A Randomized Comparison of Plasma Levobupivacaine Concentrations Following Thoracic Epidural Analgesia and Subpleural Paravertebral Analgesia in Open Thoracic Surgery
Abstract
:1. Introduction
2. Experimental Section
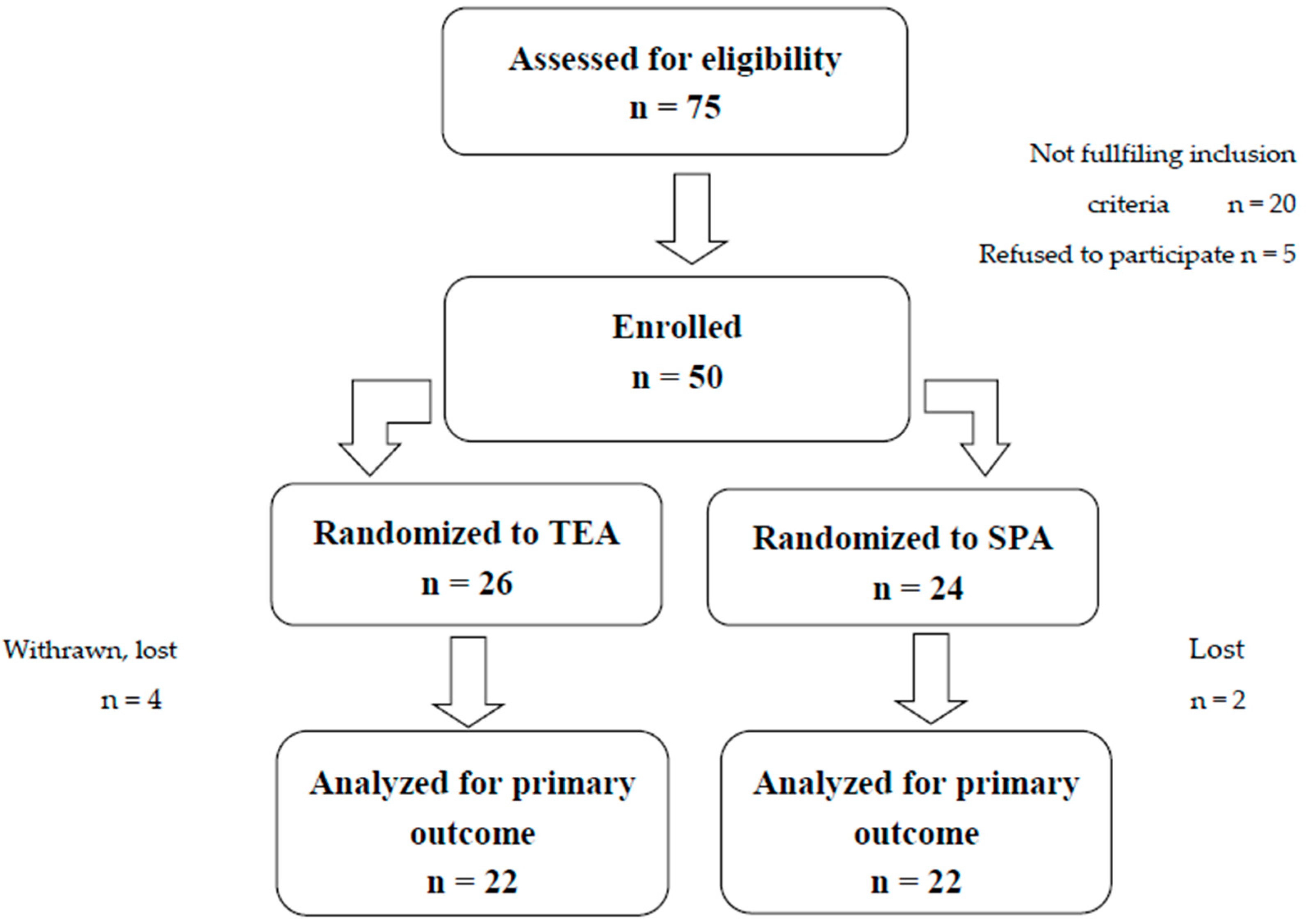
2.1. Randomization and Blinding
2.2. Procedures
2.3. Outcomes
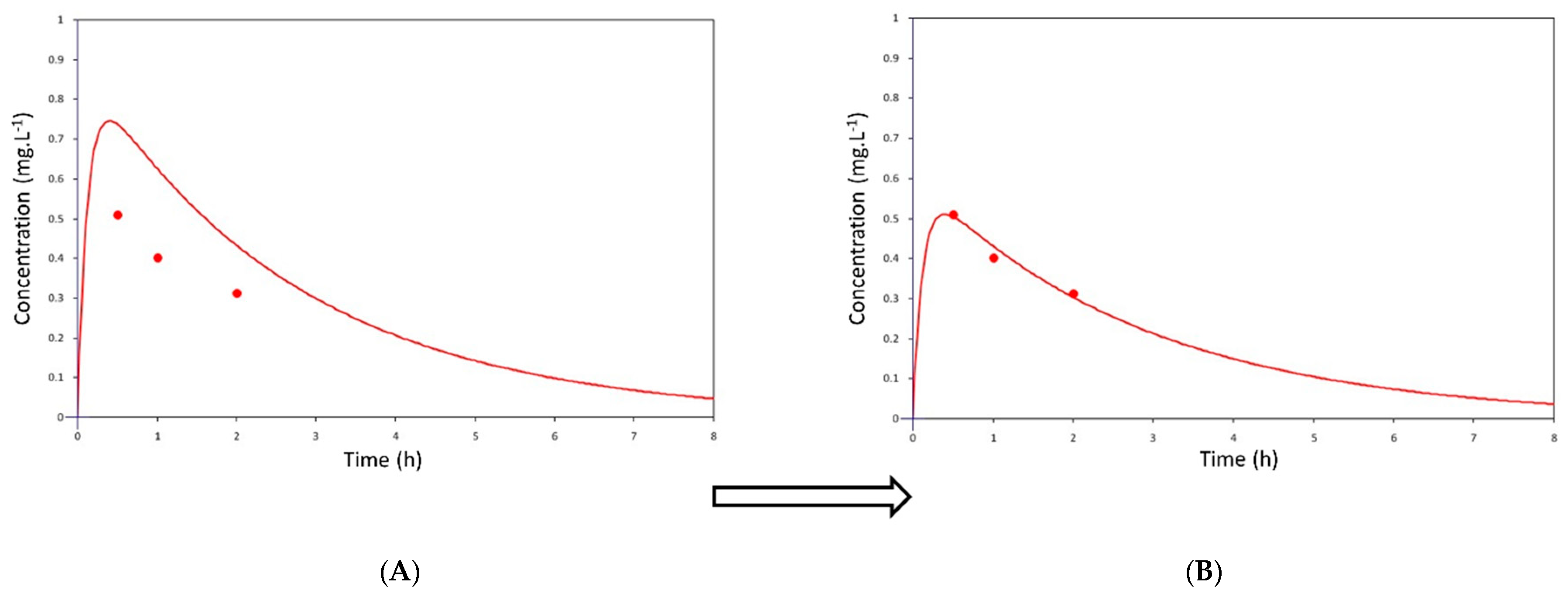
2.4. Pharmacokinetic Analysis
2.5. Pharmacokinetic Modeling
2.6. Statistics
3. Results
3.1. Patient Characteristics
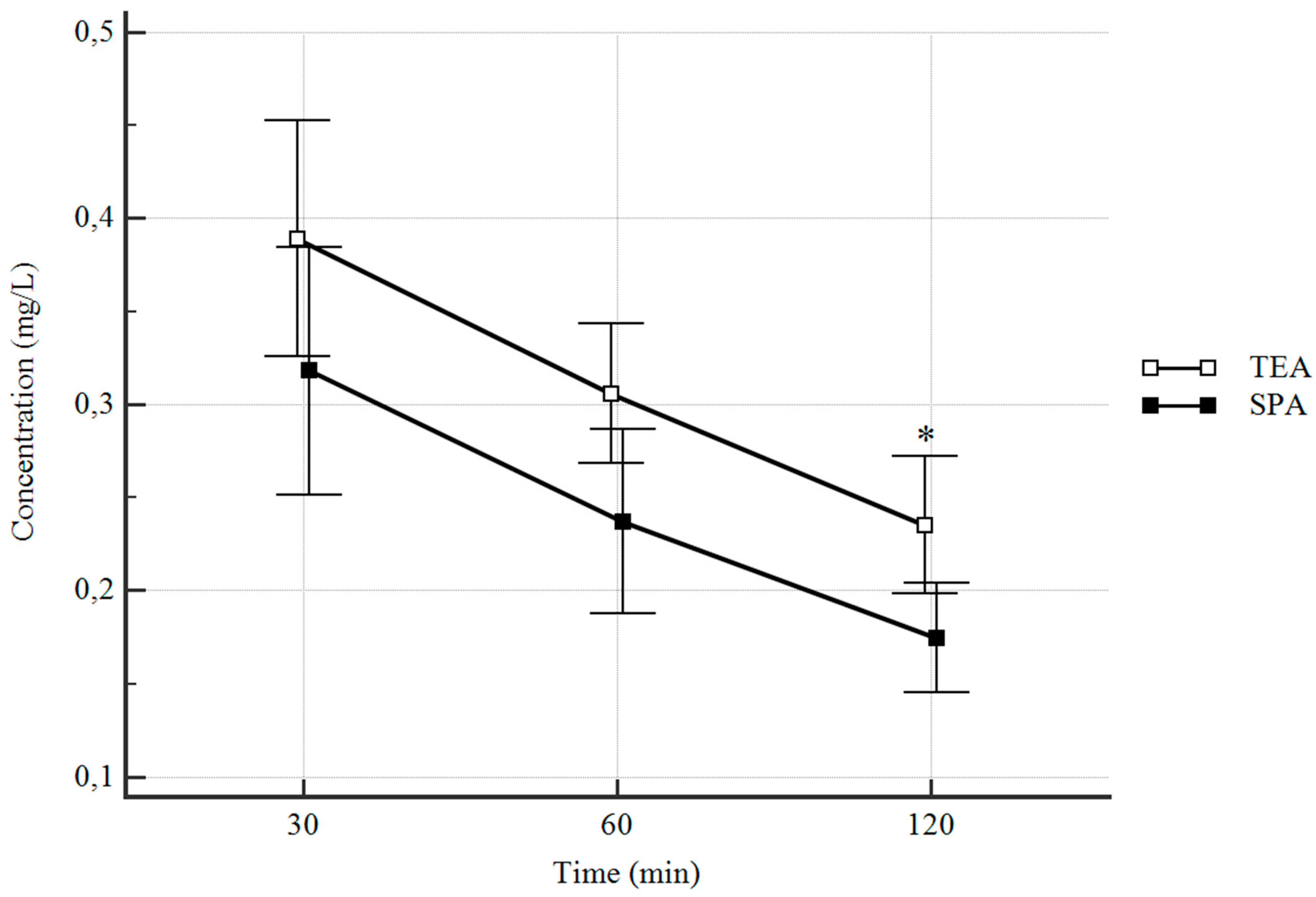
3.2. Plasma Concentrations
3.3. Clinical Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goto, T. What is the best pain control after thoracic surgery? J. Thorac. Dis. 2018, 10, 1335–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampe, S.; Wendland, M.; Welter, S.; Aigner, C.; Hachenberg, T.; Ebmeyer, U.; Weinreich, G. Independent predictors for higher postoperative pain intensity during recovery after open thoracic surgery: A retrospective analysis in 621 patients. Pain Med. 2018, 19, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Manion, S.C.; Brennan, T.J. Thoracic epidural analgesia and acute pain management. Anesthesiology 2011, 115, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y. Regional anesthesia for thoracic surgery. Anaesth. Pain Int. Care 2015, 19, 352–356. [Google Scholar]
- Fang, B.; Wang, Z.; Huang, X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: A single center randomized controlled double-blind study. Ann. Transl. Med. 2019, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Kanazi, G.E.; Ayoub, C.M.; Aouad, M.; Abdallah, F.; Sfeir, P.M.; Adham, A.F.; El-Khatib, M.F. Subpleural block is less effective than thoracic epidural analgesia for post-thoracotomy pain: A randomized controlled study. Eur. J. Anaesthesiol. 2012, 29, 186–191. [Google Scholar] [CrossRef]
