Impact of Glomerular Filtration Rate on the Incidence and Prognosis of New-Onset Atrial Fibrillation in Acute Myocardial Infarction
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Statistical Analysis
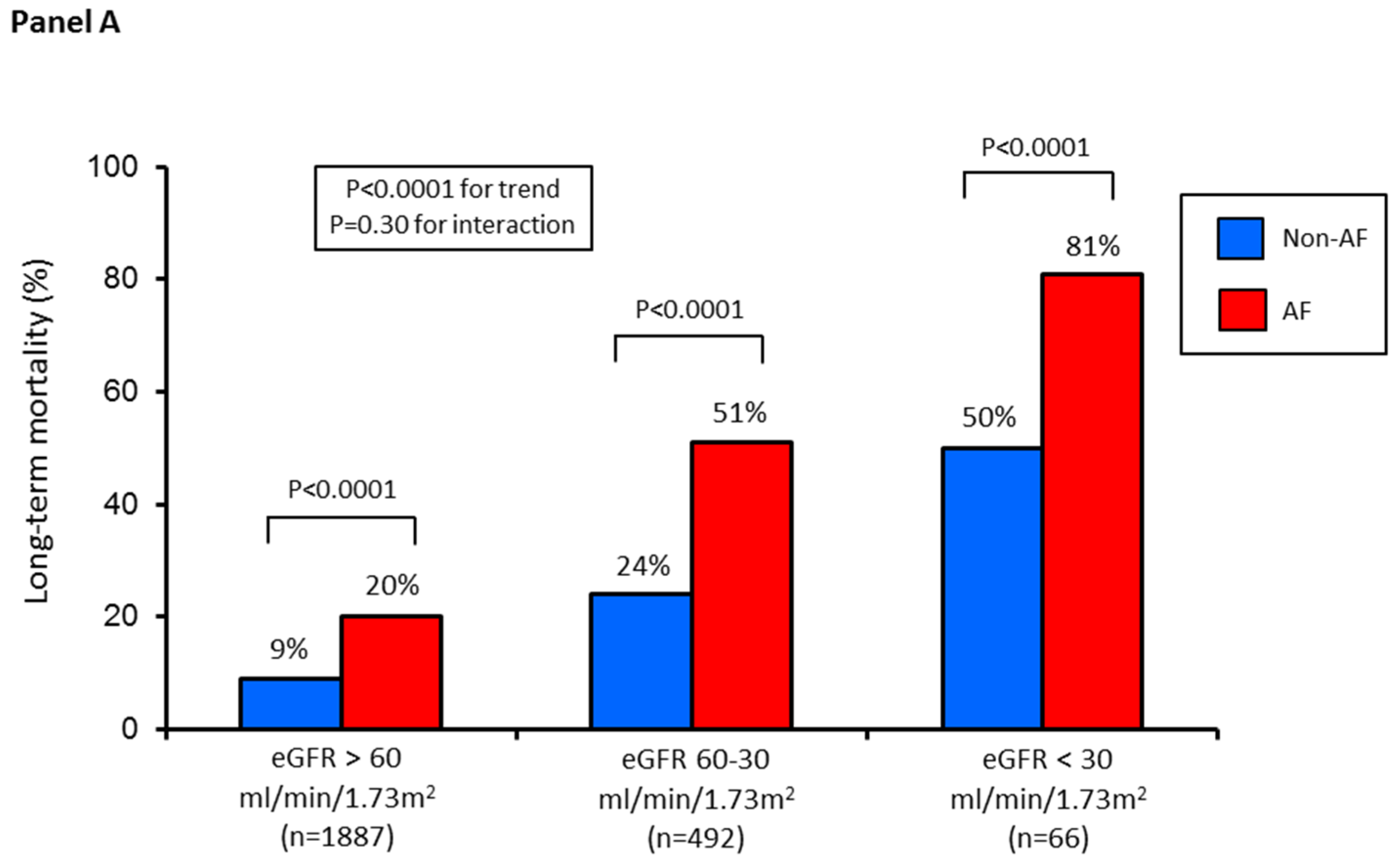
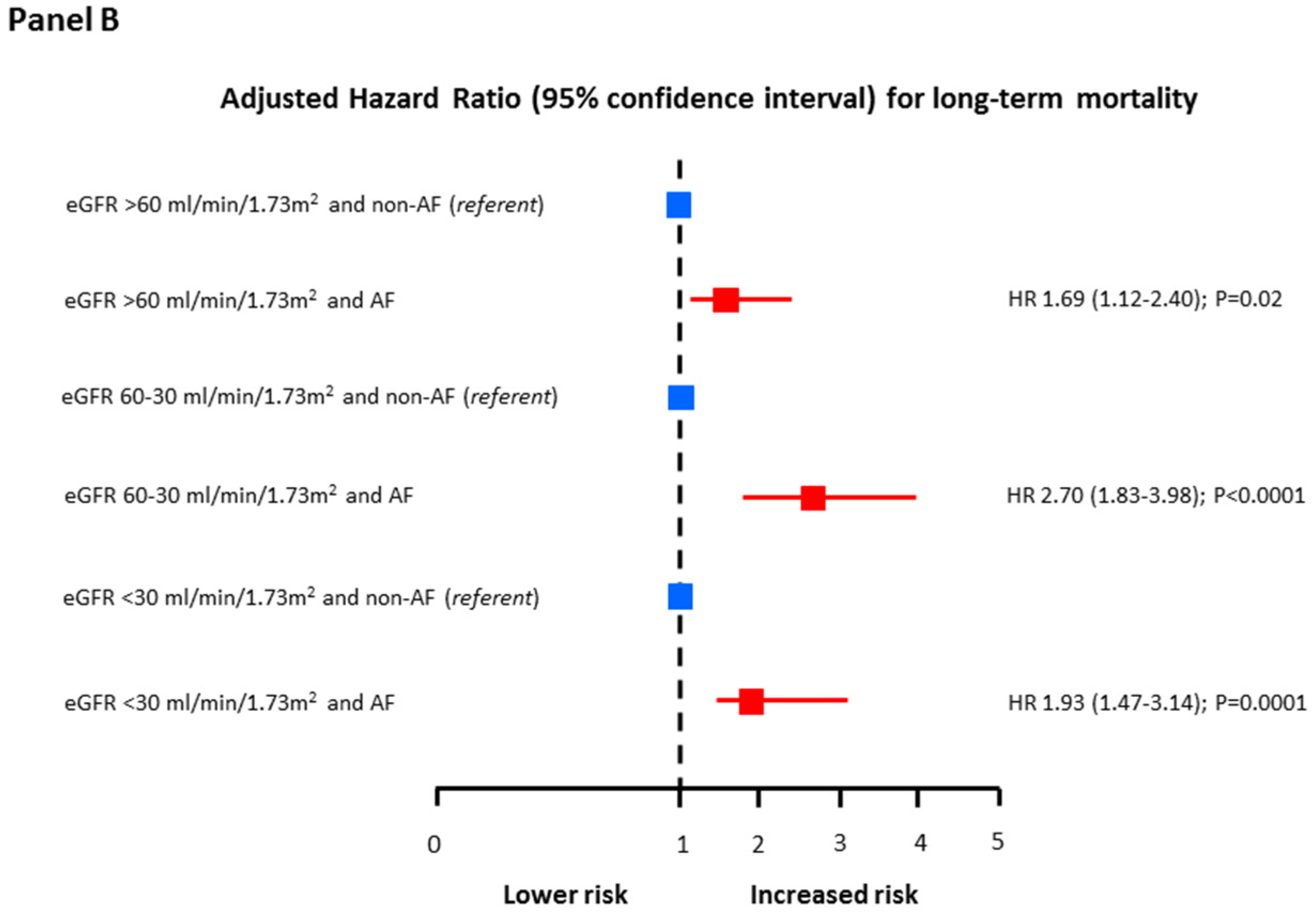
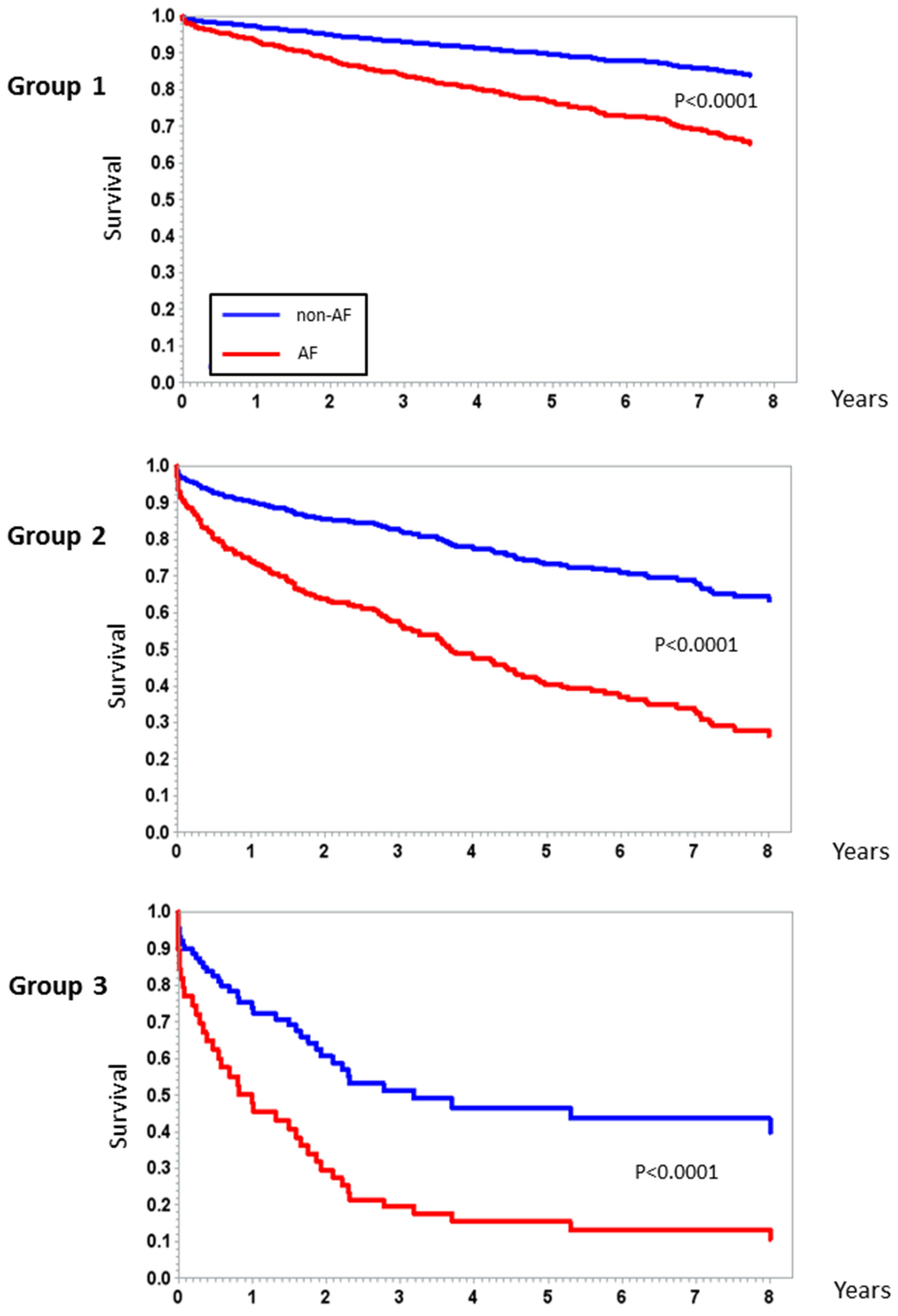
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saczynski, J.S.; McManus, D.; Zhou, Z.; Spencer, F.; Yarzebski, J.; Lessard, D.; Gore, J.M.; Goldberg, R.J. Trends in atrial fibrillation complicating acute myocardial infarction. Am. J. Cardiol. 2009, 104, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.S.; Ward, S.R.; Granger, C.B.; Stebbins, A.L.; Topol, E.J.; Califf, R.M. Atrial fibrillation in the setting of acute myocardial infarction: The GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J. Am. Coll. Cardiol. 1997, 30, 406–413. [Google Scholar] [CrossRef]
- Kinjo, K.; Sato, H.; Sato, H.; Ohnishi, Y.; Hishida, E.; Nakatani, D.; Mizuno, H.; Fukunami, M.; Koretsune, Y.; Takeda, H.; et al. Osaka Acute Coronary Insufficiency Study (OACIS) Group. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am. J. Cardiol. 2003, 92, 1150–1154. [Google Scholar] [CrossRef]
- Lopes, R.D.; Pieper, K.S.; Horton, J.R.; Al-Khatib, S.M.; Newby, L.K.; Mehta, R.H.; Van de Werf, F.; Armstrong, P.W.; Mahaffey, K.W.; Harrington, R.A.; et al. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart 2008, 94, 867–873. [Google Scholar] [CrossRef]
- Mehta, R.H.; Dabbous, O.H.; Granger, C.B.; Kuznetsova, P.; Kline-Rogers, E.M.; Anderson, F.A., Jr.; Fox, K.A.; Gore, J.M.; Goldberg, R.J.; Eagle, K.A. GRACE Investigators. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am. J. Cardiol. 2003, 92, 1031–1036. [Google Scholar] [CrossRef]
