Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study
Abstract
1. Introduction
2. Materials and Methods
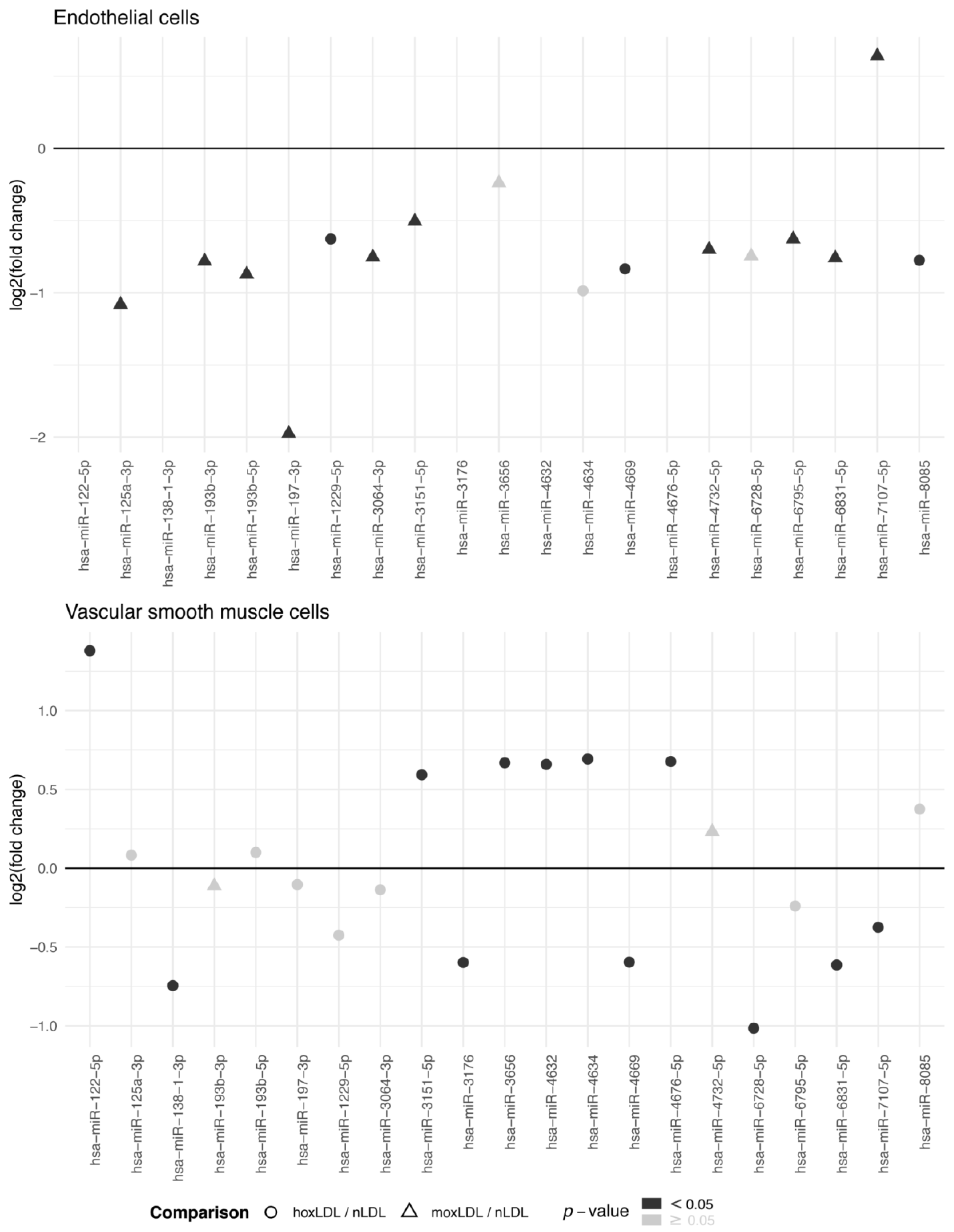
2.1. Human Arterial Cell Study
2.1.1. EC and VSMC Isolation
2.1.2. LDL Oxidation and Cell Treatment
2.2. Case-control and Case-cohort Studies
2.2.1. CV Risk Factor Data and Blood Sample Collection
2.2.2. Case-finding Procedures
2.3. RNA Extraction and Quality Control
2.4. miRNA Expression
2.4.1. Human Arterial Cell Study
2.4.2. Case-control and Case-cohort Studies
2.5. Statistical Analysis
2.5.1. miRNA Expression
2.5.2. Validation of miRNA Expression
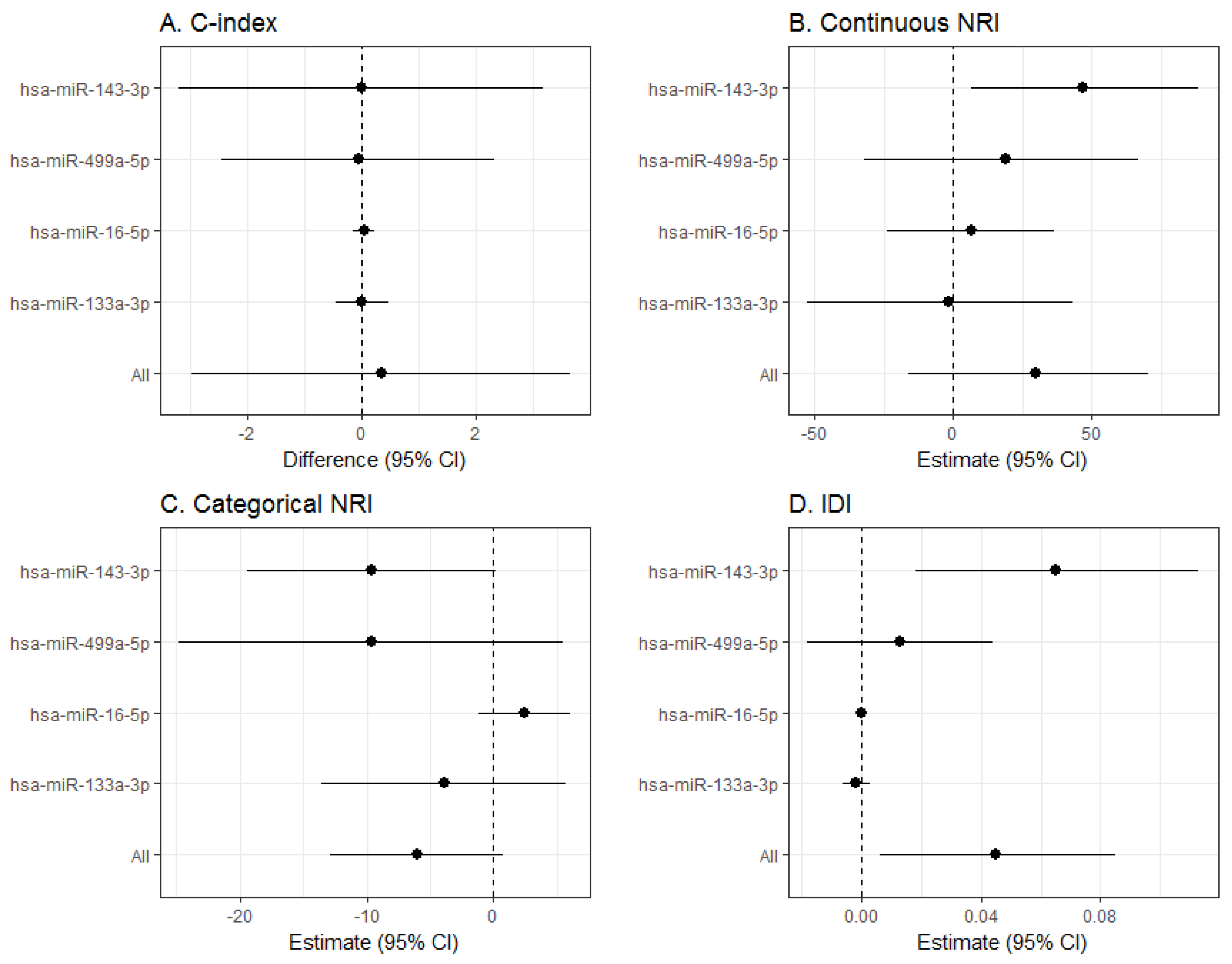
2.5.3. miRNA Predictive Capacity
2.5.4. Bioinformatics Analyses
2.6. Ethics
3. Results
4. Discussion
4.1. Main Findings
4.2. Comparison with Published Reports
4.3. Signaling Pathways
4.4. Hsa-miR-143-3p Findings and Role in the Vascular Wall
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: A report from the American heart association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Dégano, I.R.; Salomaa, V.; Veronesi, G.; Ferriéres, J.; Kirchberger, I.; Laks, T.; Havulinna, A.S.; Ruidavets, J.B.; Ferrario, M.M.; Meisinger, C.; et al. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015, 101, 1413–1421. [Google Scholar]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Marrugat, J.; Vila, J.; Baena-Díez, J.M.; Grau, M.; Sala, J.; Ramos, R.; Subirana, I.; Fitó, M.; Elosua, R. Relative validity of the 10-year cardiovascular risk estimate in a population cohort of the REGICOR study. Rev. Esp. Cardiol. 2011, 64, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1–N96. [Google Scholar]
- Muntner, P.; Colantonio, L.D.; Cushman, M.; Goff, D.C., Jr.; Howard, G.; Howard, V.J.; Kissela, B.; Levitan, E.B.; Lloyd-Jones, D.M.; Safford, M.M. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA 2014, 311, 1406–1415. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Ueda, P.; Lu, Y.; Woodward, M.; Ahmadvand, A.; Aguilar-Salinas, C.A.; Azizi, F.; Cifkova, R.; Di Cesare, M.; Eriksen, L.; et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): A pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015, 3, 339–355. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; D’Agostino, R.; Sullivan, L.; Elosua, R.; Wilson, P.; Ordovas, J.; Solanas, P.; Cordón, F.; Ramos, R.; Sala, J.; et al. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J. Epidemiol. Community Health 2003, 57, 634–638. [Google Scholar] [CrossRef]