- Kopacz, D.J.; Helman, J.D.; Nussbaum, C.E.; Hsiang, J.N.; Nora, P.C.; Allen, H.W. A comparison of epidural levobupivacaine 0.5% with or without epinephrine for lumbar spine surgery. Anesth. Analg. 2001, 93, 755–760. [Google Scholar] [CrossRef]
- Perotti, L.; Cusato, M.; Ingelmo, P.; Niebel, T.L.; Somaini, M.; Riva, F.; Tinelli, C.; de Andres, J.; Fanelli, G.; Braschi, A.; et al. A comparison of differences between the systemic pharmacokinetics of levobupivacaine and ropivacaine during continuous epidural infusion: A prospective, randomized, multicenter, double–blind controlled trial. Anesth. Analg. 2015, 121, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.J.; Veering, B.T.; Stienstra, R.; van Kleef, J.W.; Burm, A.G. Effect of age on the clinical profile and systemic absorption and disposition of levobupivacaine after epidural administration. Br. J. Anaesth. 2004, 93, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Burlacu, C.R.; Frizelle, H.P.; Moriarty, D.C.; Buggy, D.J. Pharmacokinetics of levobupivacaine, fentanyl and clonidine after administration in thoracic paravertebral analgesia. Reg. Anesth. Pain Manag. 2007, 32, 136–145. [Google Scholar] [CrossRef]
- Jette, D.U.; Stilphen, M.; Ranganathan, V.K.; Passek, S.D.; Frost, F.S.; Jette, A.M. Validity of the AM–PAC “6–Clicks” inpatient daily activity and basic mobility short forms. Phys. Ther. 2014, 94, 379–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [CrossRef] [PubMed]