- Wong, C.K.; White, H.D.; Wilcox, R.G.; Criger, D.A.; Califf, R.M.; Topol, E.J.; Ohman, E.M. Significance of atrial fibrillation during acute myocardial infarction, and its current management: Insights from the GUSTO-3 trial. Card Electrophysiol. Rev. 2003, 7, 201–207. [Google Scholar] [CrossRef]
- Pizzetti, F.; Turazza, F.M.; Franzosi, M.G.; Barlera, S.; Ledda, A.; Maggioni, A.P.; Santoro, L. GISSI-3 Investigators. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: The GISSI-3 data. Heart 2001, 86, 27–32. [Google Scholar] [CrossRef]
- Schmitt, J.; Duray, G.; Gersh, B.J.; Hohnloser, S.H. Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications. Eur. Heart J. 2009, 30, 1038–1045. [Google Scholar] [CrossRef]
- Soliman, E.Z.; Prineas, R.J.; Go, A.S.; Xie, D.; Lash, J.P.; Rahman, M.; Ojo, A.; Teal, V.L.; Jensvold, N.G.; Robinson, N.L.; et al. Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am. Heart J. 2010, 159, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Nabauer, M.; Gerth, A.; Limbourg, T.; Treszl, A.; Engelbertz, C.; Eckardt, L.; Kirchhof, P.; Wegscheider, K.; Ravens, U.; et al. Morbidity and treatment in patients with atrial fibrillation and chronic kidney disease. Kidney Int. 2015, 87, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.B.; Lip, G.Y.; Kamper, A.L.; Hommel, K.; Køber, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2016, 367, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; James, M.T.; Manns, B.J.; Quinn, R.R.; Ravani, P.; Tonelli, M.; Perkovic, V.; Winkelmayer, W.C.; Ma, Z.; Hemmelgarn, B.R.; et al. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: Population based observational study. BMJ 2015, 350, h246. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Cabiati, A.; Cosentino, N.; Assanelli, E.; Milazzo, V.; Rubino, M.; Lauri, G.; Morpurgo, M.; Moltrasio, M.; Marana, I.; et al. Prognostic significance of serum creatinine and its change patterns in patients with acute coronary syndromes. Am. Heart J. 2015, 169, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D.; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Marenzi, G.; Cabiati, A.; Bertoli, S.V.; Assanelli, E.; Marana, I.; De Metrio, M.; Rubino, M.; Moltrasio, M.; Grazi, M.; Campodonico, J.; et al. Incidence and relevance of acute kidney injury in patients hospitalized with acute coronary syndromes. Am. J. Cardiol. 2013, 111, 816–822. [Google Scholar] [CrossRef]
- Fox, K.A.; Carruthers, K.F.; Dunbar, D.R.; Graham, C.; Manning, J.R.; Manning, J.R.; De Raedt, H.; Buysschaert, I.; Buysschaert, I.; Lambrechts, D.; et al. Underestimated and under-recognized: The late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur. Heart J. 2010, 31, 2755–2764. [Google Scholar] [CrossRef]
- Lehto, M.; Snapinn, S.; Dickstein, K.; Swedberg, K.; Nieminen, M.; OPTIMAAL investigators. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: The OPTIMAAL experience. Eur. Heart J. 2005, 26, 350–356. [Google Scholar] [CrossRef]
- Zeymer, U.; Annemans, L.; Danchin, N.; Pocock, S.; Newsome, S.; Van de Werf, F.; Medina, J.; Bueno, H. Impact of known or new-onset atrial fibrillation on 2-year cardiovascular event rate in patients with acute coronary syndromes: Results from the prospective EPICOR Registry. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Jons, C.; Jacobsen, U.G.; Joergensen, R.M.; Olsen, N.T.; Dixen, U.; Johannessen, A.; Huikuri, H.; Messier, M.; McNitt, S.; Thomsen, P.E.; et al. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: A CARISMA substudy. Heart Rhythm. 2011, 8, 342–348. [Google Scholar]
