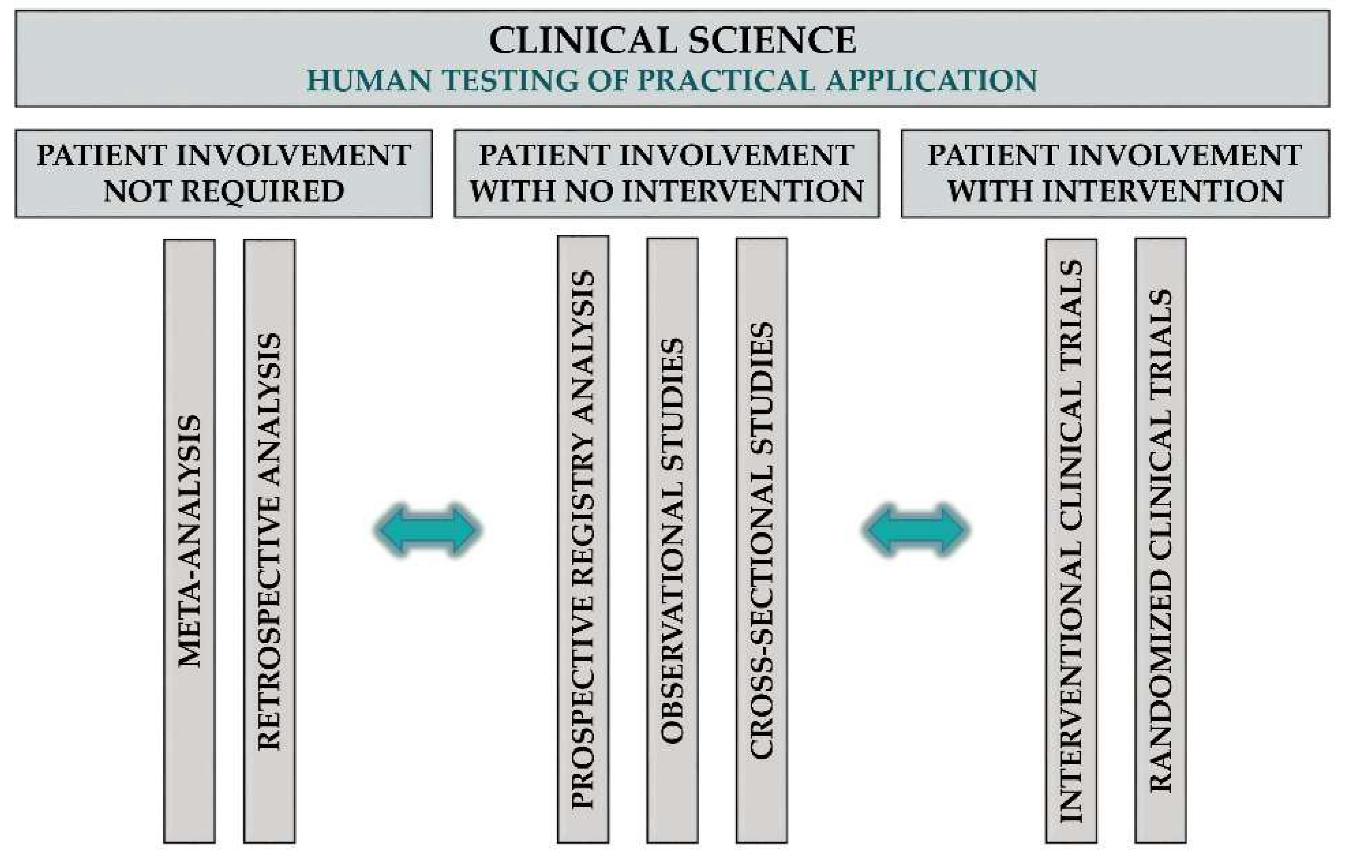
3.1.2. TM Science
Definition of TM Science
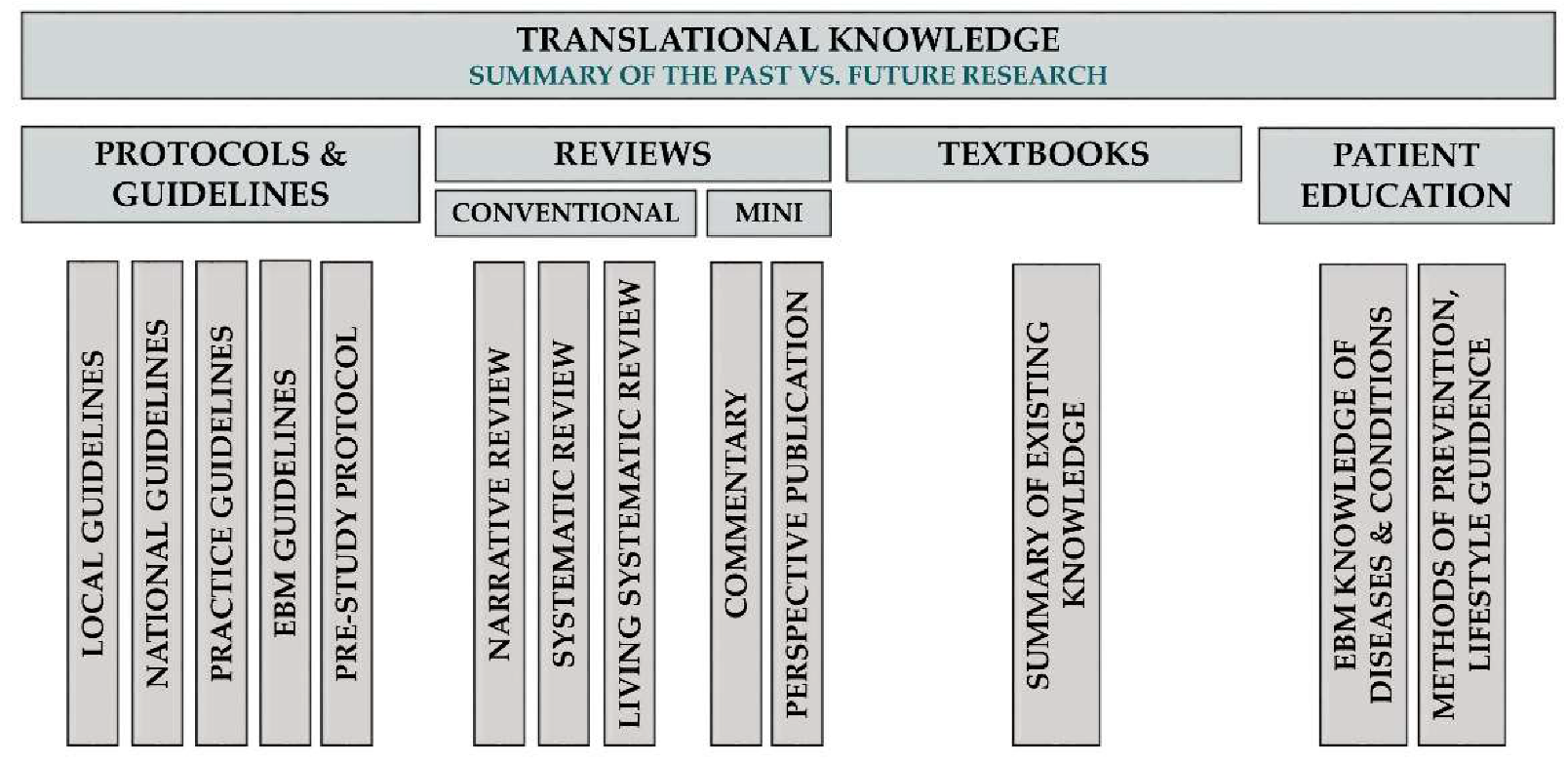
3.1.3. TM Knowledge
Definition of TM Knowledge
Classification of TM Knowledge Articles
Evaluation of TM Knowledge Articles
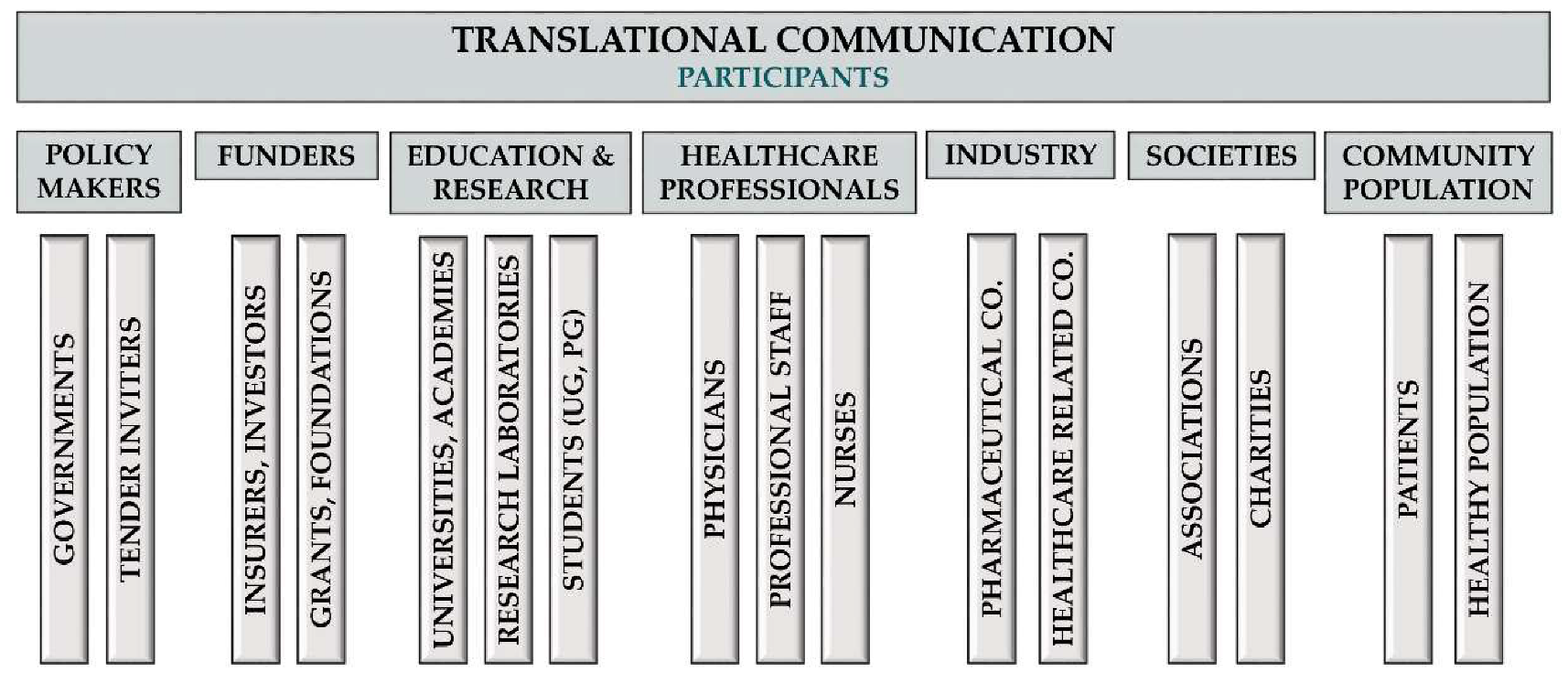
3.1.4. TM Communication
Definition of TM Communication
The Importance of Bilateral Communication
The Importance of Responsible Communication
Channels Used for Communications
Benefits for Patients
Benefits for Healthcare Professionals
3.2. Interdisciplinary Support
3.2.1. The Interdisciplinary Unit in TM
Medical Support Team
Information Technology (IT) Team
Biostatistics Team
Healthcare Economics Team
Clinical Research Administration Team
Legal Team
Communication Team
3.2.2. Quality Requirements
3.3. Academic Aspects
3.3.1. Undergraduate Education
3.3.2. Postgraduate Education
4. Discussion and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Narin, F.; Pinski, G.; Gee, H.H. Structure of the Biomedical Literature. J. Am. Soc. Inf. Sci. 1976, 27, 25–45. [Google Scholar] [CrossRef]
- Scimago Journal and Country Rank (SJR). Available online: https://www.scimagojr.com/ (accessed on 18 May 2020).
- Research Excellence Framework 2014: Overview Report by Main Panel A and Sub-Panels 1 to 6; REF: Bristol, UK, 2014.