- Marrugat, J.; Subirana, I.; Ramos, R.; Vila, J.; Marín-Ibañez, A.; Guembe, M.J.; Rigo, F.; Tormo Díaz, M.J.; Moreno-Iribas, C.; Cabré, J.J.; et al. FRESCO Investigators. FRESCO Investigators. Derivation and validation of a set of 10-year cardiovascular risk predictive functions in Spain: The FRESCO Study. Prev. Med. 2014, 61, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Antonicelli, R.; Lorenzi, M.; D’Alessandra, Y.; Lazzarini, R.; Santini, G.; Spazzafumo, L.; Lisa, R.; La Sala, L.; Galeazzi, R.; et al. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int. J. Cardiol. 2013, 167, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Zhu, J.Q.; Zhang, J.T.; Li, Q.; Li, Y.; He, J.; Qin, Y.W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Carena, M.C.; Spazzafumo, L.; Martinelli, F.; Bassetti, B.; Devanna, P.; Rubino, M.; Marenzi, G.; Colombo, G.I.; Achilli, F.; et al. Diagnostic potential of plasmatic microRNA signatures in stable and unstable angina. PLoS ONE 2013, 8, e80345. [Google Scholar] [CrossRef]
- Bye, A.; Røsjø, H.; Nauman, J.; Silva, G.J.; Follestad, T.; Omland, T.; Wisløff, U. Circulating microRNAs predict future fatal myocardial infarction in healthy individuals—The HUNT study. J. Mol. Cell Cardiol. 2016, 97, 162–168. [Google Scholar] [CrossRef]
- Velle-Forbord, T.; Eidlaug, M.; Debik, J.; Sæther, J.C.; Follestad, T.; Nauman, J.; Gigante, B.; Røsjø, H.; Omland, T.; Langaas, M.; et al. Circulating microRNAs as predictive biomarkers of myocardial infarction: Evidence from the HUNT study. Atherosclerosis 2019, 289, 1–7. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Karathanasis, S.K.; Ding, Z.; Arulandu, A.; Varughese, K.I.; Mehta, J.L. LOX-1 in atherosclerosis and myocardial ischemia. J. Am. Coll. Cardiol. 2017, 69, 2759–2768. [Google Scholar] [CrossRef]
- Pérez, G.; Pena, A.; Sala, J.; Roset, P.; Masiá, R.; Marrugat, J. Acute myocardial infarction case fatality, incidence and mortality rates in a population registry in Gerona, Spain, 1990-1992. REGICOR Investigators. Int. J. Epidemiol. 1998, 27, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Agüero, F.; Dégano, I.R.; Subirana, I.; Grau, M.; Zamora, A.; Sala, J.; Ramos, R.; Treserras, R.; Marrugat, J.; Elosua, R. Impact of a partial smoke-free legislation on myocardial infarction incidence, mortality and case-fatality in a population-based registry: The REGICOR Study. PLoS ONE 2013, 8, e53722. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Subirana, I.; Elosua, R.; Solanas, P.; Ramos, R.; Masiá, R.; Cordón, F.; Sala, J.; Juvinyà, D.; Cerezo, C.; et al. Trends in cardiovascular risk factor prevalence (1995-2000-2005) in northeastern Spain. