Combined Use of Febuxostat and Colchicine Does Not Increase Acute Hepatotoxicity in Patients with Gout: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Definition of Hepatotoxicity
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Gout Patients with or without Colchicine
3.2. Comparison of Baseline Characteristics According to Hepatotoxicity in Gout Patients on Febuxostat
3.3. Logistic Regression Analysis for Hepatotoxicity in Gout Patients on Febuxostat
3.4. Side Effects of Colchicine
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Kim, J.W.; Kwak, S.G.; Lee, H.; Kim, S.K.; Choe, J.Y.; Park, S.H. Prevalence and incidence of gout in korea: Data from the national health claims database 2007–2015. Rheumatol. Int. 2017, 37, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and metabolic syndrome: A tangled web. Curr. Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 american college of rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef] [Green Version]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H.; et al. 2016 updated eular evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 29–42. [Google Scholar] [CrossRef]
- Todd, B.A.; Billups, S.J.; Delate, T.; Canty, K.E.; Kauffman, A.B.; Rawlings, J.E.; Wagner, T.M. Assessment of the association between colchicine therapy and serious adverse events. Pharmacotherapy 2012, 32, 974–980. [Google Scholar] [CrossRef]
- Lee, J.S.; Won, J.; Kwon, O.C.; Lee, S.S.; Oh, J.S.; Kim, Y.G.; Lee, C.K.; Yoo, B.; Hong, S. Hepatic safety of febuxostat compared with allopurinol in gout patients with fatty liver disease. J. Rheumatol. 2019, 46, 527–531. [Google Scholar] [CrossRef]
- Abbott, C.E.; Xu, R.; Sigal, S.H. Colchicine-induced hepatotoxicity. ACG Case Rep. J. 2017, 4, e120. [Google Scholar] [CrossRef]
- Sharma, P.; Tyagi, P.; Singla, V.; Bansal, N.; Kumar, A.; Arora, A. Clinical and biochemical profile of tuberculosis in patients with liver cirrhosis. J. Clin. Exp. Hepatol. 2015, 5, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Febuxostat: A review of its use in the treatment of hyperuricaemia in patients with gout. Drugs 2015, 75, 427–438. [Google Scholar] [CrossRef]
