Prenatal Exposure to Preeclampsia and Long-Term Ophthalmic Morbidity of the Offspring
Abstract
1. Introduction
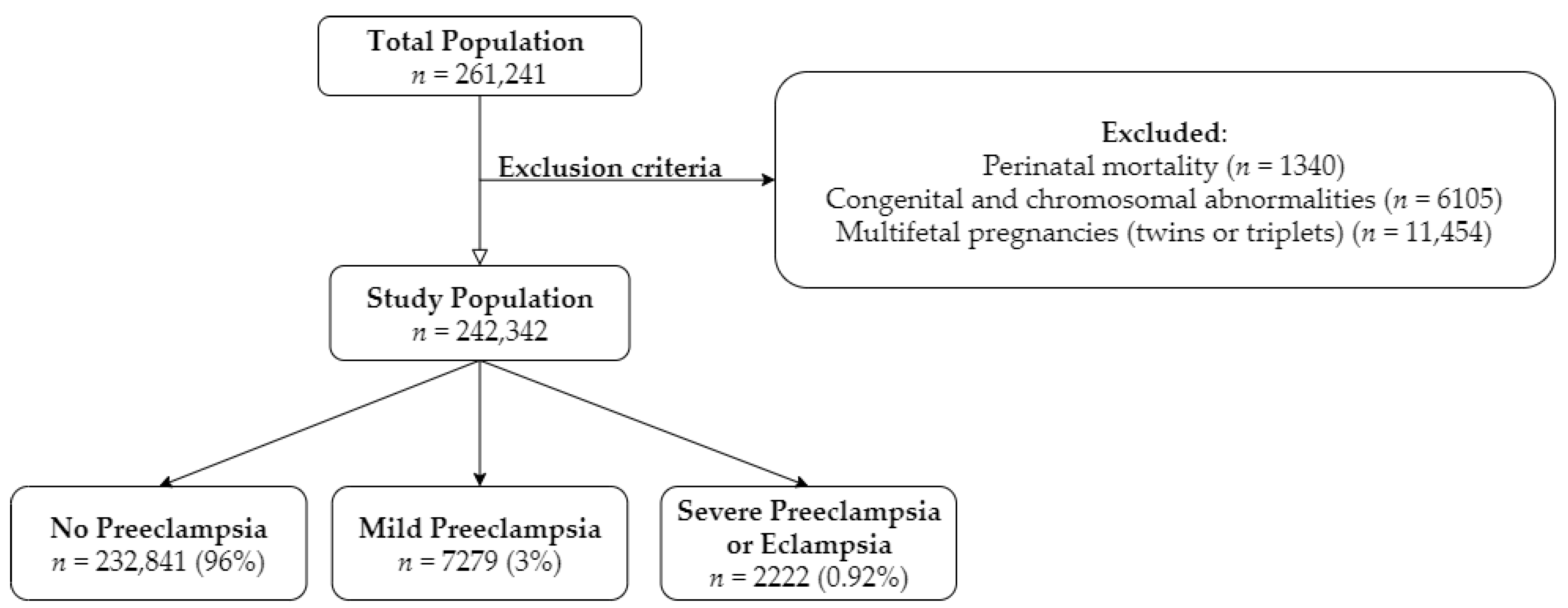
2. Experimental Section
Statistical Analysis
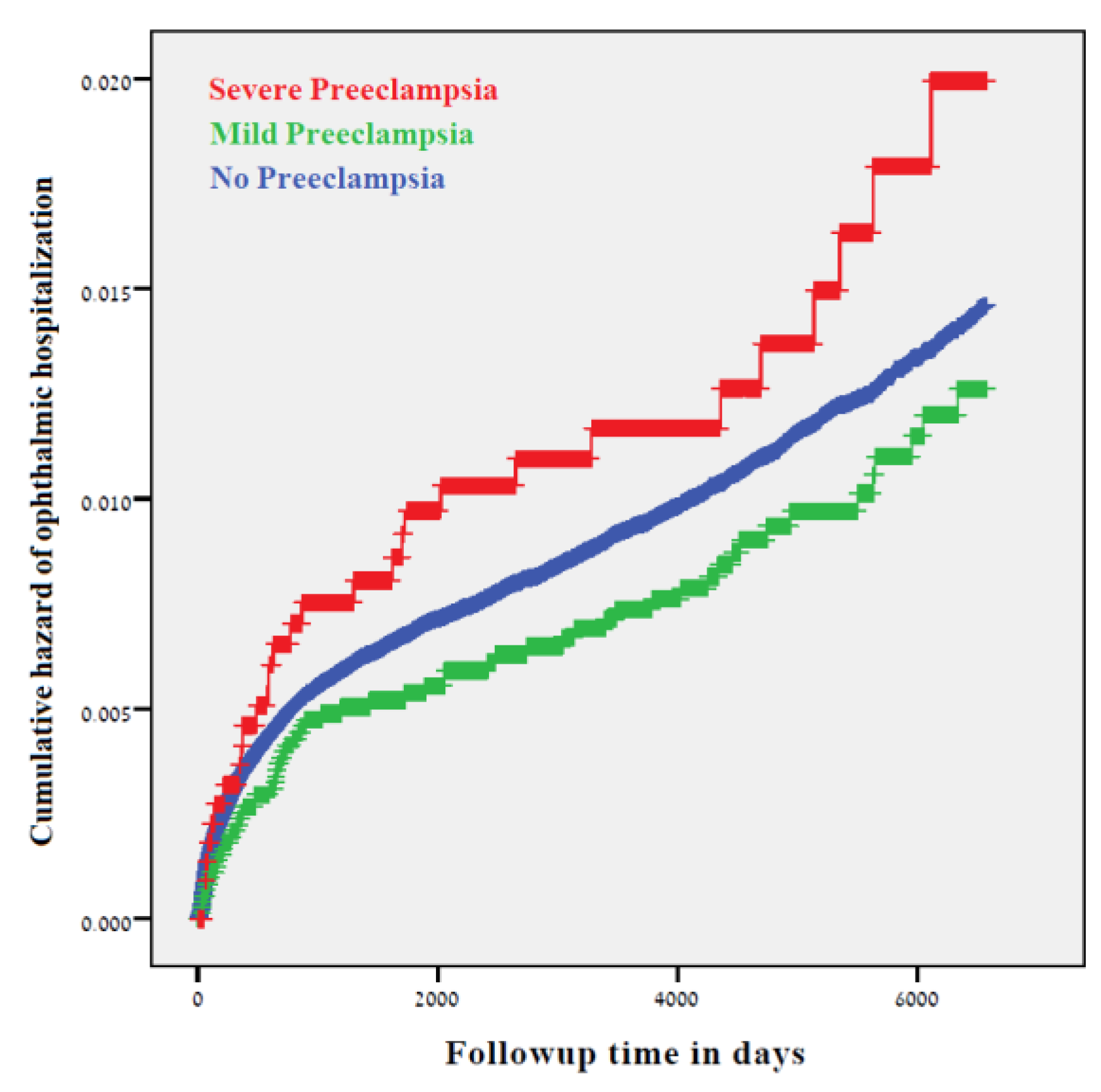
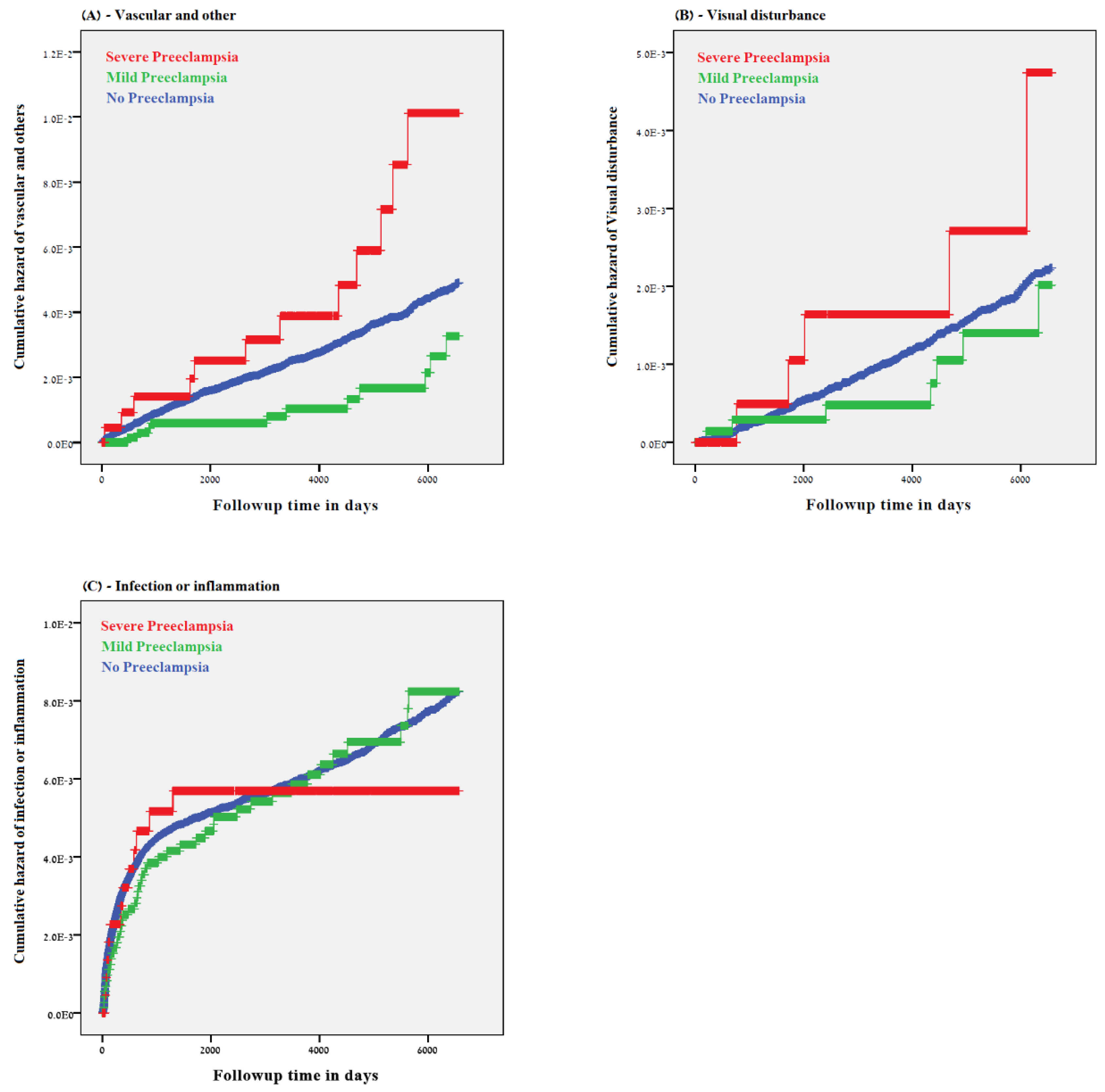
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Groups | Diagnostic Codes | Diagnosis Description |
|---|---|---|
| Infection and Inflammation | 370 | Keratitis |
| 3709 | Unspecified keratitis | |
| 3720 | Acute conjunctivitis | |
| 3723 | Other and unspecified conjunctivitis | |
| 3729 | Unspecified disorders of conjunctiva | |
| 3732 | Chalazion | |
| 3736 | Parasitic infestation of eyelid | |
| 3739 | Unspecified inflammation of eyelid | |
| 37021 | Punctate keratitis | |
| 37031 | Phlyctenular keratoconjunctivitis | |
| 37040 | Keratoconjunctivitis, unspecified | |
| 37049 | Other keratoconjunctivitis | |
| 37055 | Corneal abscess | |
| 37059 | Other interstitial and deep keratitis | |
| 37200 | Acute conjunctivitis, unspecified | |
| 37202 | Acute follicular conjunctivitis | |
| 37203 | Other mucopurulent conjunctivitis | |
| 37205 | Acute atopic conjunctivitis | |
| 37213 | Vernal conjunctivitis | |
| 37214 | Other chronic allergic conjunctivitis | |
| 37220 | Blepharoconjunctivitis, unspecified | |
| 37230 | Conjunctivitis, unspecified | |
| 37240 | Pterygium, unspecified | |
| 37261 | Granuloma of conjunctiva | |
| 37300 | Blepharitis, unspecified | |
| 37311 | Hordeolum externum | |
| 37312 | Hordeolum internum | |
| 37313 | Abscess of eyelid | |
| 37400 | Entropion, unspecified | |
| 37502 | Chronic dacryoadenitis | |
| 37530 | Dacryocystitis, unspecified | |
| 37532 | Acute dacryocystitis | |
| 37541 | Chronic canaliculitis | |
