Selective Fetal Growth Restriction in Dichorionic Twin Pregnancies: Diagnosis, Natural History, and Perinatal Outcome
Abstract
:1. Introduction
2. Experimental Section
Statistical Analysis
3. Results
3.1. Key Findings
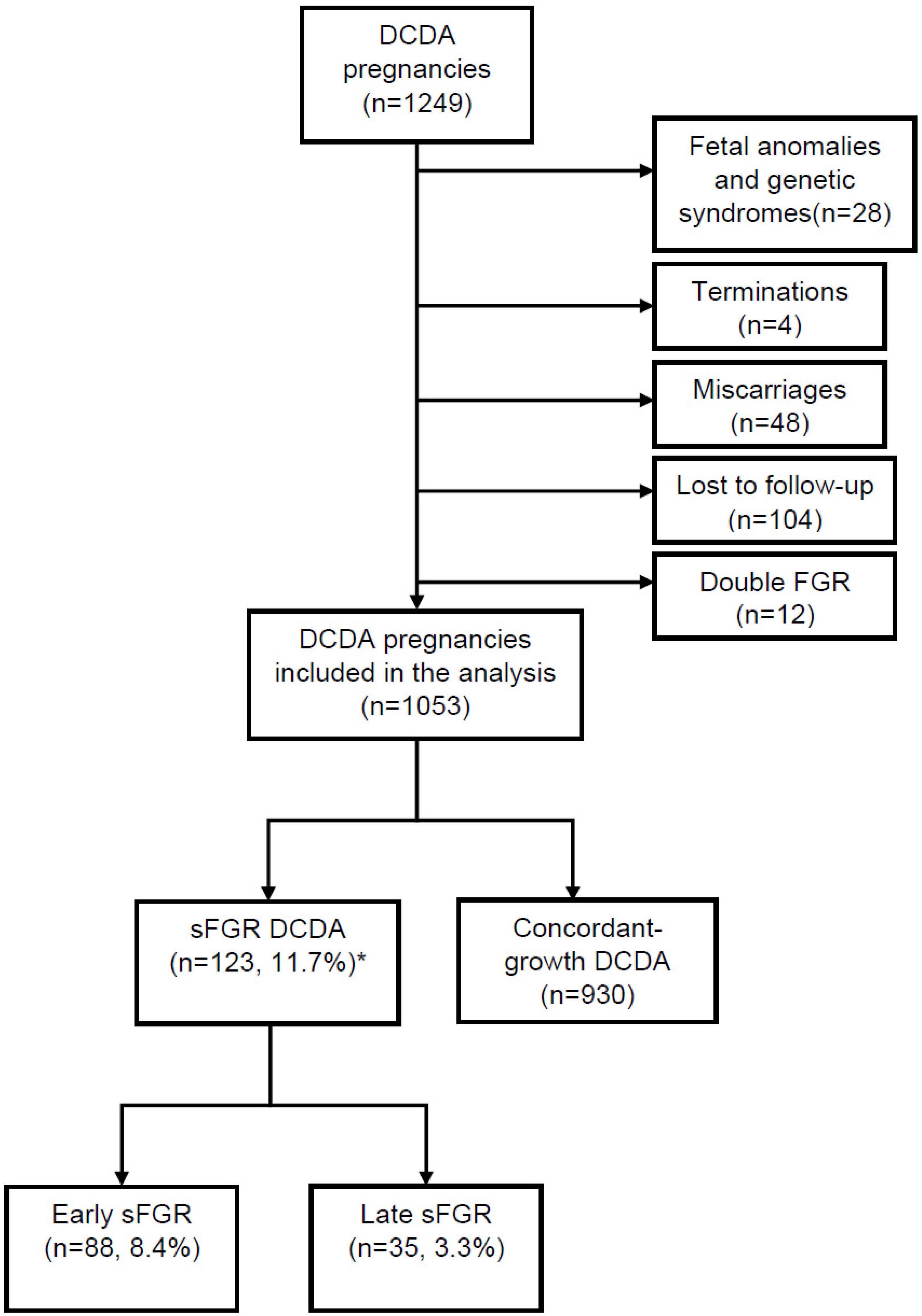
3.1.1. Study Population
3.1.2. Incidence of sFGR According to Diagnostic Criteria and Gestational Age at Diagnosis
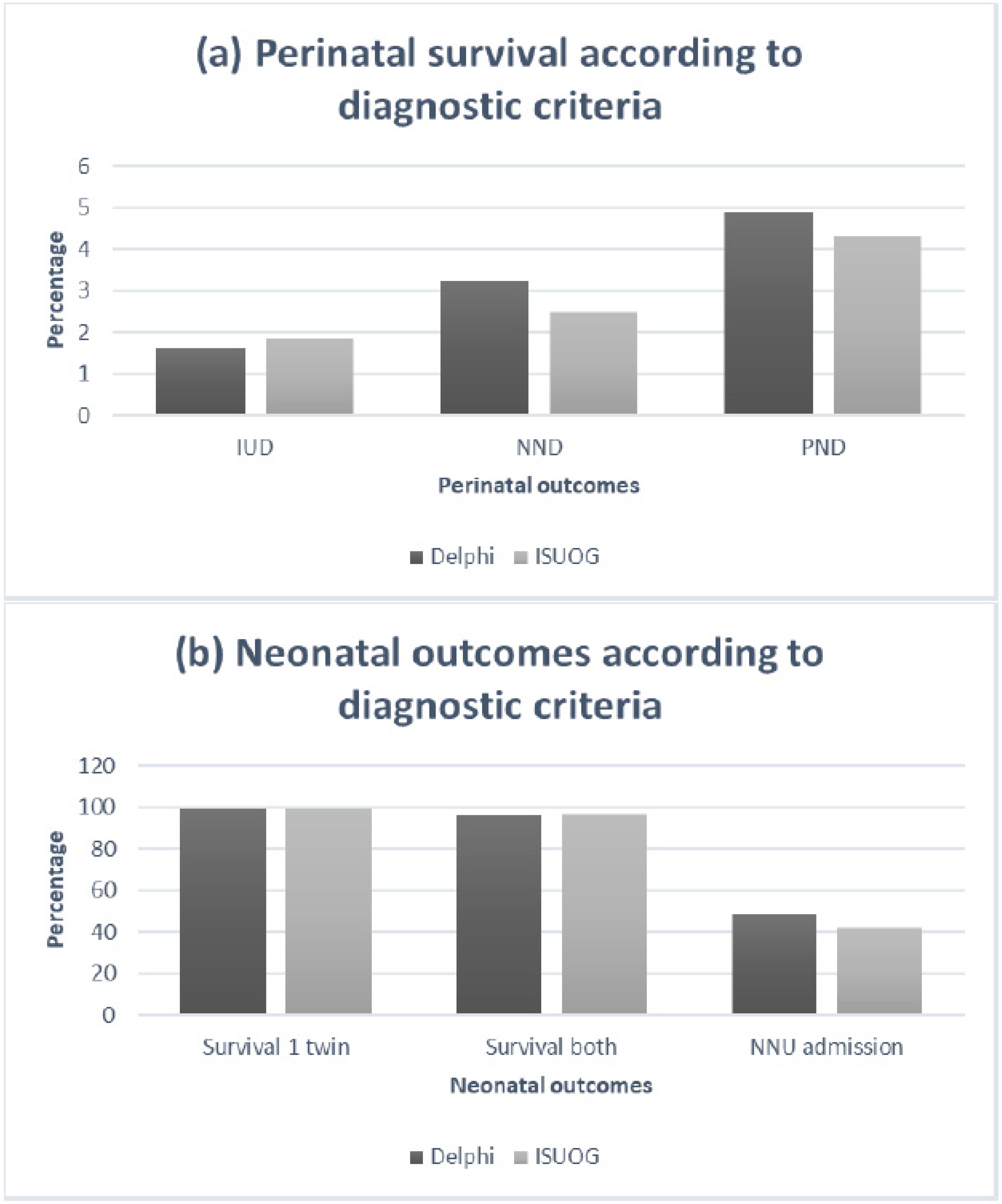
3.1.3. Disease Progression, Clinical Deterioration and Perinatal Outcomes
4. Discussion
4.1. Summary of the Study Findings
4.2. Clinical and Research Implications
4.3. Interpretation of Study Findings and Comparison with Existing Literature
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Townsend, R.; Khalil, A. Fetal growth restriction in twins. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 49, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.C.; Domingues, A.P.; Tavares, M.V.; Belo, A.; Ferreira, C.; Fonseca, E.; Moura, P. Monochorionic versus dichorionic twins: Are obstetric outcomes always different? J. Obstet. Gynaecol. 2016, 36, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Glinianaia, S.V.; Obeysekera, M.A.; Sturgiss, S.; Bell, R. Stillbirth and Neonatal Mortality in Monochorionic and Dichorionic Twins. Obstet. Gynecol. Surv. 2012, 67, 16–17. [Google Scholar] [CrossRef]
- Townsend, R.; Khalil, A. Twin pregnancy complicated by selective growth restriction. Curr. Opin. Obstet. Gynecol. 2016, 28, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.H.; Oepkes, D.; et al. ISUOG Practice Guidelines: The Role of Ultrasound in Twin Pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaku, S.; Kimura, F.; Murakami, T. Management of Fetal Growth Arrest in One of Dichorionic Twins: Three Cases and a Literature Review. Obstet. Gynecol. Int. 2015, 2015, 289875. [Google Scholar] [CrossRef] [Green Version]
- Valsky, D.V.; Eixarch, E.; Martinez, J.M.; Crispi, F.; Gratacos, E. Selective intrauterine growth restriction in monochorionic twins: Pathophysiology, diagnostic approach and management dilemmas. Semin. Fetal Neonatal Med. 2010, 15, 342–348. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.; He, Z.; Huang, X.; Fang, Q.; Luo, Y. Pre-eclampsia in twin pregnancies: Association with selective intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2016, 29, 1967–1971. [Google Scholar]