- Fisher, M.C.; Pillinger, M.H.; Keenan, R.T. Inpatient gout: A review. Curr. Rheumatol. Rep. 2014, 16, 458. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.H.; Lee, E.Y.; Lee, Y.J.; Song, Y.W.; Lee, E.B. Clinical features and risk factors of postsurgical gout. Ann. Rheum. Dis. 2008, 67, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.A.; Oude Voshaar, M.A.H.; Ten Klooster, P.M.; Vonkeman, H.E.; van de Laar, M. Prognostic factors associated with early gout flare recurrence in patients initiating urate-lowering therapy during an acute gout flare. Clin. Rheumatol. 2019, 38, 2233–2239. [Google Scholar] [CrossRef] [Green Version]
- Bursill, D.; Taylor, W.J.; Terkeltaub, R.; Abhishek, A.; So, A.K.; Vargas-Santos, A.B.; Gaffo, A.L.; Rosenthal, A.; Tausche, A.K.; Reginato, A.; et al. Gout, hyperuricaemia and crystal-associated disease network (g-can) consensus statement regarding labels and definitions of disease states of gout. Ann. Rheum. Dis. 2019, 78, 1592–1600. [Google Scholar] [CrossRef]
- Tatlock, S.; Rudell, K.; Panter, C.; Arbuckle, R.; Harrold, L.R.; Taylor, W.J.; Symonds, T. What outcomes are important for gout patients? In-depth qualitative research into the gout patient experience to determine optimal endpoints for evaluating therapeutic interventions. Patient 2017, 10, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, K.; Gow, P.; Vanderpyl, J.; Logo, P.; Dalbeth, N. The experience and impact of living with gout: A study of men with chronic gout using a qualitative grounded theory approach. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2011, 17, 1–6. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, C.H.; Lee, J.H.; Yoon, B.Y.; Kim, H.A.; Suh, C.H.; Choi, S.T.; Song, J.S.; Joo, H.Y.; Choi, S.J.; et al. Current gout treatment and flare in south korea: Prophylactic duration associated with fewer gout flares. Int. J. Rheum. Dis. 2017, 20, 497–503. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Gao, W. Prophylaxis on gout flares after the initiation of urate-lowering therapy: A retrospective research. Int. J. Clin. Exp. Med. 2015, 8, 21460–21465. [Google Scholar]
- Dalbeth, N.; Lauterio, T.J.; Wolfe, H.R. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 2014, 36, 1465–1479. [Google Scholar] [CrossRef] [Green Version]
- Nuki, G. Colchicine: Its mechanism of action and efficacy in crystal-induced inflammation. Curr. Rheumatol. Rep. 2008, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine--update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alayli, G.; Cengiz, K.; Canturk, F.; Durmus, D.; Akyol, Y.; Menekse, E.B. Acute myopathy in a patient with concomitant use of pravastatin and colchicine. Ann. Pharmacother. 2005, 39, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H. Colchicine induced toxicity and pancytopenia at usual doses and treatment with granulocyte colony-stimulating factor. J. Rheumatol. 2001, 28, 1199–1200. [Google Scholar] [PubMed]
- Terkeltaub, R.A.; Furst, D.E.; Digiacinto, J.L.; Kook, K.A.; Davis, M.W. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome p450 3a4/p-glycoprotein inhibitors. Arthritis Rheum. 2011, 63, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, D.; Li, W.; Wang, K.; Peng, Y.; Zheng, J. Electrophilicities and protein covalent binding of demethylation metabolites of colchicine. Chem. Res. Toxicol. 2016, 29, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, Y.; Li, Q.; Yang, X.; Zhao, G.; Peng, Y.; Zheng, J. Studies on hepatotoxicity and toxicokinetics of colchicine. J. Biochem. Mol. Toxicol. 2019, 33, e22366. [Google Scholar] [CrossRef]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Ye, Z.; Gu, S.W.; Jiang, Z.Y.; Zhao, L. The efficacy and tolerability of febuxostat treatment in a cohort of chinese han population with history of gout. J. Int. Med Res. 2020, 48, 300060520902950. [Google Scholar] [CrossRef]