- Simon, M.J.; Veering, B.T.; Stienstra, R.; van Kleef, J.W.; Williams, S.G.; McGuire, G.M.; Burm, A.G. The systemic absorption and disposition of levobupivacaine 0.5% after epidural administration in surgical patients: A stable-isotope study. Eur. J. Anaesthesiol. 2004, 21, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, H.; Gristwood, R.; Baker, H.; Watson, N.; Nimmo, W. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br. J. Clin. Pharmacol. 1998, 46, 245–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gristwood, R.W. Cardiac and CNS toxicity of levobupivacaine: Strengths of evidence for advantage over bupivacaine. Drug Saf. 2002, 25, 153–163. [Google Scholar] [CrossRef]
- Stewart, J.; Kellett, N.; Castro, D. The central nervous system and cardiovascular effects of levobupivacaine and ropivacaine in healthy volunteers. Anesth. Analg. 2003, 97, 412–416. [Google Scholar] [CrossRef]
- Corvetto, M.A.; Echevarria, G.C.; de la Fuente, N.; Mosqueira, L.; Solari, S.; Altermatt, F.R. Comparison of plasma concentrations of levobupivacaine with and without epinephrine for transversus abdominis plane block. Reg. Anesth. Pain Med. 2012, 37, 633–637. [Google Scholar] [CrossRef]
- Miranda, P.; Corvetto, M.A.; Altermatt, F.R.; Araneda, A.; Echevarria, G.C.; Cortinez, L.I. Levobupivacaine absorption pharmacokinetics with and without epinephrine during TAP block: Analysis of doses based on the associated risk of local anaesthetic toxicity. Eur. J. Clin. Pharmacol. 2016, 72, 1221–1227. [Google Scholar] [CrossRef]
- Ishida, T.; Sakamoto, A.; Tanaka, H.; Ide, S.; Ishida, K.; Tanaka, S.; Mori, T.; Kawamata, M. Transversus abdominis plane block with 0.25% levobupivacaine: A prospective, randomized, double-blinded clinical study. J. Anesth. 2015, 29, 557–561. [Google Scholar] [CrossRef]