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Luepker, R.V.; Apple, F.S.; Christenson, R.H.; Crow, R.S.; Fortmann, S.P.; Goff, D.; Goldberg, R.J.; Hand, M.M.; Jaffe, A.S.; Julian, D.G.; et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: A statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003, 108, 2543–2549. [Google Scholar] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sanderson, J.; Tompson, S.G.; White, I.A.; Aspelund, T.; Pennels, L. Derivation and assessment of risk prediction models using case-cohort data. BMC Med. Res. Methodol. 2013, 13, 113. [Google Scholar] [CrossRef]
- Raitoharju, E.; Seppälä, I.; Lyytikäinen, L.P.; Viikari, J.; Ala-Korpela, M.; Soininen, P.; Kangas, A.J.; Waldenberger, M.; Klopp, N.; Illig, T.; et al. Blood hsa-miR-122-5p and hsa-miR-885-5p levels associate with fatty liver and related lipoprotein metabolism-The Young Finns Study. Sci. Rep. 2016, 6, 38262. [Google Scholar] [CrossRef]
- Yeh, C.L.; Cheng, I.C.; Hou, Y.C.; Wang, W.; Yeh, S.L. MicroRNA-125a-3p expression in abdominal adipose tissues is associated with insulin signalling gene expressions in morbid obesity: Observations in Taiwanese. Asia Pac. J. Clin. Nutr. 2014, 23, 331–337. [Google Scholar]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Semik, E.; Zabek, T.; Wnuk, M. Reduced levels of methyltransferase DNMT2 sensitize human fibroblasts to oxidative stress and DNA damage that is accompanied by changes in proliferation-related miRNA expression. Redox Biol. 2018, 14, 20–34. [Google Scholar] [CrossRef]
- Karolina, D.S.; Tavintharan, S.; Armugam, A.; Sepramaniam, S.; Pek, S.L.; Wong, M.T.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. Circulating miRNA profiles in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E2271–E2276. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Chen, J.; Li, X.; Gou, D. miR-4632 mediates PDGF-BB-induced proliferation and antiapoptosis of human pulmonary artery smooth muscle cells via targeting cJUN. Am. J. Physiol. Cell Physiol. 2017, 313, C380–C391. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Tamini, S.; Bresolin, S.; Giraudi, P.; Basso, G.; Minocci, A.; Tiribelli, C.; Grugni, G.; Sartorio, A. Differences in circulating microRNA signature in Prader-Willi syndrome and non-syndromic obesity. Endocr. Connect. 2018, 7, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wu, L.J.; Li, J.J.; Xiao, H.B.; He, Y.; Yan, Y.X. A meta-analysis of dysregulated miRNAs in coronary heart disease. Life Sci. 2018, 215, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Bergmark, C.; Beyer, R.W.; Patel, R.; Pattison, J.; Miller, E.; Juliano, J.; Witztum, J.L. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, 360–370. [Google Scholar] [CrossRef]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef]