| Colchicine User (N = 121) | Colchicine No-User (N = 57) | p Value | |
|---|---|---|---|
| Age, years | 51.0 (37.0–62.0) | 56.0 (43.5–68.5) | 0.203 |
| Male | 119 (98.3) | 49 (86.0) | 0.002 |
| Disease duration, months | 26.6 (15.4–61.7) | 23.9 (16.4–41.6) | 0.748 |
| Symptom duration, months | 36.1 (5.2–73.3) | 13.6 (0.8–55.2) | 0.134 |
| Duration of febuxostat use, months | 17.6 (10.3–27.7) | 20.8 (14.5–31.0) | 0.165 |
| Dosage of febuxostat, mg/day | 59.2 ± 21.5 | 56.8 ± 19.9 | 0.491 |
| Duration of colchicine use, months | 13.3 (6.9–21.5) | ||
| Dosage of colchicine, mg/day | 0.6 ± 0.2 | ||
| Presence of tophi | 24 (19.8) | 9 (15.8) | 0.680 |
| Renal stone | 9 (7.4) | 5 (8.8) | 1.0 |
| Family history | 9 (7.4) | 4 (7.0) | 1.0 |
| Previous history of cancer | 8 (6.6) | 4 (7.0) | 1.0 |
| Gout flares within 3 months | 14/113 (12.4) | 24/51 (47.1) | <0.001 |
| Comorbidities | |||
| Hypertension | 46 (38.0) | 34 (59.6) | 0.010 |
| Diabetes mellitus | 10 (8.3) | 14 (24.6) | 0.005 |
| Cerebrovascular disease | 15 (12.4) | 14 (24.6) | 0.051 |
| Heart failure | 1 (0.8) | 4 (7.0) | 0.037 |
| Dyslipidemia | 31 (25.6) | 14 (24.6) | 1.0 |
| Hypertriglyceridemia | 56 (46.3) | 23 (40.4) | 0.589 |
| Liver disease | 24 (19.8) | 13 (22.8) | 0.694 |
| Chronic kidney disease (eGFR < 60 mL/min/1.73 m2) | 8 (6.6) | 19 (33.3) | <0.001 |
| Dementia | 1 (0.8) | 1 (1.8) | 0.539 |
| Laboratory findings | |||
| Uric acid (mg/dL) | 8.6 (7.0–9.9) | 8.4 (6.9–9.8) | 0.618 |
| AST (IU/L) | 29.0 (23.0–36.0) | 26.5 (23.0–37.8) | 0.163 |
| ALT (IU/L) | 31.5 (22.0–44.3) | 27.5 (19.0–43.0) | 0.465 |
| BUN (mg/dL) | 15.3 (12.9–19.3) | 18.9 (13.3–27.1) | 0.139 |
| Cr (mg/dL) | 1.0 (0.8–1.1) | 1.1 (0.9–1.8) | 0.006 |
| eGFR (mL/min/1.73 m2) | 89.0 (75.0–104.5) | 70.0 (34.0–96.5) | 0.020 |
| Total cholesterol (mg/dL) | 190.0 (154.0–216.0) | 171.5 (146.5–203.8) | 0.078 |
| Triglyceride (mg/dL) | 207.0 (123.0–292.0) | 199.5 (129.0–260.5) | 0.897 |