- Yasamura, R.; Kobayashi, Y.; Ochiai, R. A comparison of plasma levobupivacaine concentrations following transversus abdominis plane block and rectus sheath block. Anaesthesia 2016, 71, 544–549. [Google Scholar] [CrossRef]
- Odor, P.M.; Cavalier, A.G.; Reynolds, N.D.; Ang, K.S.; Parrington, S.J.; Xu, H.; Johnston, A.; Sage, F.J. Safety and pharmacokinetics of levobupivacaine following fascia iliaca compartment block in elderly patients. Drugs Aging 2019, 36, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Crews, J.C.; Weller, R.S.; Moss, J.; James, R.L. Levobupivacaine for axillary brachial plexus block: A pharmacokinetic and clinical comparison in patients with normal renal function or renal disease. Anesth. Analg. 2002, 95, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, F.; Cortinez, L.I.; Munoz, H. Plasma levels of levobupivacaine after combined posterior lumbar plexus and sciatic nerve blocks. Anesth. Analg. 2006, 102, 1597. [Google Scholar] [CrossRef] [PubMed]
| How Much Difficulty Does the Patient Currently Have? | Unable | A Lot | A Little | None |
|---|---|---|---|---|
| Turning over in bed (including adjusting bedclothes, sheets and blankets)? | 1 | 2 | 3 | 4 |
| Sitting down on and standing up from a chair with arms (e.g., wheelchair, bedside commode, etc.)? | 1 | 2 | 3 | 4 |
| Moving from lying on back to sitting on the side of the bed? | 1 | 2 | 3 | 4 |
| How much help from another person does the patient currently need? | Total | A lot | A little | None |
| Moving to and from a bed to a chair (including a wheelchair)? | 1 | 2 | 3 | 4 |
| To walk in hospital room? | 1 | 2 | 3 | 4 |
| Climbing 3–5 steps with a railing? | 1 | 2 | 3 | 4 |