- Vanlieferinghen, S.; Anselem, O.; Le Ray, C.; Shen, Y.; Marcellin, L.; Goffinet, F. Prognostic Value of Umbilical and Cerebral Doppler in Fetal Growth Restriction: Comparison of Dichorionic Twins and Singletons. PLoS ONE 2015, 10, e0123067. [Google Scholar] [CrossRef]
- Khalil, A.; Beune, I.; Hecher, K.; Wynia, K.; Ganzevoort, W.; Reed, K.; Lewi, L.; Oepkes, D.; Gratacos, E.; Thilaganathan, B.; et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: A Delphi procedure. Ultrasound Obstet. Gynecol. 2019, 53, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Warsof, S.L.; Gohari, P.; Berkowitz, R.L.; Hobbins, J.C. The estimation of fetal weight by computer-assisted analysis. Am. J. Obstet. Gynecol. 1977, 128, 881–892. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Stirrup, O.; Khalil, A.; D’Antonio, F.; Thilaganathan, B. Fetal growth reference ranges in twin pregnancy: Analysis of the Southwest Thames Obstetric Research Collaborative (STORK) multiple pregnancy cohort. Ultrasound Obstet. Gynecol. 2014, 45, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Vintzileos, A.M.; Shen-Schwarz, S.; Smulian, J.C.; Lai, Y.L. Standards of birth weight in twin gestations stratified by placental chorionicity. Obstet. Gynecol. 1998, 91, 917–924. [Google Scholar]
- Gordijn, S.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Højsgaard, S.; Halekoh, U.; Yan, J. The r package geepack for generalized estimating equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar]
- Gielen, M.; Lindsey, P.J.; Derom, C.; Loos, R.J.F.; Souren, N.Y.; Paulussen, A.D.C.; Zeegers, M.P.; Derom, R.; Vlietinck, R.; Nijhuis, J.G. Twin-Specific Intrauterine ‘Growth’ Charts Based on Cross-Sectional Birthweight Data. Twin Res. Hum. Genet. 2008, 11, 224–235. [Google Scholar] [CrossRef]
- Hamilton, E.F.; Platt, R.W.; Morin, L.; Usher, R.; Kramer, M. How small is too small in a twin pregnancy? Am. J. Obstet. Gynecol. 1998, 179, 682–685. [Google Scholar] [CrossRef]
- Kalafat, E.; Sebghati, M.; Thilaganathan, B.; Khalil, A.; Bahamie, A.; Bhide, A.; Deans, A.; Egbor, M.; Ellis, C.; Gandhi, H.; et al. Predictive accuracy of Southwest Thames Obstetric Research Collaborative (STORK) chorionicity-specific twin growth charts for stillbirth: A validation study. Ultrasound Obstet. Gynecol. 2019, 53, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Stirrup, O.; Khalil, A.; D’Antonio, F.; Thilaganathan, B.; Stork, O.B.O.T. Patterns of Second- and Third-Trimester Growth and Discordance in Twin Pregnancy: Analysis of the Southwest Thames Obstetric Research Collaborative (STORK) Multiple Pregnancy Cohort. Fetal Diagn. Ther. 2016, 41, 100–107. [Google Scholar] [CrossRef]