- Lopes, R.D.; Elliott, L.E.; White, H.D.; Hochman, J.S.; Van de Werf, F.; Ardissino, D.; Nielsen, T.T.; Weaver, W.D.; Widimsky, P.; Armstrong, P.W.; et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: Results from the APEX-AMI trial. Eur. Heart J. 2009, 30, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Berger, A.K.; Weinfurt, K.P.; Schulman, K.A.; Oetgen, W.J.; Gersh, B.J.; Solomon, A.J. Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation 2000, 101, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Jons, C.; Joergensen, R.M.; Hassager, C.; Gang, U.J.; Dixen, U.; Johannesen, A.; Olsen, N.T.; Hansen, T.F.; Messier, M.; Huikuri, H.V.; et al. Diastolic dysfunction predicts new-onset atrial fibrillation and cardiovascular events in patients with acute myocardial infarction and depressed left ventricular systolic function: A CARISMA substudy. Eur. J. Echocardiogr. 2010, 11, 602–607. [Google Scholar] [CrossRef]
- Jons, C.; Raatikainen, P.; Gang, U.J.; Huikuri, H.V.; Joergensen, R.M.; Johannesen, A.; Dixen, U.; Messier, M.; McNitt, S.; Thomsen, P.E.; et al. Autonomic dysfunction and new-onset atrial fibrillation in patients with left ventricular systolic dysfunction after acute myocardial infarction: A CARISMA substudy. J. Cardiovasc. Electrophysiol. 2010, 21, 983–990. [Google Scholar] [PubMed]
- Grassi, G.; Bertoli, S.; Seravalle, G. Sympathetic nervous system: Role in hypertension and in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Grisk, O. The sympathetic nervous system in acute kidney injury. Acta Physiol. 2019, 14, e13404. [Google Scholar] [CrossRef]
- Hwang, H.J.; Ha, J.W.; Joung, B.; Choi, E.H.; Kim, J.; Ahn, M.S.; Lee, M.H.; Jang, Y.; Chung, N.; Kim, S.S.; et al. Relation of inflammation and left atrial remodeling in atrial fibrillation occurring in early phase of acute myocardial infarction. Int. J. Cardiol. 2011, 146, 28–31. [Google Scholar] [CrossRef]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef]
- Floria, M.; Tanase, D.M. Atrial fibrillation type and renal dysfunction: New challenges in thromboembolic risk assessment. Heart 2019, 105, 1295–1297. [Google Scholar] [CrossRef]