- Opthof, T. Differences in citation frequency of clinical and basic science papers in cardiovascular research. Med. Biol. Eng. Comput. 2011, 49, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Boral, A.L.; Multani, P. Translational research: Walking the bridge between idea and cure—Seventeenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1998, 58, 4211–4216. [Google Scholar] [PubMed]
- Sung, N.S.; Crowley, W.F., Jr.; Genel, M.; Salber, P.; Sandy, L.; Sherwood, L.M.; Johnson, S.B.; Catanese, V.; Tilson, H.; Getz, K.; et al. Central challenges facing the national clinical research enterprise. JAMA 2003, 289, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, G.A. Opinion: Anticipating change in drug development: The emerging era of translational medicine and therapeutics. Nat. Rev. Drug Discov. 2005, 4, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Saijo, N. Translational study in cancer research. Intern. Med. 2002, 41, 770–773. [Google Scholar] [CrossRef][Green Version]
- Woolf, S.H. The meaning of translational research and why it matters. JAMA 2008, 299, 211–213. [Google Scholar] [CrossRef]
- Chan, J.Y.; Chang, A.Y.; Chan, S.H. New insights on brain stem death: From bedside to bench. Prog. Neurobiol. 2005, 77, 396–425. [Google Scholar] [CrossRef]
- Goldblatt, E.M.; Lee, W.H. From bench to bedside: The growing use of translational research in cancer medicine. Am. J. Transl. Res. 2010, 2, 1–18. [Google Scholar]
- Recke, A.; Ludwig, R.J. From bedside to bench—Reverse translational medicine. Scientific lessons from revertant mosaicism in ‘knockout’ humans. Exp. Dermatol. 2014, 23, 549–550. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Martin, T.; Ghahramani, P.; Bidaut, L.; Higgins, P.J.; Shahzad, A. Translational Medicine definition by the European Society for Translational Medicine. New Horiz. Transl. Med. 2015, 2, 86–88. [Google Scholar] [CrossRef]
- Surkis, A.; Hogle, J.A.; DiazGranados, D.; Hunt, J.D.; Mazmanian, P.E.; Connors, E.; Westaby, K.; Whipple, E.C.; Adamus, T.; Mueller, M.; et al. Classifying publications from the clinical and translational science award program along the translational research spectrum: A machine learning approach. J. Transl. Med. 2016, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.S.; Williams, N.A.; Richmond, A.; Brown, J.; Strelnick, A.H.; Calhoun, K.; De Loney, E.H.; Allen, S.; Pirie, A.; Wilkins, C.H. Community Experiences and Perceptions of Clinical and Translational Research and Researchers. Prog. Community Health Partnersh. 2018, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Puljak, L.; Sapunar, D. Acceptance of a systematic review as a thesis: Survey of biomedical doctoral programs in Europe. Syst. Rev. 2017, 6, 253. [Google Scholar] [CrossRef]
- FitzGerald, G.A. Anecdotes from ITMAT: Building capacity for translational science. Clin. Pharmacol. Ther. 2013, 94, 291–296. [Google Scholar] [CrossRef]
- Reis, S.E.; Berglund, L.; Bernard, G.R.; Califf, R.M.; Fitzgerald, G.A.; Johnson, P.C.; National, C.; Translational Science Awards, C. Reengineering the national clinical and translational research enterprise: The strategic plan of the National Clinical and Translational Science Awards Consortium. Acad. Med. 2010, 85, 463–469. [Google Scholar] [CrossRef]
- Gristwood, A.; Breithaupt, H. A focus on excellence: An interview with Iain Mattaj, former Director-General of EMBL and Director of the Human Technopole. EMBO Rep. 2019, 20, e47613. [Google Scholar] [CrossRef]
- Petersen, O.H. Reproducibility-again. J. Physiol. 2019, 597, 657–658. [Google Scholar] [CrossRef]
- Boaz, A.; Hanney, S.; Jones, T.; Soper, B. Does the engagement of clinicians and organisations in research improve healthcare performance: A three-stage review. BMJ Open 2015, 5, e009415. [Google Scholar] [CrossRef]
- Rochon, J.; du Bois, A.; Lange, T. Mediation analysis of the relationship between institutional research activity and patient survival. BMC Med. Res. Methodol. 2014, 14, 9. [Google Scholar] [CrossRef]
- Selby, P.; Autier, P. The impact of the process of clinical research on health service outcomes. Ann. Oncol. 2011, 22 (Suppl. 7), vii5–vii9. [Google Scholar] [CrossRef]
- Godi, S.; Eross, B.; Gyomber, Z.; Szentesi, A.; Farkas, N.; Parniczky, A.; Sarlos, P.; Bajor, J.; Czimmer, J.; Miko, A.; et al. Centralized care for acute pancreatitis significantly improves outcomes. J. Gastrointest. Liver Dis. 2018, 27, 151–157. [Google Scholar] [CrossRef]
- Ringelstein, E.B.; Chamorro, A.; Kaste, M.; Langhorne, P.; Leys, D.; Lyrer, P.; Thijs, V.; Thomassen, L.; Toni, D. European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke 2013, 44, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.D.; Smith, D.F.; FitzGerald, G.A.; Hogenesch, J.B. Dosing time matters. Science 2019, 365, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Building the Future of Health Research. Proposal for a European Council for Health Research. Available online: https://ec.europa.eu/programmes/horizon2020/sites/horizon2020/files/building_the_future_of_health_research_sph_22052018_final.pdf (accessed on 18 May 2020).