- Sorrentino, S.; Iaconetti, C.; De Rosa, S.; Polimeni, A.; Sabatino, J.; Gareri, C.; Passafaro, F.; Mancuso, T.; Tammè, L.; Mignogna, C.; et al. Hindlimb ischemia impairs endotelial recovery and increases neointimal proliferation in the carotid artery. Sci. Rep. 2018, 8, 761. [Google Scholar] [CrossRef]
- Frangiogiannis, N.G. The inflammatory response in myocardial injury, repair and remodeling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Heliste, J.; Jokilammi, A.; Paatero, I.; Chakroborty, D.; Stark, C.; Savunen, T.; Laaksonen, M.; Elenius, K. Receptor tyrosine kinase profiling of ischemic heart identifies ROR1 as a potential therapeutic target. BMC Cardiovasc. Disord. 2018, 18, 196. [Google Scholar] [CrossRef]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef]
- Ramanujam, D.; Engelhardt, S. Intercellular miRNA traffic. Circ. Res. 2015, 116, 1726–1728. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, Y.; Zhang, H.; Xie, D.; Nie, W.; Shi, K. Inhibition of microRNA-143-3p attenuates myocardial hypertrophy by inhibiting inflammatory response. Cell Biol. Int. 2018, 42, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Böck, J.; Northoff, B.H.; Klein, M. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Quintavalle, M.; Miragoli, M.; Chen, J.; Condorelli, G.; Elia, L. TGFβ Triggers miR-143/145 Transfer from Smooth Muscle Cells to Endothelial Cells, Thereby Modulating Vessel Stabilization. Circ. Res. 2015, 116, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Small, E.M.; Sutherland, L.B.; Qi, X.; McAnally, J.; Plato, C.F.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009, 23, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Vilades, D.; Martínez-Camblor, P.; Vea, À.; Nasarre, L.; Sanchez Vega, J.; Leta, R.; Carreras, F.; Llorente-Cortés, V. Circulating microRNAs in suspected stable coronary artery disease: A coronary computed tomography angiography study. J. Intern. Med. 2019, 286, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Aldi, S.; Matic, L.P.; Hamm, G.; van Keulen, D.; Tempel, D.; Holmstrøm, K.; Szwajda, A.; Nielsen, B.S.; Emilsson, V.; Ait-Belkacem, R.; et al. Integrated Human Evaluation of the Lysophosphatidic Acid Pathway as a Novel Therapeutic Target in Atherosclerosis. Mol. Ther. Methods Clin. Dev. 2018, 10, 17–28. [Google Scholar] [CrossRef]
- Sun, X.; Belkin, N.; Feinberg, M.W. Endothelial microRNAs and atherosclerosis. Curr. Atheroscler Rep. 2013, 15, 372. [Google Scholar] [CrossRef]
- Heo, K.S.; Chang, E.; Le, N.T.; Cushman, H.; Yeh, E.T.; Fujiwara, K.; Abe, J. De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ. Res. 2013, 112, 911–923. [Google Scholar] [CrossRef]
