Contemporary Cardiovascular Risk Assessment for Type 2 Diabetes Including Heart Failure as an Outcome: The Fremantle Diabetes Study Phase II
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Epidemiological Setting, and Approvals
2.2. Clinical and Laboratory Methods
2.3. Ascertainment of Incident Myocardial Infarction, Stroke, and Heart Failure
2.4. Validation Dataset
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of FDS1 Versus FDS2 Type 2 Diabetes Cohorts
3.2. Performance of the FDS1 Five-Year CVD Risk Equation in the FDS2 Type 2 Diabetes Cohort
3.3. Performance of the ADVANCE CVD Risk Equation in the FDS2 Type 2 Diabetes Cohort
3.4. Participant Characteristics and Outcome
3.5. Independent Associates of First Incident Three-point MACE
3.6. Independent Associates of First Incident Four-point MACE
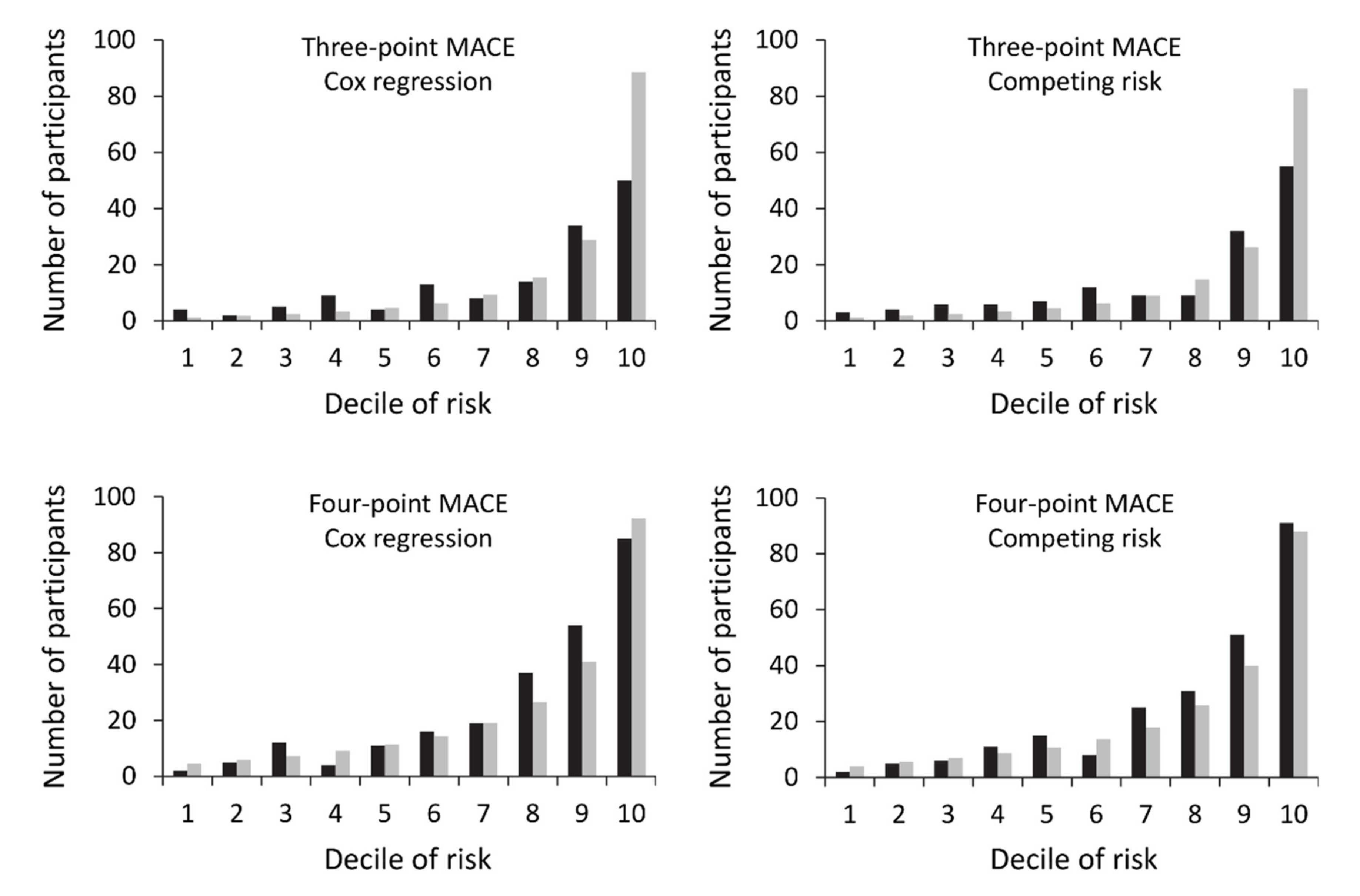
3.7. Model Performance
3.8. External Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooney, M.T.; Dudina, A.L.; Graham, I.M. Value and limitations of existing scores for the assessment of cardiovascular risk: A review for clinicians. J. Am. Coll. Cardiol. 2009, 54, 1209–1227. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; Nouri, F.; Korownyk, C.; Kolber, M.R.; Vandermeer, B.; McCormack, J. Agreement among cardiovascular disease risk calculators. Circulation 2013, 127, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Van der Leeuw, J.; van Dieren, S.; Beulens, J.W.; Boeing, H.; Spijkerman, A.M.; van der Graaf, Y.; van der, A.D.; Nothlings, U.; Visseren, F.L.; Rutten, G.E.; et al. The validation of cardiovascular risk scores for patients with type 2 diabetes mellitus. Heart 2015, 101, 222–229. [Google Scholar] [CrossRef] [PubMed]
- National Vascular Disease Prevention Alliance. Guidelines for the Management of Absolute Cardiovascular Disease Risk. 2012. Available online: http://cvdcheck.org.au/pdf/Absolute_CVD_Risk_Full_Guidelines.pdf (accessed on 13 April 2020).
- Davis, W.A.; Colagiuri, S.; Davis, T.M.E. Comparison of the performance of the UKPDS and Framingham cardiovascular risk equations in an independent cohort of type 2 diabetic patients without prior cardiovascular disease: The Fremantle Diabetes Study. Med. J. Aust. 2009, 90, 180–184. [Google Scholar] [CrossRef]
- Kothari, V.; Stevens, R.J.; Adler, A.I.; Stratton, I.M.; Manley, S.E.; Neil, H.A.; Holman, R.R. UKPDS 60: Risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke 2002, 33, 1776–1781. [Google Scholar] [CrossRef]
- Stevens, R.J.; Kothari, V.; Adler, A.I.; Stratton, I.M. The UKPDS risk engine: A model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin. Sci. 2001, 101, 671–679. [Google Scholar] [CrossRef]