- Physicians, R.C.O. Benefiting from the ‘Research Effect’. Available online: https://www.rcplondon.ac.uk/file/15901/download (accessed on 18 May 2020).
- Austin, C.P. Translating translation. Nat. Rev. Drug Discov. 2018, 17, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Waldman, S.A.; Terzic, A. Clinical and translational science: From bench-bedside to global village. Clin. Transl. Sci. 2010, 3, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.H. Medical leadership in an increasingly complex world. JAMA 2010, 304, 465–466. [Google Scholar] [CrossRef]
- FitzGerald, G.; Botstein, D.; Califf, R.; Collins, R.; Peters, K.; Van Bruggen, N.; Rader, D. The future of humans as model organisms. Science 2018, 361, 552–553. [Google Scholar] [CrossRef]
- Melamud, E.; Taylor, D.L.; Sethi, A.; Cule, M.; Baryshnikova, A.; Saleheen, D.; van Bruggen, N.; FitzGerald, G.A. The promise and reality of therapeutic discovery from large cohorts. J. Clin. Investig. 2020, 130, 575–581. [Google Scholar] [CrossRef]
- Fontelo, P.; Liu, F. A review of recent publication trends from top publishing countries. Syst. Rev. 2018, 7, 147. [Google Scholar] [CrossRef]
- Van Noorden, R. The science that’s never been cited. Nature 2017, 552, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, C.M.; Crawford, J.M. The impact of review articles. Lab. Investig. 2007, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- The impact factor game. It is time to find a better way to assess the scientific literature. PLoS Med. 2006, 3, e291.
- Garfield, E. The impact factor. Curr. Contents 1994, 25, 3–7. [Google Scholar]
- Simons, K. The misused impact factor. Science 2008, 322, 165. [Google Scholar] [CrossRef]
- Petersen, O.H. FUNCTION Is Now Functional; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Ioannidis, J.P.A.; Thombs, B.D. A user’s guide to inflated and manipulated impact factors. Eur. J. Clin. Investig. 2019, 49, e13151. [Google Scholar] [CrossRef]
- Callaway, E. Preprints come to life. Nature 2013, 503, 180. [Google Scholar] [CrossRef]
- EQUATOR Network-Enhancing the QUAlity and Transparency of Health Research. Available online: https://www.equator-network.org/ (accessed on 18 May 2020).
