Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur
Abstract
1. Introduction
2. Surgical Technique
2.1. Indications
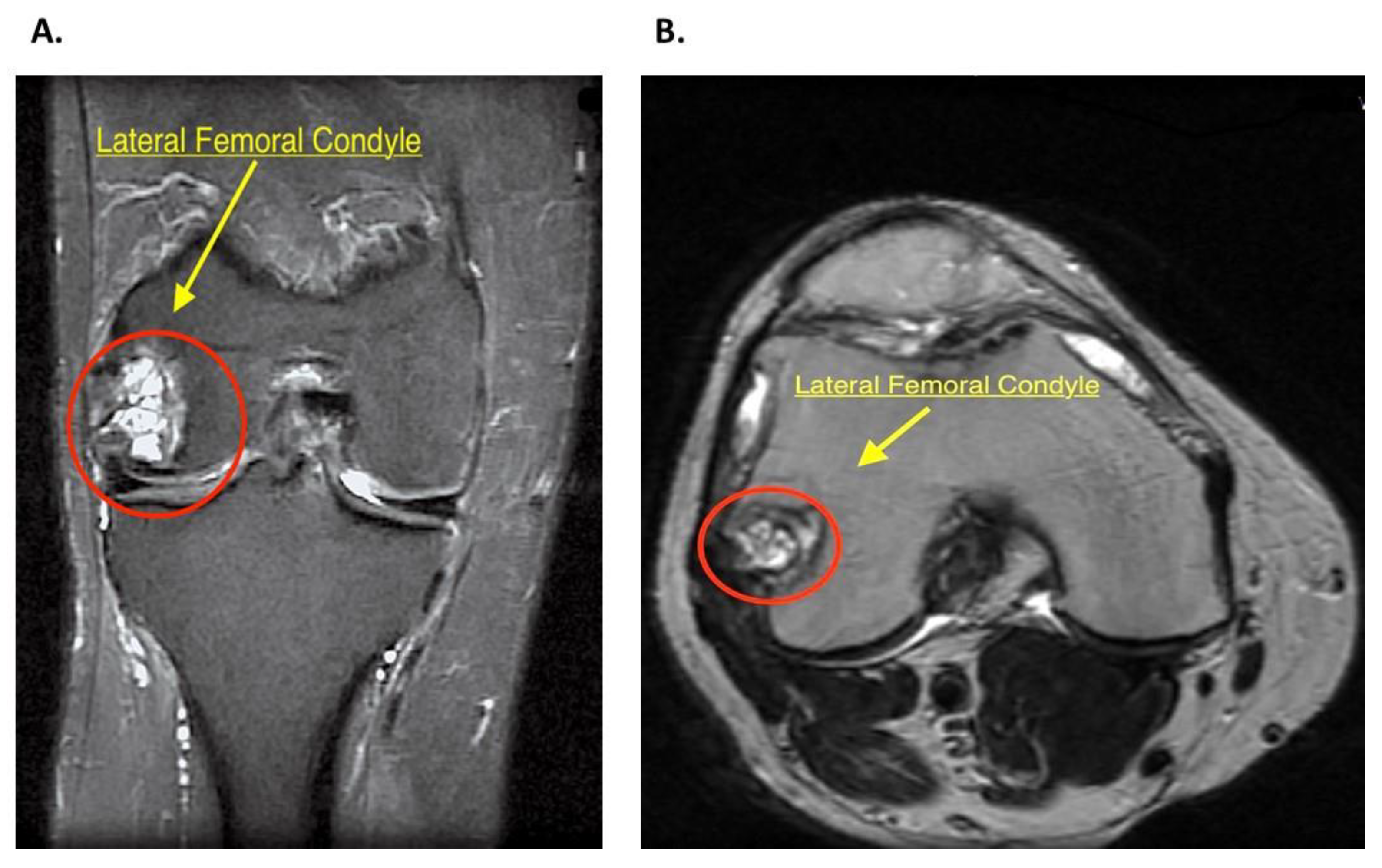
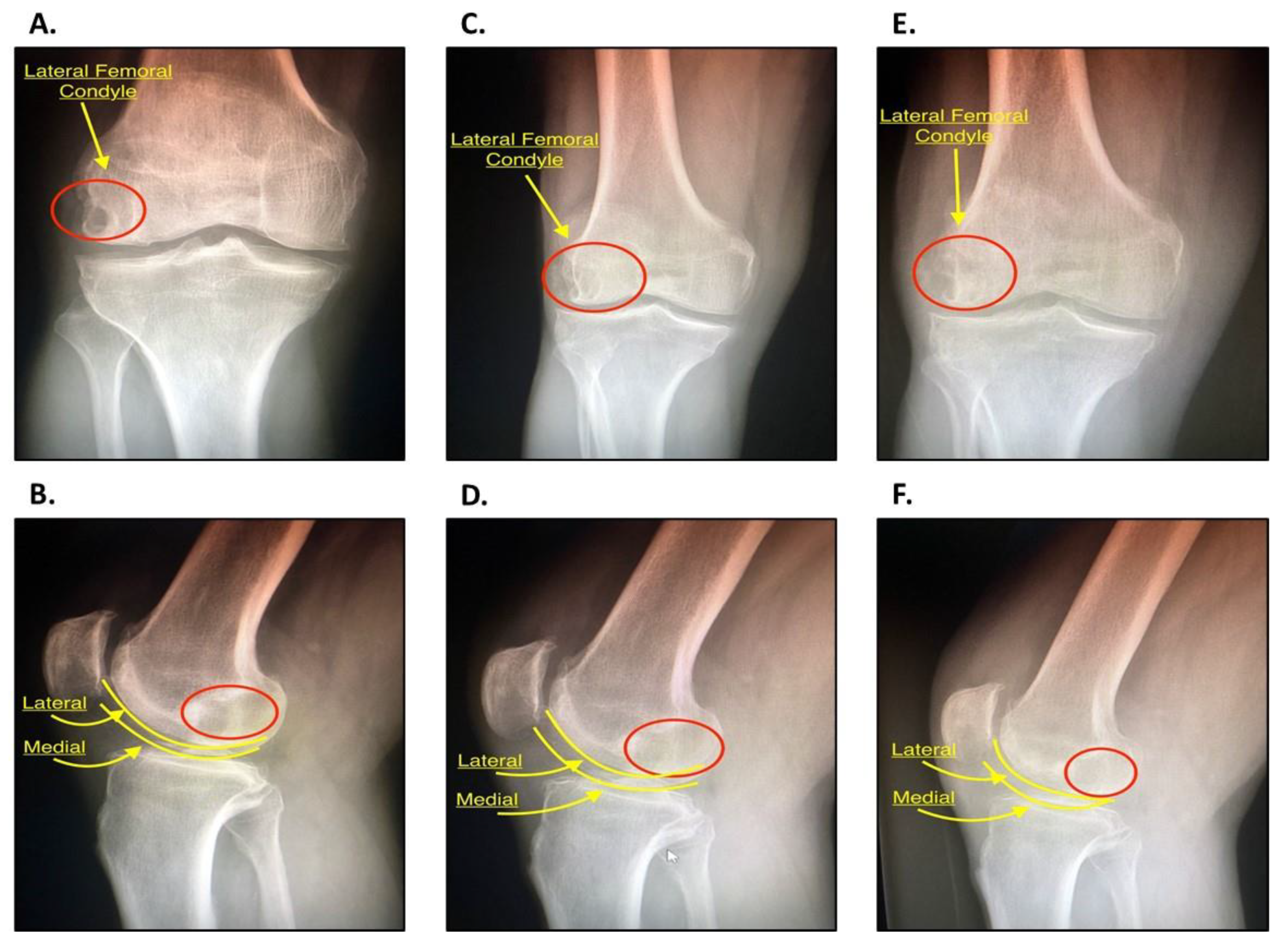
2.2. History and Diagnosis
2.3. Patient Setting
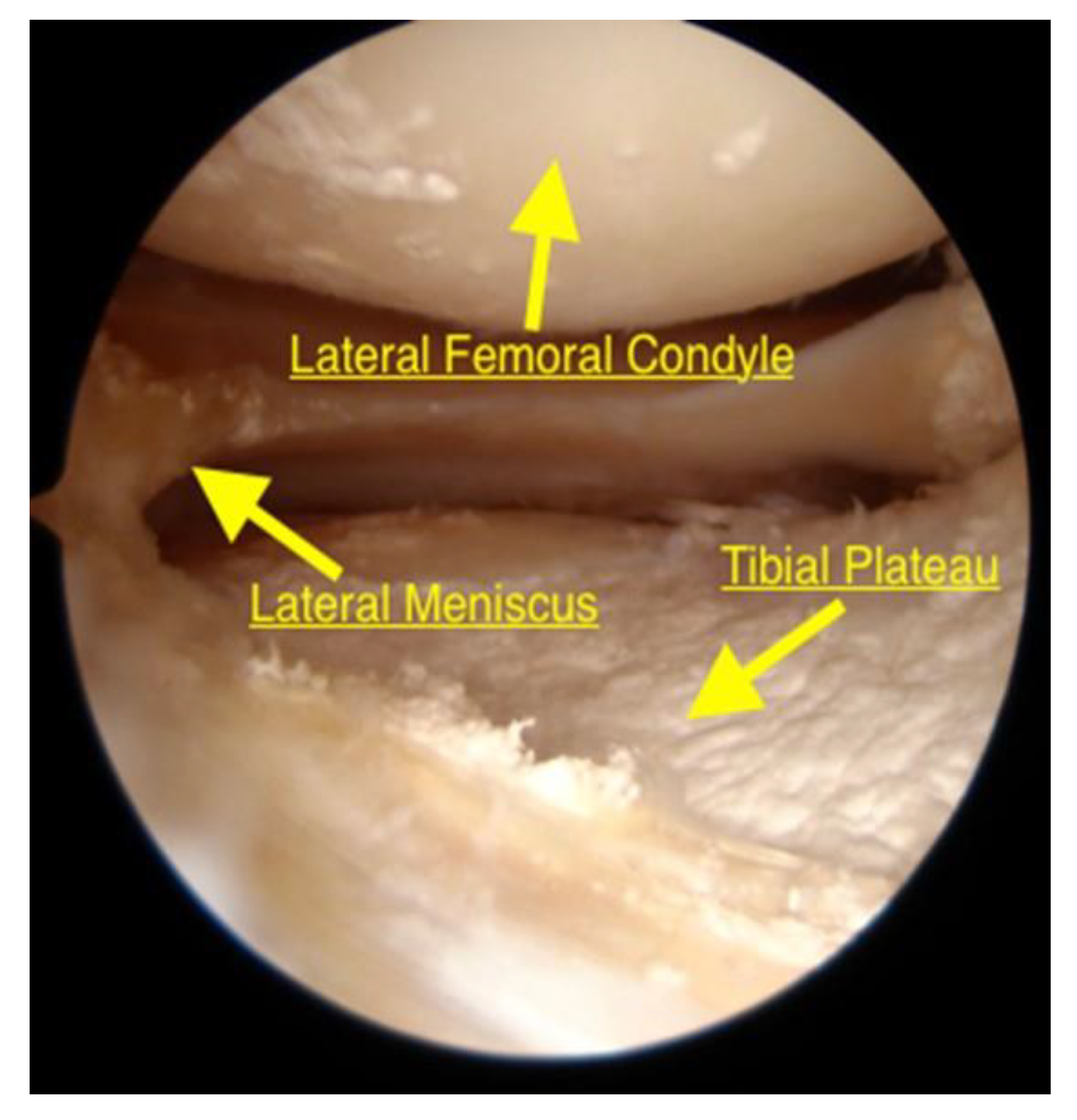
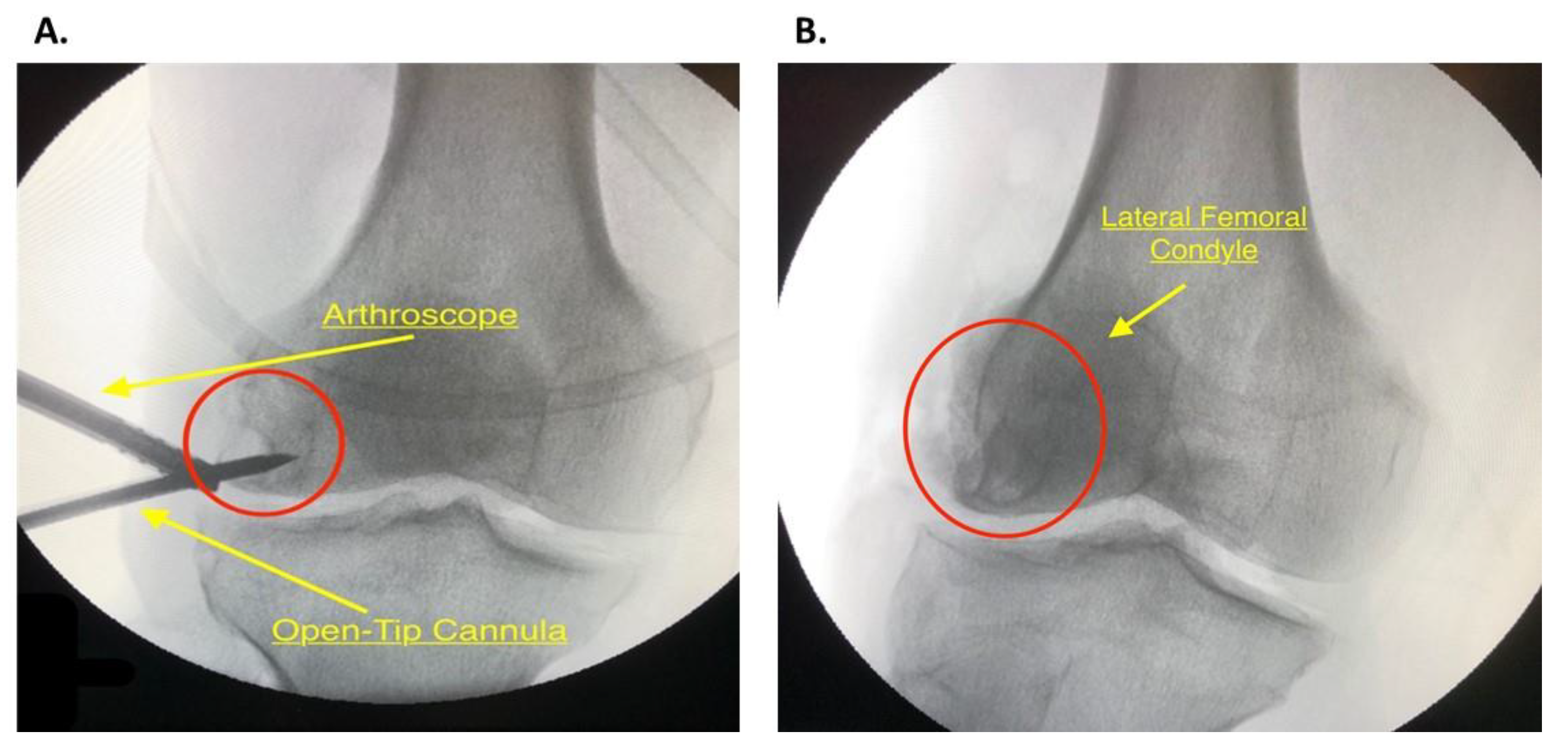
2.4. Diagnostic Arthroscopy
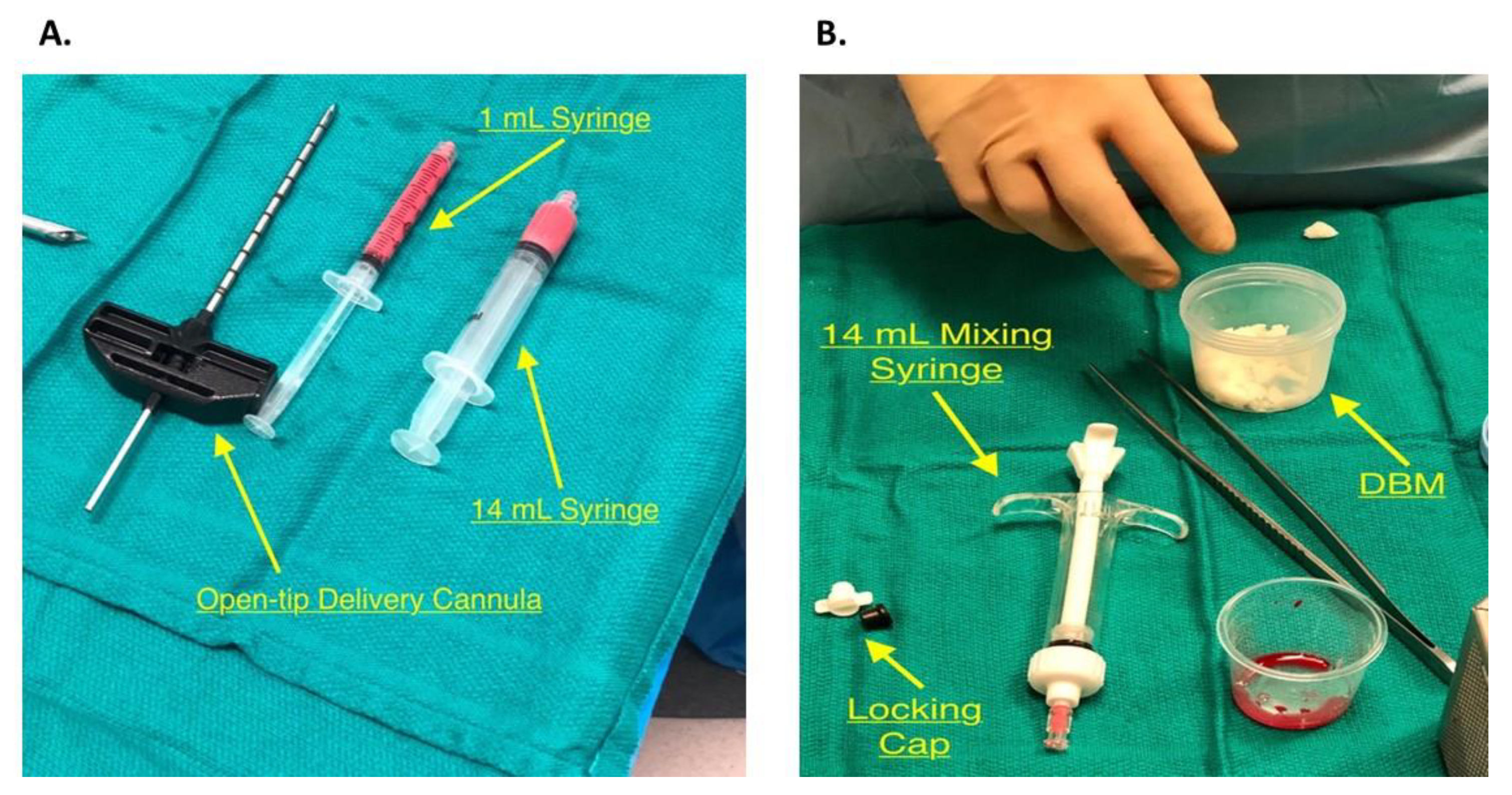
2.5. Bone Marrow Harvest and Preparation
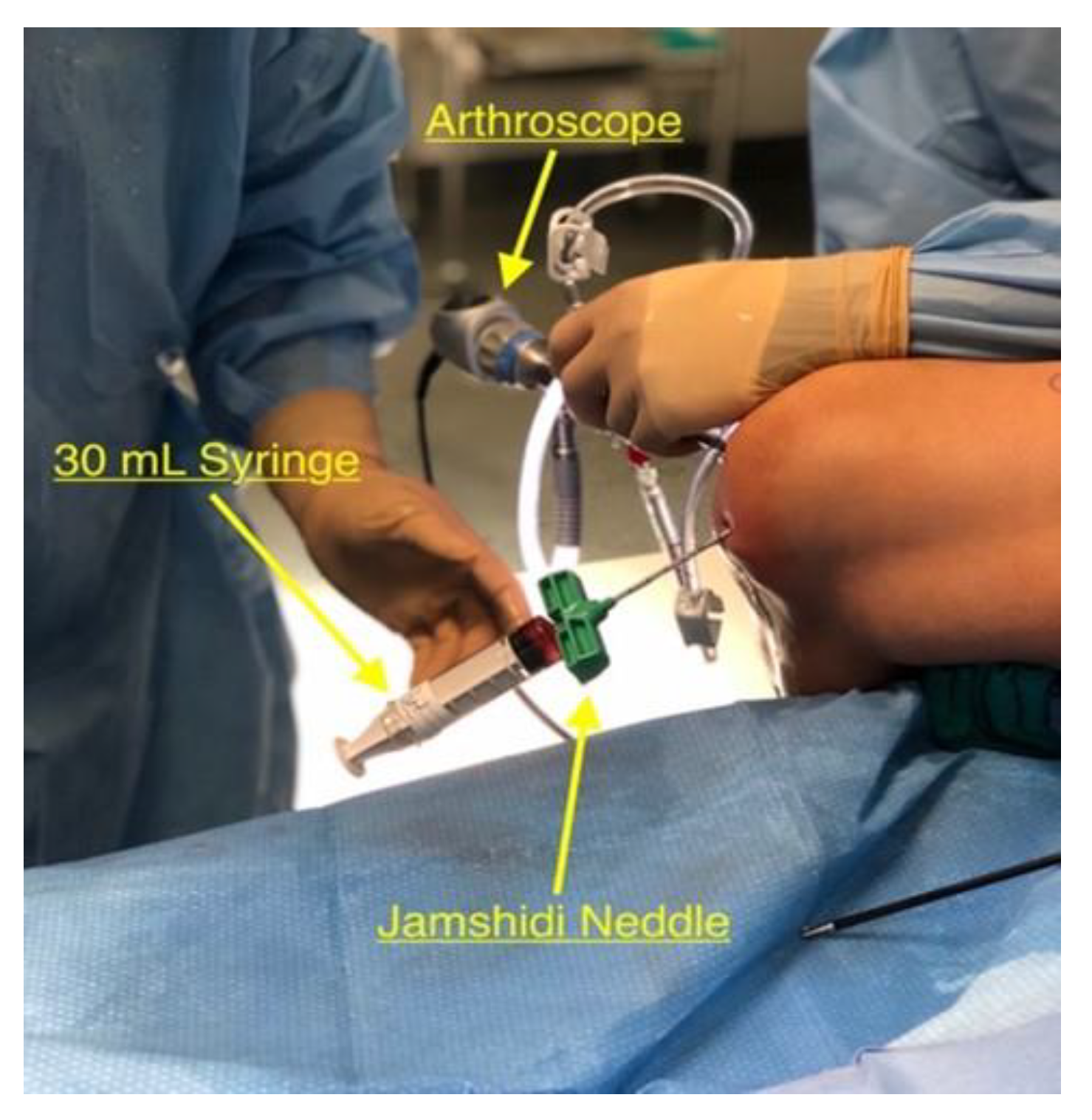
2.6. Core Decompression
2.7. PRP Inoculation and Bone Impaction
2.8. Postoperative Protocol
3. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Audrey, H.X.; Abd Razak, H.R.; Andrew, T.H. The truth behind subchondral cysts in osteoarthritis of the knee. Open Orthop. J. 2014, 8, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Magyar, E.; Talerman, A.; Fehér, M.; Wouters, H.W. The