- Davis, W.A.; Knuiman, M.W.; Davis, T.M. An Australian cardiovascular risk equation for type 2 diabetes: The Fremantle Diabetes Study. Intern. Med. J. 2010, 40, 286–292. [Google Scholar] [CrossRef]
- Davis, W.A.; Davis, T.M. Cardiovascular risk prediction in adults with type 1 diabetes: The Fremantle Diabetes Study. Diabetes Res. Clin. Pract. 2010, 90, e75–e78. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Yeasmin, F.; Rabi, D.M.; Ronksley, P.E.; Turin, T.C. Prognostic tools for cardiovascular disease in patients with type 2 diabetes: A systematic review and meta-analysis of C-statistics. J. Diabetes Complicat. 2019, 33, 98–111. [Google Scholar] [CrossRef]
- Read, S.H.; van Diepen, M.; Colhoun, H.M.; Halbesma, N.; Lindsay, R.S.; McKnight, J.A.; McAllister, D.A.; Pearson, E.R.; Petrie, J.R.; Philip, S.; et al. Performance of cardiovascular disease risk scores in people diagnosed with type 2 diabetes: External validation using data from the National Scottish Diabetes Register. Diabetes Care 2018, 41, 2010–2018. [Google Scholar] [CrossRef]
- Ford, E.S. Trends in the control of risk factors for cardiovascular disease among adults with diagnosed diabetes: Findings from the National Health and Nutrition Examination Survey 1999–2008. J. Diabetes 2011, 3, 337–347. [Google Scholar] [CrossRef]
- Harding, J.L.; Shaw, J.E.; Peeters, A.; Guiver, T.; Davidson, S.; Magliano, D.J. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997–2010. Diabetes Care 2014, 37, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.A.; Peters, K.E.; Makepeace, A.; Griffiths, S.; Bundell, C.; Grant, S.F.A.; Ellard, S.; Hattersley, A.T.; Paul Chubb, S.A.; Bruce, D.G.; et al. The prevalence of diabetes in Australia: Insights from the Fremantle Diabetes Study Phase II. Intern. Med. J. 2018, 48, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaduganathan, M.; Butler, J. Glucose-lowering therapies and heart failure in type 2 diabetes mellitus: Mechanistic links, clinical data, and future Directions. Circulation 2018, 137, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Geba, D.; Cordova, J.M.; Shetty, S.S. A risk prediction model for heart failure hospitalization in type 2 diabetes mellitus. Clin. Cardiol. 2020, 43, 275–283. [Google Scholar] [CrossRef]
- Davis, T.M.; Bruce, D.G.; Davis, W.A. Cohort profile: The Fremantle Diabetes Study. Int. J. Epidemiol. 2013, 42, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.; Hunter, M.; Peters, K.; Davis, T.; Davis, W. Fear of falling is common in patients with type 2 diabetes and is associated with increased risk of falls. Age Ageing 2015, 44, 687–690. [Google Scholar] [CrossRef]