- Kavanagh, B.P. The GRADE system for rating clinical guidelines. PLoS Med. 2009, 6, e1000094. [Google Scholar] [CrossRef]
- Zadori, N.; Parniczky, A.; Szentesi, A.; Hegyi, P. Insufficient implementation of the IAP/APA guidelines on aetiology in acute pancreatitis: Is there a need for implementation managers in pancreatology? United Eur. Gastroenterol. J. 2020, 8, 246–248. [Google Scholar] [CrossRef]
- Baron, D.M.; Metnitz, P.G.H.; Rhodes, A.; Kozek-Langenecker, S.A. Clinical guidelines: How can we improve adherence and implementation? Eur. J. Anaesthesiol. 2017, 34, 329–331. [Google Scholar] [CrossRef]
- Nordback, I.; Pelli, H.; Lappalainen-Lehto, R.; Jarvinen, S.; Raty, S.; Sand, J. The recurrence of acute alcohol-associated pancreatitis can be reduced: A randomized controlled trial. Gastroenterology 2009, 136, 848–855. [Google Scholar] [CrossRef]
- Horbach, S.; Halffman, W.W. The changing forms and expectations of peer review. Res. Integr. Peer Rev. 2018, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- King, S.R. Consultative review is worth the wait. Elife 2017, 6, e32012. [Google Scholar] [CrossRef] [PubMed]
- NIHR Dissemination Centre. Care at the Scene-Research for Ambulance Services; NIHR Dissemination Centre: Rockville, MD, USA, 2016. [Google Scholar] [CrossRef]
- van der Heide, I.; Uiters, E.; Sorensen, K.; Rothlin, F.; Pelikan, J.; Rademakers, J.; Boshuizen, H. Health literacy in Europe: The development and validation of health literacy prediction models. Eur. J. Public Health 2016, 26, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Rajah, R.; Ahmad Hassali, M.A.; Jou, L.C.; Murugiah, M.K. The perspective of healthcare providers and patients on health literacy: A systematic review of the quantitative and qualitative studies. Perspect. Public Health 2018, 138, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, S.; Kok, G.; Ledford, J.G.; Sandalova, E.; Stevelink, R. Possibilities and Pitfalls of Social Media for Translational Medicine. Front. Med. (Lausanne) 2018, 5, 345. [Google Scholar] [CrossRef]
- da Costa, D.W.; Bouwense, S.A.; Schepers, N.J.; Besselink, M.G.; van Santvoort, H.C.; van Brunschot, S.; Bakker, O.J.; Bollen, T.L.; Dejong, C.H.; van Goor, H.; et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): A multicentre randomised controlled trial. Lancet 2015, 386, 1261–1268. [Google Scholar] [CrossRef]
- Hanna, E.; Toumi, M.; Dussart, C.; Borissov, B.; Dabbous, O.; Badora, K.; Auquier, P. Funding breakthrough therapies: A systematic review and recommendation. Health Policy 2018, 122, 217–229. [Google Scholar] [CrossRef]
- Madon, T.; Hofman, K.J.; Kupfer, L.; Glass, R.I. Public health. Implementation science. Science 2007, 318, 1728–1729. [Google Scholar] [CrossRef]
- Gilliland, C.T.; Zuk, D.; Kocis, P.; Johnson, M.; Hay, S.; Hajduch, M.; Bietrix, F.; Aversa, G.; Austin, C.P.; Ussi, A.E. Putting translational science on to a global stage. Nat. Rev. Drug Discov. 2016, 15, 217–218. [Google Scholar] [CrossRef][Green Version]
- Alving, B.; Dai, K.; Chan, S.H.H. (Eds.) Translational Medicine–What, Why and How: An International Perspective; Translational Research in Biomedicine, Karger: Basel, Switzerland, 2013; Volume 3. [Google Scholar]
- Denne, S.C.; Sajdyk, T.; Sorkness, C.A.; Drezner, M.K.; Shekhar, A. Utilizing Pilot Funding and Other Incentives to Stimulate Interdisciplinary Research; Translational Research in Biomedicine, Karger: Basel, Switzerland, 2013; Volume 3, pp. 63–73. [Google Scholar]
- Tarantal, A.F.; Rainwater, J.; Wun, T.; Berglund, L. The US Initiative: Clinical and Translational Science Awards–The UC Davis Perspective; Translational Research in Biomedicine, Karger: Basel, Switzerland, 2013; Volume 3, pp. 18–28. [Google Scholar]
- PRC State Counsil. National Long-Term Science and Technology Development Outline (2006–2020); The State Council: Beijing, China, 2006.