pathogenesis of the subchondral pseudocysts in rheumatoid arthritis. Clin. Orthop. Relat. Res. 1974, 100, 341–344. [Google Scholar] [CrossRef]
- Resnick, D.; Niwayama, G.; Coutts, R.D. Subchondral cysts (geodes) in arthritic disorders: Pathologic and radiographic appearance of the hip joint. AJR Am. J. Roentgenol. 1977, 128, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.; Niwayama, G.; Goergen, T.G.; Utsinger, P.D.; Shapiro, R.F.; Haselwood, D.H.; Wiesner, K.B. Clinical, radiographic and pathologic abnormalities in calcium pyrophosphate dihydrate deposition disease (CPPD): Pseudogout. Radiology 1977, 122, 1–15. [Google Scholar] [CrossRef]
- Crema, M.D.; Roemer, F.W.; Zhu, Y.; Marra, M.D.; Niu, J.; Zhang, Y.; Lynch, J.A.; Javaid, M.K.; Lewis, C.E.; El-Khoury, G.Y.; et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: Detection with MR imaging—The MOST study. Radiology 2010, 256, 855–862. [Google Scholar] [CrossRef]
- Noordin, S.; Allana, S.; Umer, M.; Jamil, M.; Hilal, K.; Uddin, N. Unicameral bone cysts: Current concepts. Ann. Med. Surg. (Lond.) 2018, 34, 43–49. [Google Scholar] [CrossRef]
- Perry, T.A.; Parkes, M.J.; Hodgson, R.J.; Felson, D.T.; Arden, N.K.; O’Neill, T.W. Association between Bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2020, 28, 316–323. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Guan, M.; Zhao, W.; Leung, F.K.; Pan, H.; Cao, X.; Guo, X.E.; Lu, W.W. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2174–2183. [Google Scholar] [CrossRef]
- Freund, E. The pathological significance of intra-articular pressure. Edinb. Med. J. 1940, 47, 192–203. [Google Scholar]