- Kengne, A.P.; Patel, A.; Marre, M.; Travert, F.; Lievre, M.; Zoungas, S.; Chalmers, J.; Colagiuri, S.; Grobbee, D.E.; Hamet, P.; et al. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 393–398. [Google Scholar] [CrossRef]
- Advance Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Socio-Economic Indexes for Areas. Available online: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa (accessed on 13 April 2020).
- Davis, T.M.E.; Hunt, K.; McAullay, D.; Chubb, S.A.P.; Sillars, B.A.; Bruce, D.G.; Davis, W.A. Continuing disparities in cardiovascular risk factors and complications between Aboriginal and Anglo-Celt Australians with type 2 diabetes: The Fremantle Diabetes Study. Diabetes Care 2012, 35, 2005–2011. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef] [PubMed]
- Holman, C.D.; Bass, A.J.; Rouse, I.L.; Hobbs, M.S. Population-based linkage of health records in Western Australia: Development of a health services research linked database. Aust. N. Z. J. Public Health 1999, 23, 453–459. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of A WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Koller, M.T.; Leening, M.J.G.; Wolbers, M.; Steyerberg, E.W.; Hunink, M.G.M.; Schoop, R.; Hofman, A.; Bucher, H.C.; Psaty, B.M.; Lloyd-Jones, D.M.; et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann. Intern. Med. 2012, 157, 389–397. [Google Scholar] [CrossRef]
- Almeda-Valdes, P.; Cuevas-Ramos, D.; Mehta, R.; Gomez-Perez, F.J.; Aguilar-Salinas, C.A. UKPDS Risk Engine, decode and diabetes PHD models for the estimation of cardiovascular risk in patients with diabetes. Curr. Diabetes Rev. 2010, 6, 1–8. [Google Scholar] [CrossRef]
- Thompson, P.L.; Bradshaw, P.J.; Veroni, M.; Wilkes, E.T. Cardiovascular risk among urban Aboriginal people. Med. J. Aust. 2003, 179, 143–146. [Google Scholar] [CrossRef]
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; O’Hartaigh, B.; Janszky, I.; Romundstad, P.R.; Tonstad, S.; Vatten, L.J. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 504–517. [Google Scholar] [CrossRef]
- Bertoluci, M.C.; Rocha, V.Z. Cardiovascular risk assessment in patients with diabetes. Diabetol. Metab. Syndr. 2017, 9, 25. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Fuchs, S.C. Blood pressure targets in the treatment of high blood pressure: A reappraisal of the J-shaped phenomenon. J. Hum. Hypertens. 2014, 28, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sussman, J.B.; Berkowitz, S.A.; Hayward, R.A.; Yudkin, J.S. Development and validation of Risk Equations for Complications of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 788–798. [Google Scholar] [CrossRef]