- Serger, S.S.; Breidne, M. China’s Fifteen-Year Plan for Science and Technology: An Assessment. Asia Policy 2007, 4, 64. [Google Scholar] [CrossRef]
- Shi, T.Z.; Dai, K. Training Translational Investigators in China; Translational Research in Biomedicine, Karger: Basel, Switzerland, 2013; Volume 3, pp. 47–55. [Google Scholar]
- Soderquest, K.; Lord, G.M. Strategies for translational research in the United Kingdom. Sci. Transl. Med. 2010, 2, 53cm28. [Google Scholar] [CrossRef] [PubMed]
- Skarke, C.; FitzGerald, G.A. Training translators for smart drug discovery. Sci. Transl. Med. 2010, 2, 26cm12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meagher, E.A.; Fitzgerald, G.A. Efficient drug approval and monitoring must rely on sound regulatory science. Nat. Med. 2011, 17, 1535. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 3rd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Rogowski, W.; John, J.; IJzerman, M.J. Translational Health Economics. In World Scientific Handbook of Global Health Economics and Public Policy; Scheffler, R.M., Ed.; World Scientific Publishing Company: Singapore, 2016. [Google Scholar]
- Hastings, C.E.; Fisher, C.A.; McCabe, M.A.; Allison, J.; Brassil, D.; Offenhartz, M.; Browning, S.; DeCandia, E.; Medina, R.; Duer-Hefele, J.; et al. Clinical research nursing: A critical resource in the national research enterprise. Nurs. Outlook 2012, 60, 149–156.e1–3. [Google Scholar] [CrossRef]


| TM HEALTHCARE | IDENTIFIED PROBLEMS | |
| 1 | Lack of hospital-level quality assessment and feedback makes it impossible to define where the best quality treatment is available. | |
| 2 | Although we know that the multidisciplinary approach is essential to ensure good quality and effective patient care, it is still not applied in many cases. | |
| 3 | Only a narrow area of patient care is involved in scientific activities or clinical research. | |
| 4 | Funding is not sufficient for covering the costs of scientific activities (staffing, consumables, facility development) in many healthcare centers. | |
| SUGGESTED SOLUTIONS | ||
| 1 | The widespread adoption of science in patient-care is needed for improving the quality and cost-effectiveness of healthcare. | |
| 2 | Multidisciplinary teams should be formed everywhere to increase safety and achieve efficiency and added value in patient care. | |
| 3 | Continuous data recording and monitoring are needed for hospital-level assessment of quality and effectiveness to define the necessary changes in the structure of healthcare (treatment centers) or funding. | |
| 4 | Biological sample collection and cooperation of healthcare centers and research centers are necessary to promote scientific activity. | |
| 5 | Tertiary centers may be ideal locations to start implementing the TM Healthcare system | |
| 6 | All healthcare providers and other disciplines should agree on evidence (science)-based patient management and work together in a multidisciplinary approach. | |
| 7 | General patient care should be financed by national and private health insurance. Activities intended to achieve new scientific results should be funded by institutional, national and international grants and funds. Current budgets should be elevated as well. | |
| TM SCIENCE | IDENTIFIED PROBLEMS | |
| 1 | Many discoveries do not reach patient care. | |
| 2 | Clinically relevant questions are not addressed through the arsenal of basic research methodologies. | |
| 3 | The regional differences in funding and opportunities. (In Western Europe, funding of basic science is inadequate in relation to the scientific opportunities that exist. Whereas clinical science is less developed in Eastern Europe). | |
| SUGGESTED SOLUTIONS | ||
| 1 | Close cooperation of healthcare centers and research centers are necessary to promote scientific activity. | |
| 2 | All publications describing new, previously unknown results should be considered as original publications, regardless of whether these analyses are based on newly generated or already existing data | |
| 3 | Funding has to be tailored to research needs. A special emphasis should be put on raising budgets for science in Eastern and Central European countries. | |
| TM KNOWLEDGE | IDENTIFIED PROBLEMS | |
| 1 | Readers, professionals cannot cope with the huge number of publications, knowledge released. | |
| 2 | Many review articles are inaccurate or biased. | |
| 3 | There is a huge gap between the guideline, knowledge and its implementation. | |
| 4 | Summary publication and standardization is not rewarded in the academic carrier, neither in impact factors. | |
| SUGGESTED SOLUTIONS | ||
| 1 | A proper classification of summary publication is needed, our paper recommends one. | |
| 2 | Quality assessment of summary publications is extremely important. Several measure are recommended in the peer review process (consultative peer review, technical innovations, etc.) | |