| Variable | Atrial Fibrillation | p Value | |
|---|---|---|---|
| No (n = 2204) | Yes (n = 241) | ||
| Age (years) | 66 ± 12 | 75 ± 10 | <0.0001 |
| Male sex, n (%) | 1647 (75%) | 159 (66%) | 0.003 |
| Body weight (kg) | 76 ± 14 | 74 ± 16 | 0.01 |
| Diabetes mellitus, n (%) | 467 (21%) | 81 (34%) | <0.0001 |
| Hypertension, n (%) | 1398 (63%) | 194 (80%) | <0.0001 |
| Smokers, n (%) | 1191 (54%) | 98 (31%) | <0.0001 |
| Hyperlipidemia, n (%) | 1105 (50%) | 123 (51%) | 0.80 |
| Prior myocardial infarction, n (%) | 571 (26%) | 77 (32%) | 0.004 |
| Prior CABG, n (%) | 255 (12%) | 39 (16%) | 0.03 |
| Prior PCI, n (%) | 578 (26%) | 68 (28%) | 0.48 |
| Left ventricular ejection fraction (%) | 51 ± 11 | 43 ± 13 | <0.0001 |
| STEMI, n (%) | 1017 (46%) | 131 (54%) | 0.01 |
| CA/PCI during hospitalization, n (%) | 2080 (95%) | 210 (87%) | <0.0001 |
| Laboratory values at hospital admission | |||
| Serum creatinine (mg/dL) | 1.02 ± 0.44 | 1.23 ± 0.82 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 79 ± 26 | 68 ± 26 | <0.0001 |
| Hemoglobin (g/dL) | 13.8 ± 1.8 | 13.2 ± 1.9 | <0.0001 |
| Blood glucose (mg/dL) | 147 ± 60 | 166 ± 72 | <0.0001 |
| hs-TnI (ng/L) | 400 (70–2340) | 1064 (130–6690) | 0.0001 * |
| hs-CRP (mg/L) | 3.39 (1.37–10.71) | 7.06 (2.12–36.41) | <0.0001 * |
| Medication before hospital admission | |||
| Aspirin, n (%) | 822 (37%) | 100 (41%) | 0.2 |
| Statins, n (%) | 747 (34%) | 99 (42%) | 0.02 |
| Beta-blockers, n (%) | 772 (35%) | 104 (43%) | 0.001 |
| ACE/AR blockers, n (%) | 859 (39%) | 121 (50%) | 0.001 |
| In-hospital complications | |||
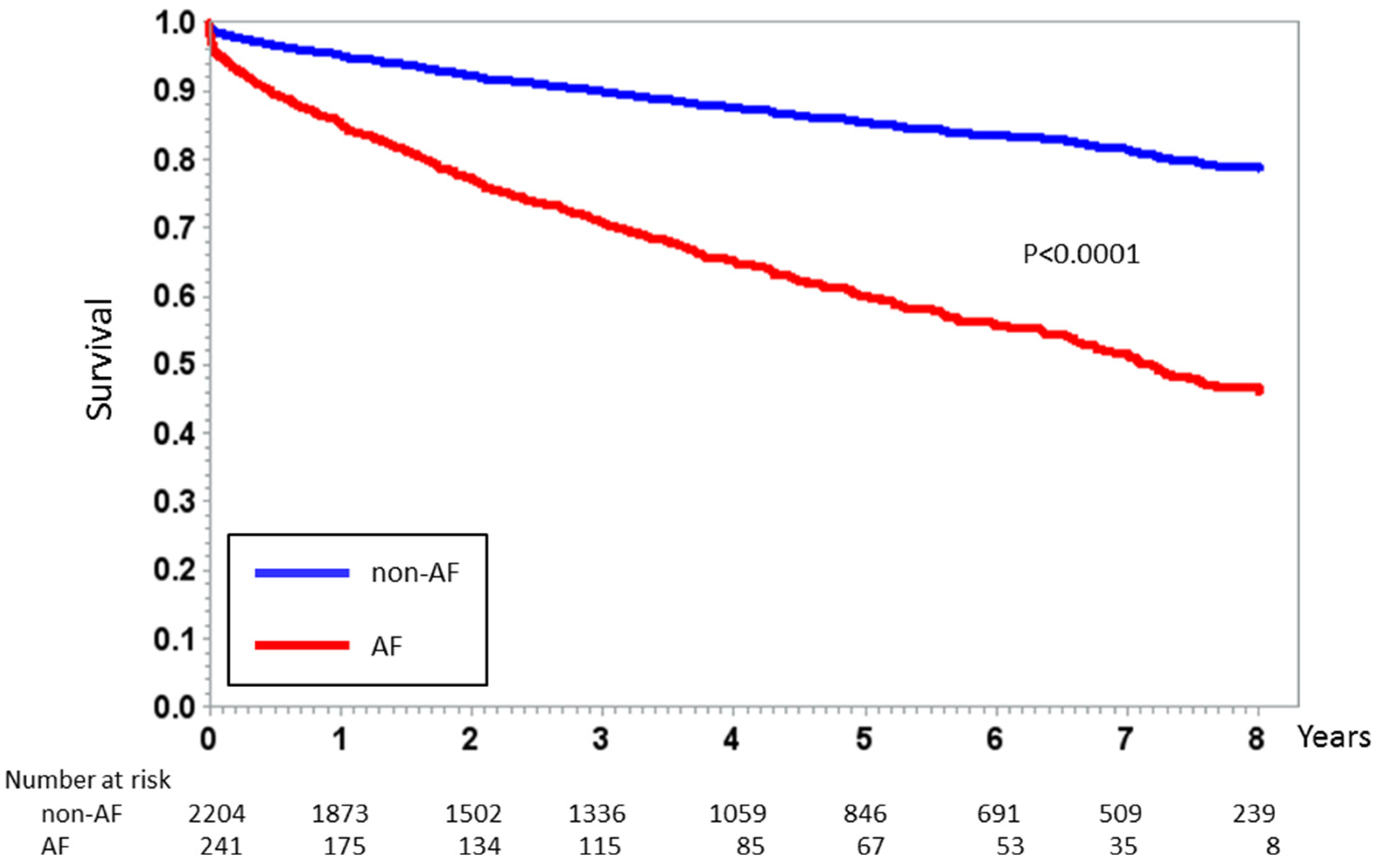
| In-hospital death, n (%) | 26 (1.2%) | 13 (5.4%) | <0.0001 |
| Cardiogenic shock, n (%) | 86 (4%) | 35 (15%) | <0.0001 |
| Acute pulmonary edema, n (%) | 150 (7%) | 76 (32%) | <0.0001 |