- Rhaney, K.; Lamb, D.W. The cysts of osteoarthritis of the hip; a radiological and pathological study. J. Bone Jt. Surg. Br. 1955, 37, 663–675. [Google Scholar] [CrossRef]
- Ondrouch, A.S. Cyst formation in osteoarthritis. J. Bone Jt. Surg. Br. 1963, 45, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Aaron, R.K.; Dyke, J.P.; Ciombor, D.M.; Ballon, D.; Lee, J.; Jung, E.; Tung, G.A. Perfusion abnormalities in subchondral bone associated with marrow edema, osteoarthritis, and avascular necrosis. Ann. N. Y. Acad. Sci. 2007, 1117, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Amoako, A.O.; Pujalte, G.G. Osteoarthritis in young, active, and athletic individuals. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2014, 7, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, B.J.; Smith, K.M.; Dragoo, J.L. Hitting the mark: Optimizing the use of calcium phosphate injections for the treatment of bone marrow lesions of the proximal tibia and distal femur. Arthrosc. Tech. 2018, 7, e1013–e1018. [Google Scholar] [CrossRef]
- Figgie, M.P.; Blevins, J.L.; Richardson, S.S.; Gausden, E.B.; Sculco, T.P.; Sculco, P.K. Younger patients undergoing total knee arthroplasty have higher complication rates. Orthop. Proc. 2018, 100 (Suppl. 12), 59. [Google Scholar]
- Donaldson, S.; Chundamala, J.; Yandow, S.; Wright, J.G. Treatment for unicameral bone cysts in long bones: An evidence-based review. Orthop. Rev. (Pavia) 2010, 2, e13. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Gangji, V.; De Maertelaer, V.; Hauzeur, J.P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011, 49, 1005–1009. [Google Scholar] [CrossRef]
- Hernigou, P.; Beaujean, F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin. Orthop. Relat. Res. 2002, 405, 14–23. [Google