- Brigstock, D.R. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 2002, 5, 153–165. [Google Scholar] [CrossRef]
- Wu, J.; Strawn, T.L.; Luo, M.; Wang, L.; Li, R.; Ren, M.; Xia, J.; Zhang, Z.; Ma, W.; Luo, T.; et al. Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-αVβ3 integrin cross talk. Arter. Thromb. Vasc. Biol. 2015, 35, 111–120. [Google Scholar] [CrossRef]
- Wu, C.; Gui, C.; Li, L.; Pang, Y.; Tang, Z.; Wei, J. Expression and secretion of neuregulin-1 in cardiac microvascular endothelial cells treated with angiogenic factors. Exp. Ther. Med. 2018, 15, 3577–3581. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.N.; Mazzoni, M.R.; Cleator, J.H.; Earls, L.; Perdigoto, A.L.; Brooks, J.D.; Muldowney, J.A., 3rd; Vaughan, D.E.; Hamm, H.E. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J. Biol. Chem. 2005, 280, 22172–22180. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Song, S.; Lee, J.; Yoon, J.; Park, J.; Choi, S.; Park, J.K.; Choi, K.; Choi, C. Phenotypic modulation of primary vascular smooth muscle cells by short-term culture on micropatterned substrate. PLoS ONE 2014, 9, e88089. [Google Scholar] [CrossRef] [PubMed]
- Wanjare, M.; Kuo, F.; Gerecht, S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2013, 97, 321–330. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dégano, I.R.; Camps-Vilaró, A.; Subirana, I.; García-Mateo, N.; Cidad, P.; Muñoz-Aguayo, D.; Puigdecanet, E.; Nonell, L.; Vila, J.; Crepaldi, F.M.; et al. Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study. J. Clin. Med. 2020, 9, 1402. https://doi.org/10.3390/jcm9051402
Dégano IR, Camps-Vilaró A, Subirana I, García-Mateo N, Cidad P, Muñoz-Aguayo D, Puigdecanet E, Nonell L, Vila J, Crepaldi FM, et al. Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study. Journal of Clinical Medicine. 2020; 9(5):1402. https://doi.org/10.3390/jcm9051402
Chicago/Turabian StyleDégano, Irene R., Anna Camps-Vilaró, Isaac Subirana, Nadia García-Mateo, Pilar Cidad, Dani Muñoz-Aguayo, Eulàlia Puigdecanet, Lara Nonell, Joan Vila, Felipe M. Crepaldi, and et al. 2020. "Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study" Journal of Clinical Medicine 9, no. 5: 1402. https://doi.org/10.3390/jcm9051402
APA StyleDégano, I. R., Camps-Vilaró, A., Subirana, I., García-Mateo, N., Cidad, P., Muñoz-Aguayo, D., Puigdecanet, E., Nonell, L., Vila, J., Crepaldi, F. M., de Gonzalo-Calvo, D., Llorente-Cortés, V., Pérez-García, M. T., Elosua, R., Fitó, M., & Marrugat, J. (2020). Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study. Journal of Clinical Medicine, 9(5), 1402. https://doi.org/10.3390/jcm9051402