| Mechanical ventilation, n (%) | 55 (3%) | 28 (12%) | <0.0001 |
| VT/VF, n (%) | 147 (7%) | 32 (13%) | 0.0001 |
| High-degree AV block, n (%) | 69 (3%) | 18 (7%) | 0.0001 |
| Major bleeding, n (%) | 50 (2%) | 26 (11%) | <0.0001 |
| hs-TnI peak value (ng/L) | 37,442 ± 77,002 | 74,162 ± 168,968 | <0.0001 |
| Medication at hospital discharge # | |||
| Dual antiplatelet therapy, n (%) | 2014 (92%) | 209 (92%) | 0.66 |
| Statins, n (%) | 2005 (92%) | 207 (91%) | 0.50 |
| Beta-blockers, n (%) | 1702 (78%) | 175 (77%) | 0.63 |
| ACE/AR blockers, n (%) | 1401 (64%) | 133 (58%) | 0.07 |
| Oral anticoagulant | |||
| therapy, n (%) | 48 (2%) | 30 (13%) | <0.0001 |
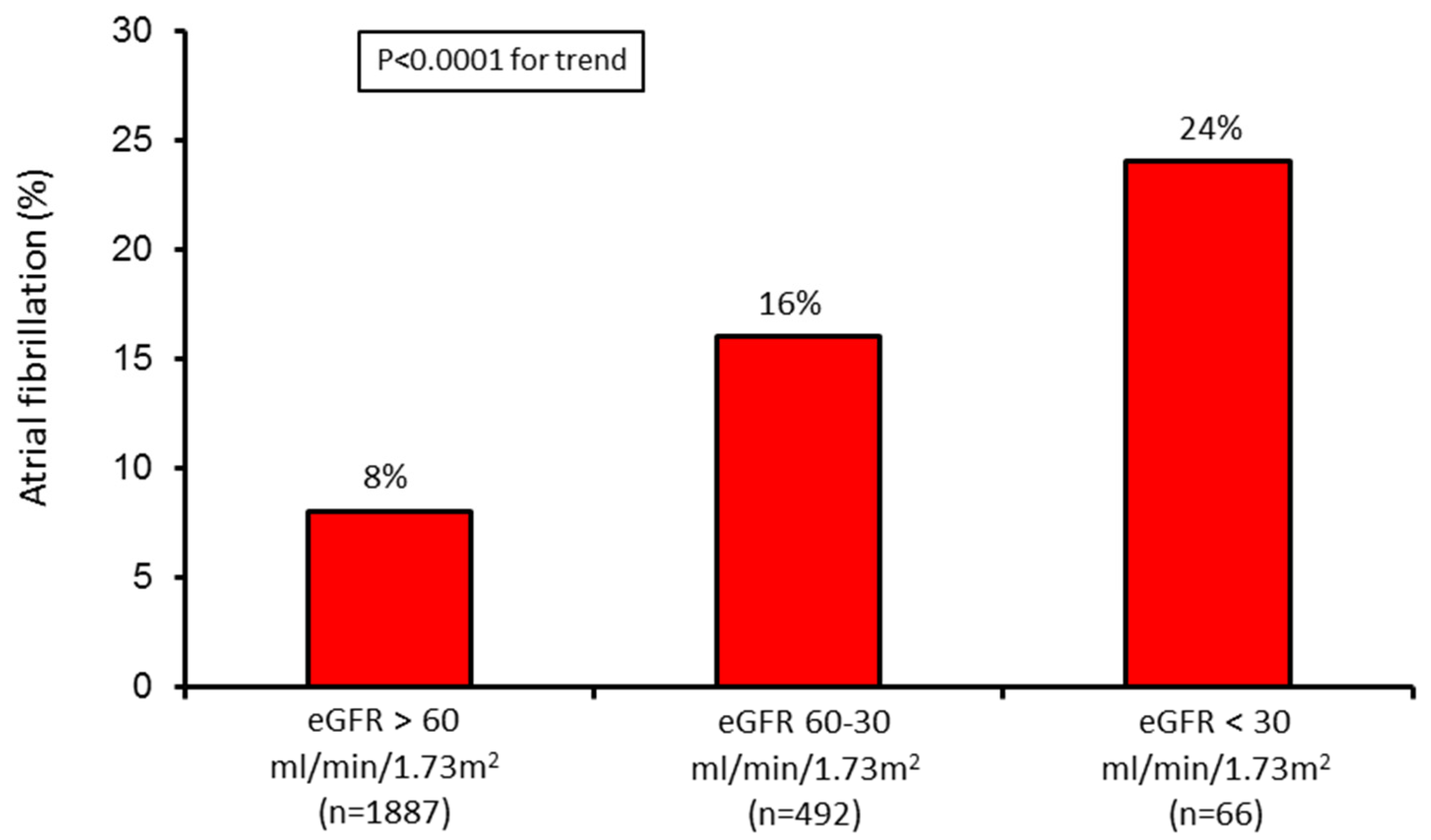
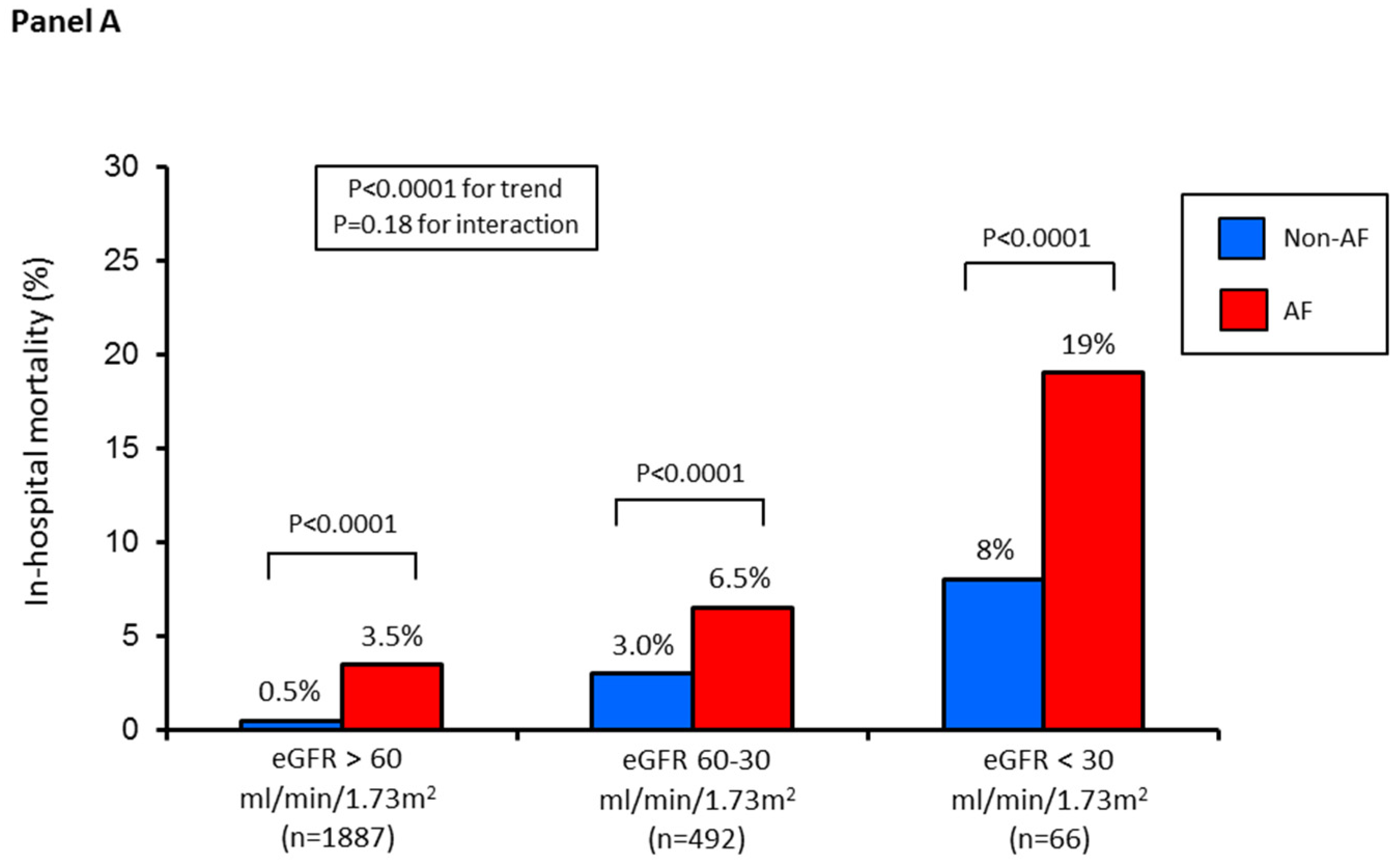
| Variable | Group 1 (n = 1887) | Group 2 (n = 492) | Group 3 (n = 66) | p Value (for Trend) |
|---|---|---|---|---|
| Age (years) | 65 ± 12 | 73 ± 11 | 75 ± 9 | <0.0001 |
| Male sex, n (%) | 1450 (77%) | 319 (65%) | 37 (56%) | <0.0001 |
| Body weight (kg) | 76 ± 14 | 76 ± 16 | 73 ± 12 | 0.69 |
| Diabetes mellitus, n (%) | 354 (19%) | 162 (33%) | 32 (48%) | <0.0001 |
| Hypertension, n (%) | 1148 (61%) | 387 (79%) | 57 (87%) | <0.0001 |
| Smokers, n (%) | 1068 (57%) | 198 (40%) | 23 (36%) | <0.0001 |
| Hyperlipidemia, n (%) | 917 (49%) | 268 (54%) | 43 (65%) | 0.0009 |
| Prior myocardial infarction, n (%) | 431 (23%) | 185 (38%) | 32 (48%) | <0.0001 |
| Prior CABG, n (%) | 188 (10%) | 90 (18%) | 16 (24%) | <0.0001 |
| Prior PCI, n (%) | 433 (23%) | 188 (38%) | 25 (38%) | <0.0001 |
| Left ventricular ejection fraction (%) | 51 ± 11 | 48 ± 13 | 43 ± 14 | <0.0001 |
| STEMI, n (%) | 915 (48%) | 211 (43%) | 22 (33%) | 0.002 |
| CA/PCI during hospitalization, n (%) | 1796 (95%) | 439 (89%) | 55 (83%) | <0.0001 |
| Laboratory values at hospital admission | ||||