- D’Antonio, F.; Odibo, A.O.; Prefumo, F.; Khalil, A.; Buca, D.; Flacco, M.E.; Liberati, M.; Manzoli, L.; Acharya, G. Weight discordance and perinatal mortality in twin pregnancy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 52, 11–23. [Google Scholar] [CrossRef]
- Hillman, S.; Morris, R.K.; Kilby, M.D. Co-Twin Prognosis After Single Fetal Death. Obstet. Gynecol. 2011, 118, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Audibert, F.; Salomon, L.J.; Castaigne-Meary, V.; Alves, K.; Frydman, R. Selective termination of a twin pregnancy as a treatment of severe preeclampsia. BJOG 2003, 110, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.; Duffy, J.M.N.; Perry, H.; Ganzevoort, W.; Reed, K.; Baschat, A.A.; Deprest, J.; Gratacos, E.; Hecher, K.; Lewi, L.; et al. A core outcome set for studies investigating the management of selective fetal growth restriction in twins. Ultrasound Obstet. Gynecol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, H.; Duffy, J.; Reed, K.; A Baschat, A.; Deprest, J.; Hecher, K.; Lewi, L.; Lopriore, E.; Oepkes, D.; Khalil, A.; et al. Core outcome set for research studies evaluating treatments for twin–twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2019, 54, 255–261. [Google Scholar] [CrossRef]
- Lam, J.R.; Liu, B.; Bhate, R.; Fenwick, N.; Reed, K.; Duffy, J.M.N.; Khalil, A.; Bartley, H.; Baschat, A.; Sans, M.B.; et al. Research priorities for the future health of multiples and their families: The Global Twins and Multiples Priority Setting Partnership. Ultrasound Obstet. Gynecol. 2019, 54, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef]
- Curado, J.; Sileo, F.G.; Bhide, A.; Thilaganathan, B.; Khalil, A. Early and late selective fetal growth restriction in monochorionic diamniotic twin pregnancies: Natural history and diagnostic criteria. Ultrasound Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Vasario, E.; Borgarello, V.; Bossotti, C.; Libanori, E.; Biolcati, M.; Arduino, S.; Spinelli, R.; Piane, L.D.; Revelli, A.; Todros, T. IVF