| Thoracic Epidural Analgesia (TEA) | Subpleural Paravertebral Analgesia (SPA) | |
|---|---|---|
| (n = 22) | (n = 22) | |
| Gender (M/F) | 10/12 | 11/11 |
| Age (years) | 72.5 [48–80] | 68 [53–84] |
| Height (cm) | 168 [154–187] | 172 [159–188] |
| Weight (kg) | 74 [51–116] | 85.5 [48–119] |
| Body surface area (BSA) | 1.81 [1.54–2.37] | 1.98 [1.47–2.44] |
| Body mass index (BMI) | 25.5 [18.1–39.7] | 28.9 [19–38.1] |
| Creatinine (µmol × L−1) | 70 [53–96] | 71 [42–144] |
| TEA (n = 22) | SPA (n = 22) | p | |
|---|---|---|---|
| Plasma concentration at 30 min | 0.389 (0.326–0.452) | 0.318 (0.252–0.385) | 0.33 * |
| [0.09–0.668] | [0.122–0.784] | ||
| Plasma concentration at 60 min | 0.306 (0.268–0.34) | 0.237 (0.188–0.288) | 0.08 * |
| [0.101–0.449] | [0.99–0.559] | ||
| Plasma concentration at 120 min | 0.235 (0.198–0.272) | 0.175 (0.145–0.204) | 0.03 * |
| [0.083–0.421] | [0.09–0.314] | ||
| Cmax | 0.396 (0.341–0.451) | 0.320 (0.256–0.384) | 0.069 |
| [0.107–0.605] | [0.149–0.777] | ||
| tmax | 0.46 (0.39–0.53) | 0.40 (0.36–0.44) | 0.17 |
| [0.318–0.86] | [0.313–0.803] | ||
| AUC | 1.262 (1.04–1.48) | 0.906 (0.74–1.07) | 0.039 |
| [0.427–2.539] | [0.418–1.666] |
| TEA (n = 22) | SPA (n = 22) | p | ||
|---|---|---|---|---|
| Nurse rating scale (NRS) | 1 h | 3 [1–5] | 5 [3–6.25] | 0.036 |
| 2 h | 3 [1–5.25] | 4 [3–5] | 0.44 | |
| 6 h | 3 [1.75–4] | 4 [2.75–4] | 0.064 | |
| 12 h | 2 [1–3] | 3 [2–4] | 0.064 | |
| 24 h | 2 [1–3] | 3 [2–4] | 0.061 | |
| AMP–AC | 1 h | 6 [6–6] | 6 [6–6] | 0.99 |
| 2 h | 6 [6–7] | 6 [6–7] | 0.307 | |
| 6 h | 7 [6.75–8] | 7 [6.75–8] | 0.936 | |
| 12 h | 8 [7–9.25] | 8 [7–9] | 0.96 | |
| 24 h | 14 [11.5–15.25] | 12 [10.75–15.25] | 0.603 | |