- Yang, X.; So, W.Y.; Kong, A.P.; Ho, C.S.; Lam, C.W.; Stevens, R.J.; Lyu, R.R.; Yin, D.D.; Cockram, C.S.; Tong, P.C.; et al. Development and validation of stroke risk equation for Hong Kong Chinese patients with type 2 diabetes: The Hong Kong Diabetes Registry. Diabetes Care 2007, 30, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Vitale, C.; Seferovic, P. Heart Failure in Patients with Diabetes Mellitus. Card. Fail. Rev. 2017, 3, 52–55. [Google Scholar] [CrossRef]
- Jia, G.H.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, S.; Faghihimani, E.; Hassanzadeh, A.; Amini, M.; Mansourian, M. Predictors of all-cause and cardiovascular-specific mortality in type 2 diabetes: A competing risk modeling of an Iranian population. Adv. Biomed. Res. 2016, 5, 82. [Google Scholar] [CrossRef]
- Shastri, S.; Tangri, N.; Tighiouart, H.; Beck, G.J.; Vlagopoulos, P.; Ornt, D.; Eknoyan, G.; Kusek, J.W.; Herzog, C.; Cheung, A.K.; et al. Predictors of sudden cardiac death: A competing risk approach in the hemodialysis study. Clin. J. Am. Soc. Nephrol. 2012, 7, 123–130. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Fang, J.; Lee, D.S.; Tu, J.V.; Amir, E.; Austin, P.C.; Anderson, G.M. Importance of considering competing risks in time-to-event analyses: Application to stroke risk in a retrospective cohort study of elderly patients with atrial fibrillation. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004580. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
| Phase I | Phase II | p-Value | |
|---|---|---|---|
| Number (%) | 1296 | 1551 | |
| Age (years) | 64.0 ± 11.3 | 65.7 ± 11.6 | <0.001 |
| Sex (% male) | 48.6 | 51.9 | 0.08 |
| ApoE ε4 genotype (%) | 21.8 | 23.6 | 0.27 |
| Ethnic background (%) | <0.001 | ||
| Anglo-Celt | 63.3 | 53.4 | |
| Southern European | 18.4 | 12.6 | |
| Other European | 8.5 | 7.2 | |
| Asian | 3.3 | 4.4 | |
| Aboriginal | 1.3 | 6.7 | |
| Mixed/other | 5.2 | 15.7 | |
| Not fluent in English (%) | 15.3 | 10.6 | <0.001 |
| Currently married/de facto relationship (%) | 65.7 | 62.7 | 0.10 |
| Educational attainment beyond primary level (%) | 74.0 | 86.7 | <0.001 |
| Smoking status (%) | 0.001 | ||
| Never | 44.7 | 45.5 | |
| Ex | 40.2 | 44.0 | |
| Current | 15.1 | 10.5 | |
| Alcohol consumption (standard drinks/day) | 0 [0–0.8] | 0.1 [0–1.2] | <0.001 |
| Age at diabetes diagnosis (years) | 57.9 ±11.7 | 55.5 ± 12.3 | <0.001 |