| 37542 | Chronic dacryocystitis | |
| 37543 | Lacrimal mucocele | |
| 37600 | Acute inflammation of orbit, unspecified | |
| 37601 | Orbital cellulitis | |
| 37610 | Chronic inflammation of orbit, unspecified | |
| 37611 | Orbital granuloma | |
| 37612 | Orbital myositis | |
| 37730 | Optic neuritis, unspecified | |
| 37739 | Other optic neuritis | |
| 376010 | Periorbital cellulitis | |
| Retinopathy of Prematurity | 36221 | Retrolental Fibroplasia |
| 362211 | Retinopathy of prematurity | |
| Visual Disturbances | 3688 | Other specified visual disturbances |
| 3689 | Unspecified visual disturbance | |
| 3699 | Unspecified visual loss | |
| 3780 | Esotropia | |
| 36900 | Blindness of both eyes, impairment level not further specified | |
| 36960 | Blindness, one eye, not otherwise specified | |
| 36970 | Low vision, one eye, not otherwise specified | |
| 37775 | Cortical blindness | |
| 37800 | Esotropia, unspecified | |
| 37801 | Monocular esotropia | |
| 37805 | Alternating esotropia | |
| 37810 | Exotropia, unspecified | |
| 37815 | Alternating exotropia | |
| 37817 | Alternating exotropia with v pattern | |
| 37820 | Intermittent heterotropia, unspecified | |
| 37821 | Intermittent esotropia, monocular | |
| 37830 | Heterotropia, unspecified | |
| 37831 | Hypertropia | |
| 37832 | Hypotropia | |
| Vascular and Others | 3769 | Unspecified disorder of orbit |
| 3770 | Papilledema | |
| 37000 | Corneal ulcer, unspecified | |
| 37004 | Hypopyon ulcer | |
| 37100 | Corneal opacity, unspecified | |
| 37120 | Corneal edema, unspecified | |
| 37142 | Recurrent erosion of cornea | |
| 37150 | Hereditary corneal dystrophy, unspecified | |
| 37160 | Keratoconus, unspecified | |
| 37162 | Keratoconus, acute hydrops | |
| 37170 | Corneal deformity, unspecified | |