| Hemodynamic instability | 3 (13.6%) | 0 | 0.232 | |
| Complications - Local hematoma - Temporary paresthesia - Transient weakness of leg(s) | 4 (18.2%) 1 (4.5%) 1 (4.5%) 2 (9.1%) | 0 | 0.108 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matek, J.; Cernohorsky, S.; Trca, S.; Krska, Z.; Hoskovec, D.; Bruthans, J.; Sima, M.; Michalek, P. A Randomized Comparison of Plasma Levobupivacaine Concentrations Following Thoracic Epidural Analgesia and Subpleural Paravertebral Analgesia in Open Thoracic Surgery. J. Clin. Med. 2020, 9, 1395. https://doi.org/10.3390/jcm9051395
Matek J, Cernohorsky S, Trca S, Krska Z, Hoskovec D, Bruthans J, Sima M, Michalek P. A Randomized Comparison of Plasma Levobupivacaine Concentrations Following Thoracic Epidural Analgesia and Subpleural Paravertebral Analgesia in Open Thoracic Surgery. Journal of Clinical Medicine. 2020; 9(5):1395. https://doi.org/10.3390/jcm9051395
Chicago/Turabian StyleMatek, Jan, Stanislav Cernohorsky, Stanislav Trca, Zdenek Krska, David Hoskovec, Jan Bruthans, Martin Sima, and Pavel Michalek. 2020. "A Randomized Comparison of Plasma Levobupivacaine Concentrations Following Thoracic Epidural Analgesia and Subpleural Paravertebral Analgesia in Open Thoracic Surgery" Journal of Clinical Medicine 9, no. 5: 1395. https://doi.org/10.3390/jcm9051395
APA StyleMatek, J., Cernohorsky, S., Trca, S., Krska, Z., Hoskovec, D., Bruthans, J., Sima, M., & Michalek, P. (2020). A Randomized Comparison of Plasma Levobupivacaine Concentrations Following Thoracic Epidural Analgesia and Subpleural Paravertebral Analgesia in Open Thoracic Surgery. Journal of Clinical Medicine, 9(5), 1395. https://doi.org/10.3390/jcm9051395