| Diabetes duration (years) | 4.0 [1.0–9.0] | 9.0 [3.0–15.8] | <0.001 |
| Diabetes treatment (%) | <0.001 | ||
| Diet | 31.9 | 24.1 | |
| Oral hypoglycaemic agents (OHAs)/non-insulin injectables | 55.7 | 53.4 | |
| Insulin only | 9.5 | 5.9 | |
| Insulin + OHAs/non-insulin injectables | 2.8 | 16.6 | |
| Fasting serum glucose (mmol/L) | 8.3 (5.9–11.5) | 7.6 (5.6–10.2) | <0.001 |
| HbA1c (%) | 7.3 (5.9–9.2) | 7.1 (5.9–8.5) | <0.001 |
| HbA1c (mmol/mol) | 56 (41–77) | 54 (41–69) | <0.001 |
| BMI (kg/m2) | 29.6 ± 5.4 | 31.2 ± 6.1 | <0.001 |
| Central obesity (by waist circumference, %) | 64.5 | 71.4 | <0.001 |
| ABSI (m11/6kg−2/3) | 0.082 ± 0.005 | 0.081 ± 0.005 | 0.18 |
| Systolic blood pressure (mmHg) | 151 ± 24 | 146 ± 22 | <0.001 |
| Diastolic blood pressure (mmHg) | 80 ± 11 | 80±12 | 0.51 |
| Taking antihypertensive medication (%) | 50.9 | 73.7 | <0.001 |
| Total serum cholesterol (mmol/L) | 5.4 (4.4–6.5) | 4.2 (3.3–5.4) | <0.001 |
| Serum HDL-cholesterol (mmol/L) | 1.01 (0.75–1.38) | 1.19 (0.92–1.55) | <0.001 |
| Total:HDL-cholesterol ratio | 5.3 (3.8–7.4) | 3.5 (2.6–4.8) | <0.001 |
| Serum triglycerides (mmol/L) | 2.2 (1.2–3.9) | 1.5 (0.9–2.6) | <0.001 |
| Taking lipid-lowering medication (%) | 10.5 | 68.5 | <0.001 |
| Taking aspirin (%) | 22.0 | 37.5 | <0.001 |
| Cerebrovascular disease (%) | 10.0 | 11.4 | 0.22 |
| Coronary heart disease (%) | 29.6 | 29.5 | 0.97 |
| Peripheral arterial disease (%) | 29.3 | 22.9 | <0.001 |
| Peripheral sensory neuropathy (%) | 30.8 | 58.6 | <0.001 |
| eGFR (CKD-EPI) category (%) | 0.001 | ||
| ≥90 mL/min/1.73m2 | 32.2 | 38.3 | |
| 60–89 mL/min/1.73m2 | 49.8 | 44.7 | |
| 45–59 mL/min/1.73m2 | 11.9 | 9.1 | |
| 30–44 mL/min/1.73m2 | 4.4 | 5.2 | |
| 15–29 mL/min/1.73m2 | 1.2 | 1.9 | |
| <15 mL/min/1.73m2 | 0.5 | 0.8 | |
| Urinary albumin:creatinine ratio (mg/mmol) | 5.2 (1.5–17.8) | 3.3 (0.9–12.9) | <0.001 |
| Model 1: Cox Three-Point MACE | Model 2: Fine and Gray Three-Point MACE | Model 3: Cox Four-Point MACE | Model 4: Fine and Gray Four-Point MACE | |
|---|---|---|---|---|
| Age – 65.7 (years) | 0.0213 | 0.0133 | 0.0306 | 0.0273 |
| (Age – 65.7)2 (years2) | 0.0011 | 0.0009 | 0.0009 | 0.0006 |
| Sex (0 = female, 1 = male) | 0.2924 | |||
| Australian Aboriginal (0 = no, 1 = yes) | 0.9873 | 0.9781 | 0.6854 | 0.5830 |
| Heart rate – 70 (beats/minute) | 0.0173 | |||
| Diabetes duration – 10.2 (years) | 0.0187 | 0.0162 | ||
| loge(HbA1c) – 3.98 (mmol/mol) | 0.8371 | 0.9488 | 0.7120 | 0.5898 |
| loge(serum total:HDL-cholesterol ratio) − 1.27 (mmol/L) | 0.6137 | |||
| loge(urinary albumin:creatinine ratio) – 1.22 (mg/mmol) | 0.5342 | 0.5307 | 0.1906 | 0.1791 |