| 37172 | Descemetocele | |
| 37263 | Symblepharon | |
| 37272 | Conjunctival hemorrhage | |
| 37273 | Conjunctival edema | |
| 37274 | Vascular abnormalities of conjunctiva | |
| 37275 | Conjunctival cysts | |
| 37289 | Other disorders of conjunctiva | |
| 37430 | Ptosis of eyelid, unspecified | |
| 37451 | Xanthelasma of eyelid | |
| 37482 | Edema of eyelid | |
| 37484 | Cysts of eyelids | |
| 37489 | Other disorders of eyelid | |
| 37515 | Tear film insufficiency, unspecified | |
| 37520 | Epiphora, unspecified as to cause | |
| 37521 | Epiphora due to excess lacrimation | |
| 37552 | Stenosis of lacrimal punctum | |
| 37553 | Stenosis of lacrimal canaliculi | |
| 37555 | Obstruction of nasolacrimal duct, neonatal | |
| 37556 | Stenosis of nasolacrimal duct, acquired | |
| 37561 | Lacrimal fistula | |
| 37630 | Exophthalmos, unspecified | |
| 37633 | Orbital edema or congestion | |
| 37646 | Enlargement of orbit | |
| 37651 | Enophthalmos due to atrophy of orbital tissue | |
| 37681 | Orbital cysts | |
| 37689 | Other orbital disorders | |
| 37700 | Papilledema, unspecified | |
| 37703 | Papilledema associated with retinal disorder | |
| 37710 | Optic atrophy, unspecified | |
| 37721 | Drusen of optic disc | |
| 37724 | Pseudopapilledema | |
| 37749 | Other disorders of optic nerve | |
| 37852 | Third or oculomotor nerve palsy, total | |
| 37854 | Sixth or abducens nerve palsy |
References
- Steiner, N.; Weintraub, A.Y.; Madi, Y.; Barski, L.; Sheiner, E. The unfavorable slope from mild preeclampsia through severe preeclampsia, to eclampsia. Pregnancy Hypertens. 