| Serum creatinine (mg/dL) | 0.88 ± 0.18 | 1.39 ± 0.31 | 3.01 ± 1.370 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 88 ± 21 | 48 ± 8 | 21 ± 5 | <0.0001 |
| Hemoglobin (g/dL) | 14.0 ± 1.7 | 13.1 ± 1.9 | 11.3 ± 1.9 | <0.0001 |
| Blood glucose (mg/dL) | 143 ± 56 | 167 ± 70 | 193 ± 92 | <0.0001 |
| hs-TnI (ng/L) | 430 (76–2460) | 440 (80–2700) | 1087 (80–5024) | 0.12 |
| Medication before hospital admission | ||||
| Aspirin, n (%) | 638 (34%) | 242 (49%) | 42 (64%) | <0.0001 |
| Statins, n (%) | 587 (31%) | 223 (46%) | 36 (55%) | <0.0001 |
| Beta-blockers, n (%) | 604 (32%) | 229 (47%) | 43 (65%) | <0.0001 |
| ACE/AR blockers, n (%) | 683 (36%) | 271 (55%) | 26 (39%) | <0.0001 |
| In-hospital complications | ||||
| In-hospital death, n (%) | 14 (0.7%) | 18 (4%) | 7 (11%) | <0.0001 |
| Cardiogenic shock, n (%) | 66 (3%) | 44 (9%) | 11 (17) | <0.0001 |
| Acute pulmonary edema, n (%) | 118 (6%) | 84 (17%) | 24 (36%) | <0.0001 |
| Mechanical ventilation, n (%) | 37 (2%) | 38 (8%) | 8 (12%) | <0.0001 |
| VT/VF, n (%) | 128 (7%) | 46 (9%) | 5 (8%) | 0.11 |
| High-degree AV block, n (%) | 50 (3%) | 34 (7%) | 3 (5%) | 0.001 |
| Major bleeding, n (%) | 41 (2%) | 26 (5%) | 9 (14%) | <0.0001 |
| hs-TnI peak value (ng/L) | 37,001 ± 749,442 | 49,503 ± 108,275 | 71,774 ± 240,209 | 0.65 |
| Variable | OR * (95% CI) | p Value |
|---|---|---|
| eGFR 60–30 vs. >60 mL/min/1.73 m2 | 2.28 (1.70–3.06) | <0.0001 |
| eGFR <30 vs. >60 mL/min/1.73 m2 | 3.81 (2.12–6.87) | <0.0001 |
| STEMI vs. NSTEMI | 1.48 (1.23–3.93) | 0.0001 |
| Age (every 10-year increase) | 2.12 (1.38–3.66) | <0.0001 |
| LVEF (every 10% decrease) | 1.64 (1.37–2.97) | <0.0001 |
| Killip class III-IV vs. I-II | 1.98 (1.65–3.13) | <0.0001 |
| PCI (no vs. yes) | 1.73 (1.31–3.01) | <0.0001 |
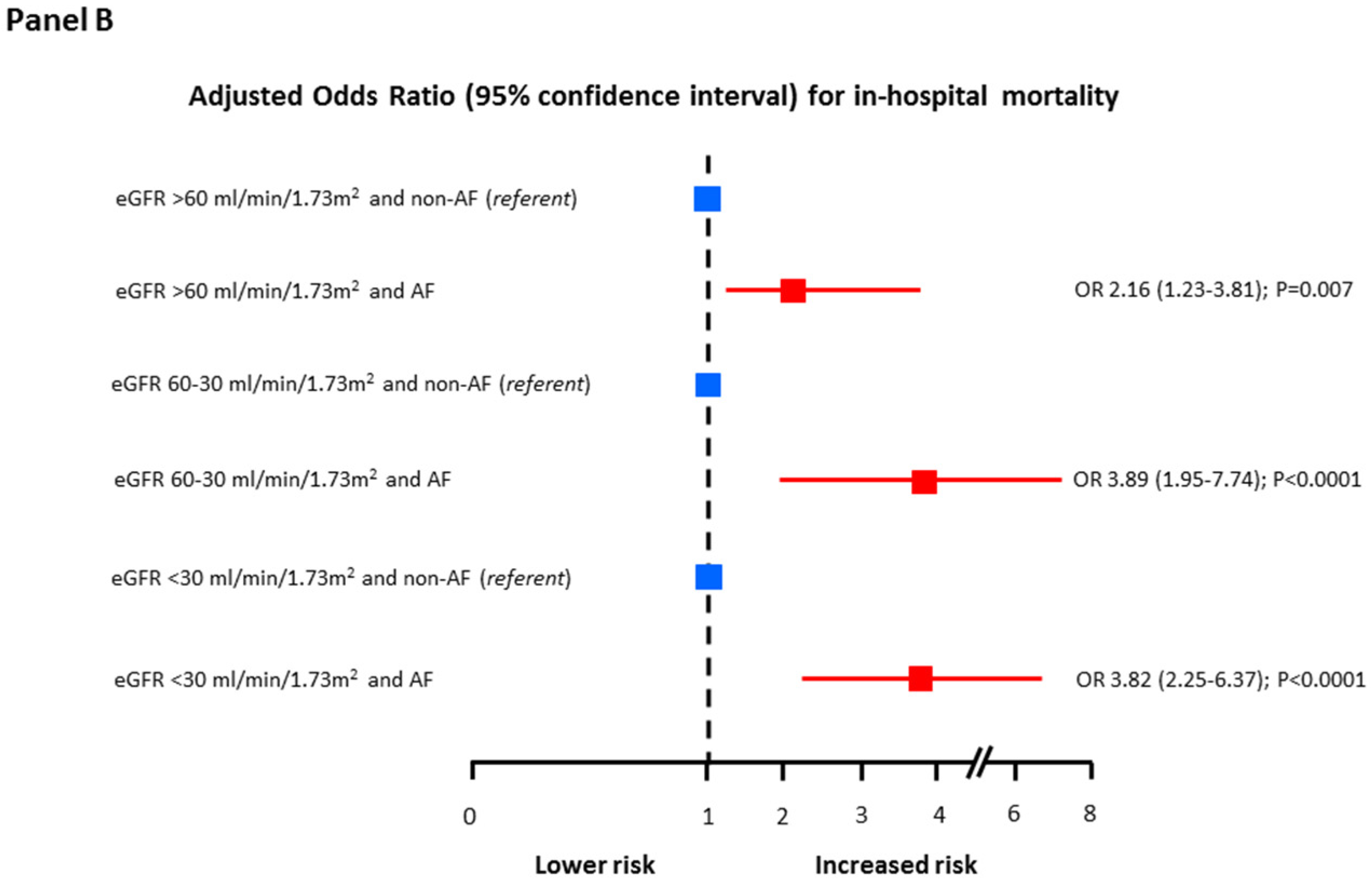
| Variable | Adjusted OR (95% CI) | p Value |
| eGFR 60–30 vs. >60 mL/min/1.73 m2 | 2.69 (1.13–6.37) | 0.003 |