twins have similar obstetric and neonatal outcome as spontaneously conceived twins: A prospective follow-up study. Reprod. Biomed. Online 2010, 21, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Geisler, M.E.; O’Mahony, A.; Meaney, S.; Waterstone, J.J.; O’Donoghue, K. Obstetric and perinatal outcomes of twin pregnancies conceived following IVF/ICSI treatment compared with spontaneously conceived twin pregnancies. Eur. J. Obstet. Gynecol. Reprod. Boil. 2014, 181, 78–83. [Google Scholar] [CrossRef]
- Moini, A.; Shiva, M.; Arabipoor, A.; Hosseini, R.; Chehrazi, M.; Sadeghi, M. Obstetric and neonatal outcomes of twin pregnancies conceived by assisted reproductive technology compared with twin pregnancies conceived spontaneously: A prospective follow-up study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, H.; Sheng, X.; Xie, Q.; Gao, S. Assisted reproductive technology and risk of adverse obstetric outcomes in dichorionic twin pregnancies: A systematic review and meta-analysis. Fertil. Steril. 2016, 105, 1180–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, R.S.; Fields, J.C.; Barberio, A.L.; Bodenlos, K.; Fox, N.S. Outcomes of Twin Pregnancies in Women 45 Years of Age or Older. Obstet. Gynecol. 2017, 129, 827–830. [Google Scholar] [CrossRef] [PubMed]
| sFGR * (n = 123) | No sFGR (n = 930) | p-Value | |
|---|---|---|---|
| Maternal age in years (mean ± SD) | 34.0 (29.0–36.0) | 34.0 (30.0–36.0) | 0.409 |
| Maternal body mass index in Kg/m2 (mean ± SD) | 24.0 (21.0–27.0) | 24.0 (22.0–27.4) | 0.231 |
| Mode of conception | |||
| Spontaneous conception,n(%) | 83 (67.5) | 586 (63.0) | 0.333 |
| IVF,n(%) | 40 (32.5) | 344 (37.0) | |
| Ethnicity,n(%) | |||
| White | 79 (64.2) | 639 (68.7) | 0.032 |
| Black | 11 (8.9) | 131 (14.1) | |
| Asian | 26 (21.1) | 113 (12.2) | |
| Other | 7 (5.7) | 47 (5.1) | |
| Diagnostic Criteria | Incidence of sFGR | Incidence of Early sFGR | GA Diagnosis Early sFGR (wks) | Incidence of Late sFGR | GA Diagnosis Late sFGR (wks) |
|---|---|---|---|---|---|
| Delphi criteria A EFW < 3rd centile of one twin | 95 (9.0) | 80 (7.6) | 27.0 (22.0–28.0) | 15 (1.4) | 34.0 (33.0–35.0) |
| Delphi criteria B EFW < 10th centile of one twin + inter-twin EFW discordance ≥ 25% | 49 (4.7) | 39 (3.7) | 26.0 (22.0–28.0) | 10 (1.0) | 34.0 (32.0–35.0) |