| eGFR (CKD-EPI) 45–59 mL/min/1.73m2 | 0.5399 | 0.5936 | ||
| eGFR (CKD-EPI) < 45 mL/min/1.73m2 | 0.8599 | 0.7998 | 0.6472 | 0.6559 |
| Peripheral arterial disease (0 = no, 1 = yes) | 0.5712 | 0.6186 | 0.3071 | 0.4006 |
| Left ventricular hypertrophy (0 = no, 1 = yes) | 1.6355 | 1.5301 | 1.0864 | 1.0617 |
| Heart failure (0 = no, 1 = yes) | 0.8803 | 0.8602 | ||
| Coronary heart disease and/or cerebrovascular disease (0 = no, 1 = yes) | 1.0245 | 0.9975 | 0.7203 | 0.7182 |
| Regression Method | Outcome | Observed N (% (95% CI)) | Predicted N (%) | AUC (95% CI) | H-L Test, p-Value | Brier Score (Range) | Sensitivity (%) * | Specificity (%) * | PPV (%) * | NPV (%) * |
|---|---|---|---|---|---|---|---|---|---|---|
| Cox | Three-point MACE | 143 (9.2 (7.9–10.8)) | 161.9 (10.4) | 0.77 (0.73–0.82) | <0.001 | 0.08 (0.00–0.999) | 64.3 | 79.3 | 24.0 | 95.6 |
| Fine and Gray | Three-point MACE | 143 (9.2 (7.9–10.8)) | 152.4 (9.8) | 0.77 (0.73–0.81) | <0.001 | 0.08 (0.00–0.999) | 62.9 | 80.0 | 24.3 | 95.5 |
| Cox | Four-point MACE | 245 (15.8 (14.0–17.7)) | 231.3 (14.9) | 0.81 (0.78–0.84) | 0.058 | 0.10 (0.00–0.96) | 81.2 | 65.2 | 30.4 | 94.9 |
| Fine and Gray | Four-point MACE | 245 (15.8 (14.0–17.7)) | 221.2 (14.3) | 0.82 (0.79–0.85) | 0.17 | 0.10 (0.00–0.95) | 79.2 | 68.1 | 31.8 | 94.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, W.A.; Hellbusch, V.; Hunter, M.L.; Bruce, D.G.; Davis, T.M.E. Contemporary Cardiovascular Risk Assessment for Type 2 Diabetes Including Heart Failure as an Outcome: The Fremantle Diabetes Study Phase II. J. Clin. Med. 2020, 9, 1428. https://doi.org/10.3390/jcm9051428
Davis WA, Hellbusch V, Hunter ML, Bruce DG, Davis TME. Contemporary Cardiovascular Risk Assessment for Type 2 Diabetes Including Heart Failure as an Outcome: The Fremantle Diabetes Study Phase II. Journal of Clinical Medicine. 2020; 9(5):1428. https://doi.org/10.3390/jcm9051428
Chicago/Turabian StyleDavis, Wendy A., Valentina Hellbusch, Michael L. Hunter, David G. Bruce, and Timothy M. E. Davis. 2020. "Contemporary Cardiovascular Risk Assessment for Type 2 Diabetes Including Heart Failure as an Outcome: The Fremantle Diabetes Study Phase II" Journal of Clinical Medicine 9, no. 5: 1428. https://doi.org/10.3390/jcm9051428
APA StyleDavis, W. A., Hellbusch, V., Hunter, M. L., Bruce, D. G., & Davis, T. M. E. (2020). Contemporary Cardiovascular Risk Assessment for Type 2 Diabetes Including Heart Failure as an Outcome: The Fremantle Diabetes Study Phase II. Journal of Clinical Medicine, 9(5), 1428. https://doi.org/10.3390/jcm9051428