| LDL (mg/dL) | 112.5 (86.3–135.0) | 106.0 (82.0–128.0) | 0.268 |
| HDL (mg/dL) | 46.0 (40.0–51.0) | 43.0 (37.3–54.0) | 0.508 |
| Medications | |||
| Aspirin | 14 (11.6) | 13 (22.8) | 0.072 |
| Diuretics | 8 (6.6) | 15 (26.3) | 0.001 |
| Hepatotoxicity (N = 17) | No Hepatotoxicity (N = 161) | p Value | |
|---|---|---|---|
| Age, years | 38.0 (34.0–60.0) | 54.0 (39.0–64.0) | 0.166 |
| Male | 17 (100.0) | 151 (93.8) | 0.601 |
| Disease duration, months | 24.3 (17.8–91.1) | 26.1 (15.4–47.5) | 1.0 |
| Symptom duration, months | 18.2 (0.8–90.2) | 25.1 (3.9–67.9) | 0.793 |
| Duration of febuxostat use, months | 17.5 (6.6–27.0) | 19.2 (11.7–29.0) | 0.645 |
| Dosage of febuxostat, mg/day | 50.6 ± 20.1 | 59.3 ± 21.0 | 0.109 |
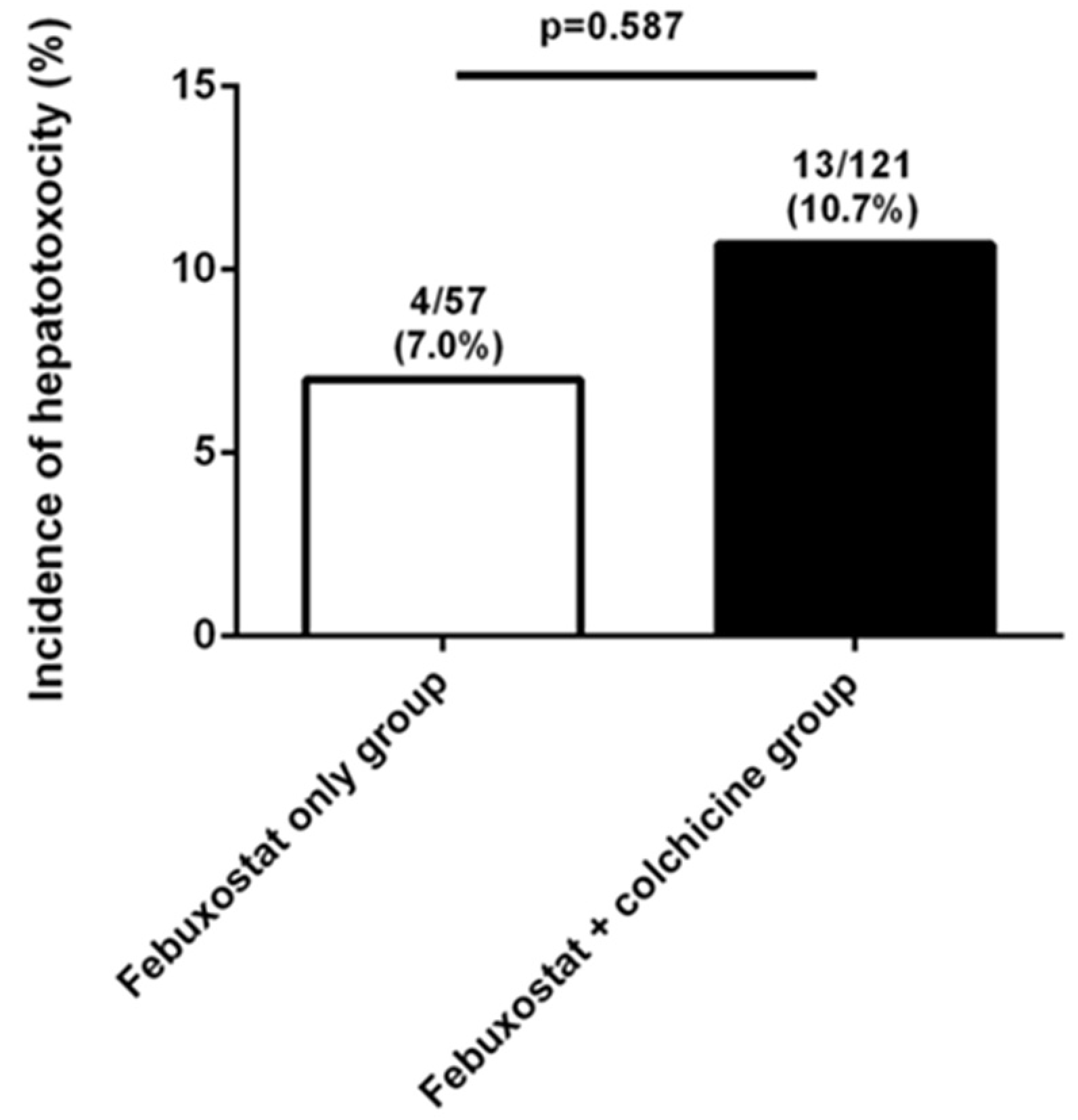
| Use of colchicine | 13 (76.5) | 108 (67.1) | 0.587 |
| Duration of colchicine use, months | 7.0 (3.9–25.3) | 13.4 (7.4–21.5) | 0.975 |
| Dosage of colchicine, mg/day | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.858 |
| Presence of tophi | 3 (17.6) | 30 (18.6) | 1.0 |
| Gout flares | 2 (11.8) | 39 (24.2) | 0.365 |
| Comorbidities | |||
| Hypertension | 6 (35.3) | 74 (46.0) | 0.452 |
| Diabetes mellitus | 3 (17.6) | 21 (13.0) | 0.706 |
| Cerebrovascular disease | 2 (11.8) | 27 (16.8) | 1.0 |
| Heart failure | 1 (5.9) | 4 (2.5) | 0.398 |
| Dyslipidemia | 7 (41.2) | 38 (23.6) | 0.142 |
| Hypertriglyceridemia | 7 (41.2) | 70 (43.4) | 1.0 |
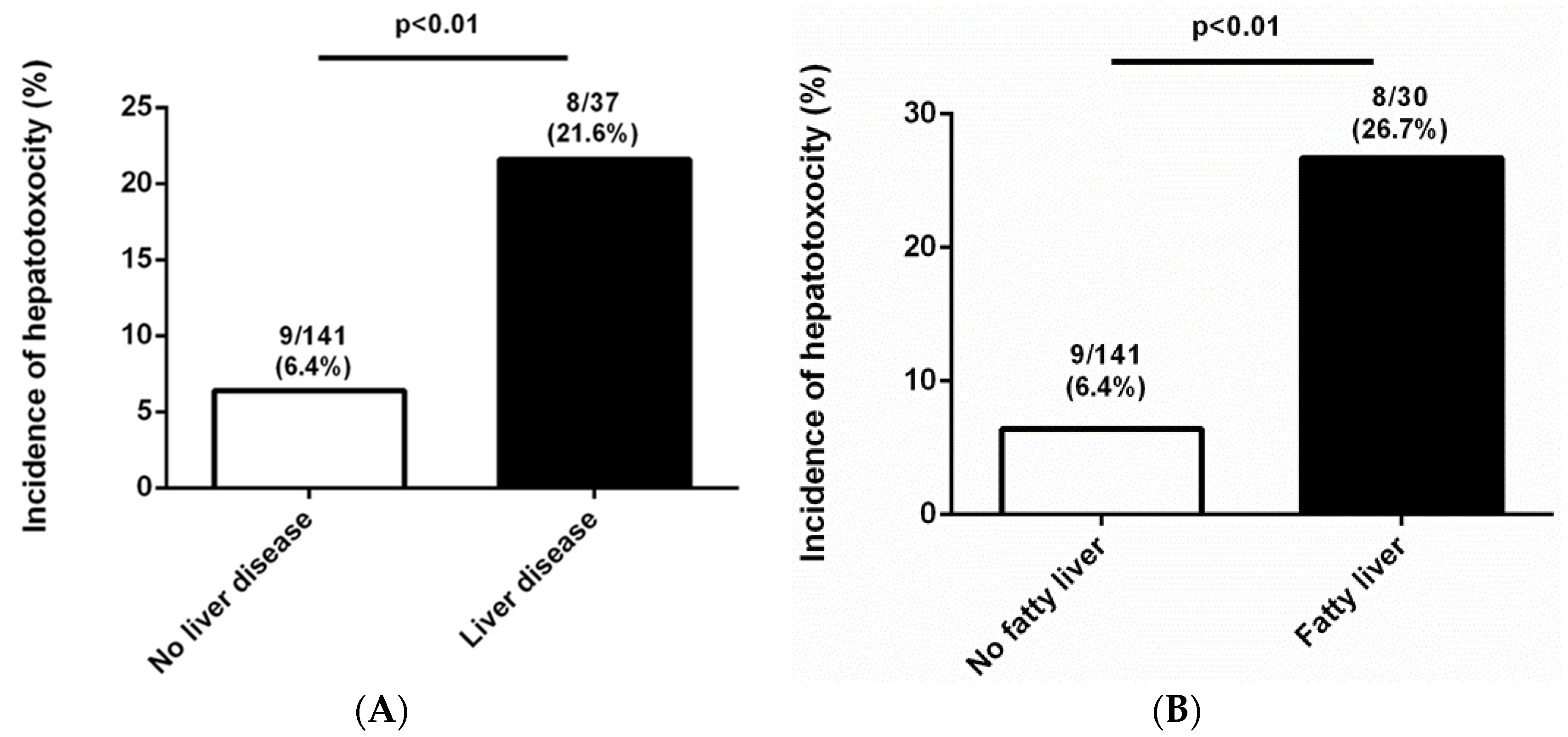
| Liver disease | 8 (47.1) | 29 (18.0) | 0.010 |
| Chronic kidney disease (eGFR < 60 mL/min/1.73 m2) | 1 (5.9) | 26 (16.1) | 0.476 |
| Dementia | 1 (5.9) | 1 (0.6) | 0.182 |
| Laboratory findings | |||
| Uric acid (mg/dL) | 8.6 (7.0–9.8) | 8.5 (6.9–9.8) | 1.0 |
| AST (IU/L) | 30.0 (26.5–44.8) | 28.0 (23.0–35.3) | 0.402 |
| ALT (IU/L) | 42.5 (20.0–76.3) | 29.0 (21.0–41.3) | 0.755 |
| BUN (mg/dL) | 13.3 (10.1–19.3) | 15.9 (13.2–22.4) | 0.793 |
| Cr (mg/dL) | 1.0 (0.8–1.2) | 1.0 (0.9–1.2) | 0.925 |