| Delphi criteria C EFW < 10th centile of one twin + umbilical artery PI > 95th centile | 60 (5.7) | 36 (3.4) | 28.0 (22.0–28.0) | 24 (2.3) | 33.5 (32.7–34.0) |
| Delphi criteria D Inter-twin EFW discordance ≥ 25% + umbilical artery PI > 95th centile | 19 (1.8) | 16 (1.5) | 25.5 (22.0–28.0) | 3 (0.3) | 32.0 (32.0–32.0) |
| ISUOG criteria EFW < 10th centile of one twin | 162 (15.4) | 116 (11.0) | 27.0 (22.0–28.0) | 46 (4.4) | 34.0 (33.0–35.0) |
| sFGR (n = 123) | Early Onset sFGR (n = 88) | Late-Onset sFGR (n = 35) | |
|---|---|---|---|
| Progression, n (%) | 44 (35.8) | 35 (39.8) | 9 (25.7) |
| Interval between diagnosis and progression in weeks, median (IQR) | 4 (2–7) | 5 (1–7.5) | 1 (1–1) |
| Stable, n (%) | 79 (64.2) | 53 (60.2) | 26 (74.3) |
| sFGR (n = 123) | No sFGR (n = 930) | p-Value | Early sFGR (n = 88) | Late sFGR (n = 35) | p-Value | |
|---|---|---|---|---|---|---|
| Gestation at birth (weeks) | 34.0 (33.0–36.0) | 37.0 (35.0–37.0) | <0.001 | 34.0 (31.8–36.0) | 35.0 (33.5–36.0) | 0.083 |
| Birth weight (g), larger baby | 2107 (1771–2339) | 2640 (2345–2920) | <0.001 | 2044 (1656–2328) | 2128 (1985–2377) | 0.011 |
| Birth weight (g), smaller baby | 1660 (1192–1846) | 2362 (2080–2601) | <0.001 | 1529 (1130–1815) | 1740 (1588–1895) | 0.074 |
| Birth weight centile, larger baby | 36.0 (18.7–55.1) | 63.5 (43.4–82.1) | <0.001 | 39.3 (11.6–80.5) | 20.7 (1.2–72.3) | 0.062 |
| Birth weight centile, smaller baby | 3.2 (1.1–7.8) | 34.3 (14.4–52.7) | <0.001 | 5.6 (0.5–12.9) | 4.6 (0.3–13.6) | 0.295 |
| Intrauterine demise (per 1000 total birth †) | 2 (8) | 10 (5) | 0.644 ‡ | 2 (11) | 0 (0) | <0.001 ‡ |
| Neonatal death (per 1000 live birth †) | 4 (16) | 19 (10) | 0.464 ‡ | 4 (23) | 0 (0) | <0.001 ‡ |
| Perinatal death (per 1000 total birth †) | 6 (24) | 29 (16) | 0.018 ‡ | 6 (34) | 0 (0) | 0.178 ‡ |
| Survival of at least one twin | 122 (99.2) | 925 (99.5) | 0.703 | 87 (98.9) | 35 (100.0) | 0.999 |
| Survival of both twins | 118 (95.9) | 896 (96.3) | 0.821 | 83 (94.3) | 35 (100.0) | 0.350 |
| Neonatal unit admission * | 133 (54.5) | 389 (21.2) | <0.001 ‡ | 105 (60.3) | 28 (40.0) | 0.005 ‡ |
| sFGR (n = 162) | No sFGR (n = 891) | p-Value | Early sFGR (n = 116) | Late sFGR (n = 46) | p-Value | |
|---|---|---|---|---|---|---|
| Gestation at birth (wks) | 35.0 (33.0–37.0) | 37.0 (35.0–37.0) | <0.001 | 35.0 (32.0–37.0) | 35.0 (34.0–36.7) | 0.131 |