| eGFR <30 vs. 60–30 mL/min/1.73 m2 | 2.53 (1.35–7.68) | 0.0001 |
| Variable | Adjusted HR (95% CI) | p Value |
| eGFR 60–30 vs. >60 mL/min/1.73 m2 | 1.44 (1.14–1.81) | 0.002 |
| eGFR <30 vs. 60–30 mL/min/1.73 m2 | 2.24 (1.55–3.23) | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosentino, N.; Ballarotto, M.; Campodonico, J.; Milazzo, V.; Bonomi, A.; Genovesi, S.; Moltrasio, M.; De Metrio, M.; Rubino, M.; Veglia, F.; et al. Impact of Glomerular Filtration Rate on the Incidence and Prognosis of New-Onset Atrial Fibrillation in Acute Myocardial Infarction. J. Clin. Med. 2020, 9, 1396. https://doi.org/10.3390/jcm9051396
Cosentino N, Ballarotto M, Campodonico J, Milazzo V, Bonomi A, Genovesi S, Moltrasio M, De Metrio M, Rubino M, Veglia F, et al. Impact of Glomerular Filtration Rate on the Incidence and Prognosis of New-Onset Atrial Fibrillation in Acute Myocardial Infarction. Journal of Clinical Medicine. 2020; 9(5):1396. https://doi.org/10.3390/jcm9051396
Chicago/Turabian StyleCosentino, Nicola, Marco Ballarotto, Jeness Campodonico, Valentina Milazzo, Alice Bonomi, Simonetta Genovesi, Marco Moltrasio, Monica De Metrio, Mara Rubino, Fabrizio Veglia, and et al. 2020. "Impact of Glomerular Filtration Rate on the Incidence and Prognosis of New-Onset Atrial Fibrillation in Acute Myocardial Infarction" Journal of Clinical Medicine 9, no. 5: 1396. https://doi.org/10.3390/jcm9051396
APA StyleCosentino, N., Ballarotto, M., Campodonico, J., Milazzo, V., Bonomi, A., Genovesi, S., Moltrasio, M., De Metrio, M., Rubino, M., Veglia, F., Assanelli, E., Marana, I., Grazi, M., Lauri, G., Bartorelli, A. L., & Marenzi, G. (2020). Impact of Glomerular Filtration Rate on the Incidence and Prognosis of New-Onset Atrial Fibrillation in Acute Myocardial Infarction. Journal of Clinical Medicine, 9(5), 1396. https://doi.org/10.3390/jcm9051396