| eGFR (mL/min/1.73 m2) | 84.0 (78.3–103.0) | 85.5 (63.8–104.0) | 0.8 |
| Total cholesterol (mg/dL) | 195.0 (157.0–230.0) | 176.5 (151.0–210.3) | 0.1 |
| Triglyceride (mg/dL) | 215.5 (137.8–282.8) | 199.0 (123.0–292.0) | 0.784 |
| LDL (mg/dL) | 142.0 (119.0–165.0) | 108.0 (82.0–129.0) | 0.01 |
| HDL (mg/dL) | 44.0 (40.0–51.0) | 46.0 (39.0–52.0) | 0.982 |
| Medications | |||
| Aspirin | 3 (17.6) | 24 (14.9) | 0.726 |
| Diuretics | 1 (5.9) | 22 (13.7) | 0.702 |
| Baseline Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 0.975 (0.946–1.006) | 0.114 | 0.976 (0.941–1.013) | 0.198 |
| Duration of febuxostat use | 0.990 (0.956–1.025) | 0.573 | ||
| Febuxostat dosage | 0.978 (0.952–1.005) | 0.113 | 0.976 (0.946–1.006) | 0.120 |
| Colchicine use | 1.595 (0.496–5.128) | 0.433 | ||
| Duration of colchicine use | 0.998 (0.954–1.043) | 0.917 | ||
| Colchicine dosage | 0.700 (0.015–32.928) | 0.856 | ||
| ALT | 1.016 (0.998–1.034) | 0.082 | 1.010 (0.987–1.033) | 0.415 |
| LDL | 1.0 (0.997–1.003) | 0.821 | ||
| Hyperlipidemia | 2.266 (0.807–6.360) | 0.120 | 1.855 (0.581–5.920) | 0.296 |
| Chronic kidney disease | 0.325 (0.041–2.555) | 0.285 | ||
| Liver disease | 4.046 (1.439–11.375) | 0.008 | 4.083 (1.326–12.577) | 0.014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.-J.; Moon, K.W. Combined Use of Febuxostat and Colchicine Does Not Increase Acute Hepatotoxicity in Patients with Gout: A Retrospective Study. J. Clin. Med. 2020, 9, 1488. https://doi.org/10.3390/jcm9051488
Oh Y-J, Moon KW. Combined Use of Febuxostat and Colchicine Does Not Increase Acute Hepatotoxicity in Patients with Gout: A Retrospective Study. Journal of Clinical Medicine. 2020; 9(5):1488. https://doi.org/10.3390/jcm9051488
Chicago/Turabian StyleOh, Yoon-Jeong, and Ki Won Moon. 2020. "Combined Use of Febuxostat and Colchicine Does Not Increase Acute Hepatotoxicity in Patients with Gout: A Retrospective Study" Journal of Clinical Medicine 9, no. 5: 1488. https://doi.org/10.3390/jcm9051488