Scholar] [CrossRef]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. Am. 2005, 87, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Arbeloa-Gutierrez, L.; Dean, C.S.; Chahla, J.; Pascual-Garrido, C. Core decompression augmented with autologous bone marrow aspiration concentrate for early avascular necrosis of the femoral head. Arthrosc. Tech. 2016, 5, e615–e620. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Elena, N.; Woodall, B.M.; Lee, K.; McGahan, P.J.; Pathare, N.P.; Shin, E.C.; Chen, J.L. Intraosseous bioplasty for chondral cyst in the lateral tibial plateau. Arthrosc. Tech. 2018, 7, e1149–e1156. [Google Scholar] [CrossRef] [PubMed]
| Indications | Contraindications | Relative Contraindications |
|---|---|---|
|
|
|
| Advantages | Disadvantages |
|---|---|
|
|
| Pearls | Pitfalls |
|---|---|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potty, A.G.R.; Gupta, A.; Rodriguez, H.C.; Stone, I.W.; Maffulli, N. Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur. J. Clin. Med. 2020, 9, 1358. https://doi.org/10.3390/jcm9051358
Potty AGR, Gupta A, Rodriguez HC, Stone IW, Maffulli N. Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur. Journal of Clinical Medicine. 2020; 9(5):1358. https://doi.org/10.3390/jcm9051358
Chicago/Turabian StylePotty, Anish G.R., Ashim Gupta, Hugo C. Rodriguez, Ian W. Stone, and Nicola Maffulli. 2020. "Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur" Journal of Clinical Medicine 9, no. 5: 1358. https://doi.org/10.3390/jcm9051358
APA StylePotty, A. G. R., Gupta, A., Rodriguez, H. C., Stone, I. W., & Maffulli, N. (2020). Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur. Journal of Clinical Medicine, 9(5), 1358. https://doi.org/10.3390/jcm9051358