| 3 | A good critical review should be rewarded with impact factors as well. | |
| 4 | Summary publication, standardization is an important part of the TM cycle, the field should be rewarded in academic progress and in publication impact. | |
| TM COMMUNICATION | IDENTIFIED PROBLEMS | |
| 1 | Often there is a lack of communication between the participants of healthcare (e.g., scientists and insurance policy-makers or scientists and patients). | |
| 2 | Guidelines are often not translated into the local language and not incorporated into insurance policies in many countries, which is an obstacle for implementation. | |
| 3 | Patient organizations and advocacy is underdeveloped in many countries. | |
| 4 | Medical students and nurses has no access to clinical research methodology knowledge as the curricula do not cover them. | |
| SUGGESTED SOLUTIONS | ||
| 1 | Bilateral/multilateral communication need to be developed between participants of healthcare. Feedback has a crucial importance. | |
| 2 | Guidelines need to be translated and incorporated into insurance policies, knowledge publication should be communicated to healthcare professionals in order to be implemented. | |
| 3 | Patient organizations have a critical role as channels for patient educations and advocacy, in most countries patient organizations have to be developed. | |
| 4 | All research methodologies (including clinical research) should be included in the curricula of medical universities and education of nurses. | |
| 5 | Policymakers need guidance to create a balance between ensuring patient access to innovation and maintaining financial sustainability | |
| TM INTERDISCIPLINARY | IDENTIFIED PROBLEMS | |
| 1 | Lack of time, resources for organizing clinical research among clinicians. | |
| 2 | Lack of clinical research methodology knowledge and special knowledge in the fields of biostatistics, IT, communication to policymakers, etc. among clinicians and researchers. | |
| 3 | A dedicated interdisciplinary team for supporting the TM cycle elements is missing from the academic organization in many countries, especially in Central and Eastern Europe. | |
| SUGGESTED SOLUTIONS | ||
| 1 | Providing funds for and establishing interdisciplinary teams in the academic environment supporting TM. | |
| 2 | The interdisciplinary team should cover the fields of biostatistics, IT, data management and patient inclusion coordination, ethical submissions, communication, patient club coordination, implementation coordination, other supporting roles like event coordination, management, administration and training in regulatory science, etc. | |
| TM ACADEMY | IDENTIFIED PROBLEMS | |
| 1 | Universities and academic institutes do not adapt fast enough to the changing environment, concerning the inclusion of new methodologies in their curricula. | |
| 2 | Clinical research nursing is not included in nursing curricula, although they play a critical role in play a role in the maintenance of participant safety, the integrity of protocol implementation and the accuracy of data collection. | |
| 3 | Because of the lack of knowledge in clinical research methodologies among supervisors and interdisciplinary support units, clinical research is not very popular among students. | |
| SUGGESTED SOLUTIONS | ||
| 1 | Medical schools are strongly advised to plan compulsory or at least elective courses for medical students to teach them the basics of TM, scientific methodologies and scientific knowledge supplemented by techniques of effective medical information translation for the different members of the community. | |
| 2 | Nursing curricula should include clinical research nursing. | |
| 3 | New form of education is needed, a ‘learning by doing’ model, which may involve practicing physicians beside student and those seeking PhD. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, 1532. https://doi.org/10.3390/jcm9051532
Hegyi P, Petersen OH, Holgate S, Erőss B, Garami A, Szakács Z, Dobszai D, Balaskó M, Kemény L, Peng S, et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. Journal of Clinical Medicine. 2020; 9(5):1532. https://doi.org/10.3390/jcm9051532
Chicago/Turabian StyleHegyi, Péter, Ole H. Petersen, Stephen Holgate, Bálint Erőss, András Garami, Zsolt Szakács, Dalma Dobszai, Márta Balaskó, Lajos Kemény, Shuang Peng, and et al. 2020. "Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits" Journal of Clinical Medicine 9, no. 5: 1532. https://doi.org/10.3390/jcm9051532
APA StyleHegyi, P., Petersen, O. H., Holgate, S., Erőss, B., Garami, A., Szakács, Z., Dobszai, D., Balaskó, M., Kemény, L., Peng, S., Monteiro, J., Varró, A., Lamont, T., Laurence, J., Gray, Z., Pickles, A., FitzGerald, G. A., Griffiths, C. E. M., Jassem, J., ... Szentesi, A. (2020). Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. Journal of Clinical Medicine, 9(5), 1532. https://doi.org/10.3390/jcm9051532