| Birth weight (g), larger baby | 2195 (1856–3450) | 2660 (2350–2950) | <0.001 | 2164 (1738–2447) | 2222 (2024–2530) | 0.062 |
| Birth weight (g), smaller baby | 1720 (1400–1920) | 2390 (2105–2615) | <0.001 | 1680 (1173–1910) | 1747 (1645–1958) | 0.062 |
| Birth weight centile, larger baby | 36.2 (21.0–54.3) | 64.4 (44.8–82.6) | <0.001 | 34.6 (22.2–51.7) | 38.0 (18.9–58.4) | 0.568 |
| Birth weight centile, smaller baby | 3.9 (1.5–8.0) | 35.8 (18.9–55.3) | <0.001 | 3.5 (1.1–7.3) | 4.8 (1.8–9.2) | 0.194 |
| Intrauterine demise (per 1000 total birth †) | 3 (9) | 9 (5) | 0.370 ‡ | 3 (13) | 0 (0) | <0.001 ‡ |
| Neonatal death (per 1000 live birth †) | 4 (12) | 19 (11) | 0.758 ‡ | 4 (17) | 0 (0) | <0.001 ‡ |
| Perinatal death (per 1000 total birth †) | 7 (22) | 28 (16) | 0.069 ‡ | 7 (30) | 0 (0) | 0.150 ‡ |
| Survival of at least one twin | 161 (99.4) | 888 (99.7) | 0.593 | 115 (99.1) | 46 (100.0) | 0.999 |
| Survival of both twins | 156 (96.3) | 866 (97.2) | 0.534 | 110 (94.8) | 46 (100.0) | 0.266 |
| Neonatal unit admission * | 155 (48.4) | 367 (20.8) | <0.001 ‡ | 121 (53.1) | 34 (37.0) | 0.009 ‡ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonakopoulos, N.; Pateisky, P.; Liu, B.; Kalafat, E.; Thilaganathan, B.; Khalil, A. Selective Fetal Growth Restriction in Dichorionic Twin Pregnancies: Diagnosis, Natural History, and Perinatal Outcome. J. Clin. Med. 2020, 9, 1404. https://doi.org/10.3390/jcm9051404
Antonakopoulos N, Pateisky P, Liu B, Kalafat E, Thilaganathan B, Khalil A. Selective Fetal Growth Restriction in Dichorionic Twin Pregnancies: Diagnosis, Natural History, and Perinatal Outcome. Journal of Clinical Medicine. 2020; 9(5):1404. https://doi.org/10.3390/jcm9051404
Chicago/Turabian StyleAntonakopoulos, Nikolaos, Petra Pateisky, Becky Liu, Erkan Kalafat, Baskaran Thilaganathan, and Asma Khalil. 2020. "Selective Fetal Growth Restriction in Dichorionic Twin Pregnancies: Diagnosis, Natural History, and Perinatal Outcome" Journal of Clinical Medicine 9, no. 5: 1404. https://doi.org/10.3390/jcm9051404
APA StyleAntonakopoulos, N., Pateisky, P., Liu, B., Kalafat, E., Thilaganathan, B., & Khalil, A. (2020). Selective Fetal Growth Restriction in Dichorionic Twin Pregnancies: Diagnosis, Natural History, and Perinatal Outcome. Journal of Clinical Medicine, 9(5), 1404. https://doi.org/10.3390/jcm9051404