2013, 3, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Okby, R.; Harlev, A.; Sacks, K.N.; Sergienko, R.; Sheiner, E. Preeclampsia acts differently in in vitro fertilization versus spontaneous twins. Arch. Gynecol. Obs. 2018, 297, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Clifton, V.L.; Stark, M.J.; Osei-Kumah, A.; Hodyl, N.A. Review: The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta 2012, 33, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K.; Kalluri, R. The Biology of Preeclampsia. Kidney Int. 2009, 76, 831–837. [Google Scholar] [CrossRef]
- Kalafat, E.; Thilaganathan, B. Cardiovascular origins of preeclampsia. Curr. Opin. Obs. Gynecol. 2017, 29, 383–389. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sharma, R.; Thilaganathan, B. Cardiovascular implications in preeclampsia: An overview. Circulation 2014, 130, 703–714. [Google Scholar] [CrossRef]
- Tal, R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol. Reprod. 2012, 87, 134. [Google Scholar] [CrossRef]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Harats, D.; Feldman, B.; Avivi, C. Effects of Hypoxia-Inducible Factor-1α Overexpression in Pregnant Mice. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef]
- Al-Jameil, N.; Aziz Khan, F.; Fareed Khan, M.; Tabassum, H. A Brief Overview of Preeclampsia. J. Clin. Med. Res. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Amaral, L.M.; Cunningham, M.W.; Cornelius, D.C.; LaMarca, B. Preeclampsia: Long-term consequences for vascular health. Vasc. Health Risk Manag. 2015, 11, 403–415. [Google Scholar]
- Abu Samra, K. The eye and visual system in the preeclampsia/eclampsia syndrome: What to expect? Saudi J. Ophthalmol. 2013, 27, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Nahum Sacks, K.; Friger, M.; Shoham-Vardi, I.; Sergienko, R.; Spiegel, E.; Landau, D.; Sheiner, E. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Hum. Dev. 2019, 130, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Roos, N.M.; Wiegman, M.J.; Jansonius, N.M.; Zeeman, G.G. Visual disturbances in (pre)eclampsia. Obstet. Gynecol. Surv. 2012, 67, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Lampinen, K.H.; Rönnback, M.; Kaaja, R.J.; Groop, P.-H. Impaired vascular dilatation in women with a history of pre-eclampsia. J. Hypertens. 2006, 24, 751–756. [Google Scholar] [CrossRef]
- Shalom, G.; Shoham-Vardi, I.; Sergienko, R.; Wiznitzer, A.; Sherf, M.; Sheiner, E. Is preeclampsia a significant risk factor for long-term hospitalizations and morbidity? J. Matern. Fetal Neonatal Med. 2013, 26, 13–15. [Google Scholar] [CrossRef]
- Kessous, R.; Shoham-Vardi, I.; Pariente, G.; Sergienko, R.; Sheiner, E. Long-term maternal atherosclerotic morbidity in women with pre-eclampsia. Heart 2015, 101, 442–446. [Google Scholar] [CrossRef]
- Beharier, O.; Davidson, E.; Sergienko, R.; Szaingurten-Solodkin, I.; Kessous, R.; Charach, R.; Sheiner, E.; Belfair, N.J. Preeclampsia and Future Risk for Maternal Ophthalmic Complications. Am. J. Perinatol. 2016, 33, 703–707. [Google Scholar]
- Sheiner, E.; Kapur, A.; Retnakaran, R.; Hadar, E.; Poon, L.C.; McIntyre, H.D.; Hod, M.; Divakar, H.; Staff, A.C.; Narula, J.; et al. FIGO (International Federation of Gynecology and Obstetrics) Postpregnancy Initiative: Long-term Maternal Implications of Pregnancy Complications—Follow-up Considerations. Int. J. Gynecol. Obstet. 2019, 147, 1–31. [Google Scholar] [CrossRef]
- Shulman, J.P.; Weng, C.; Wilkes, J.; Greene, T.; Hartnett, M.E. Association of Maternal Preeclampsia with Infant Risk of Premature Birth and Retinopathy of Prematurity. JAMA Ophthalmol. 2017, 135, 947–953. [Google Scholar] [CrossRef]
- Wu, C.S.; Nohr, E.A.; Bech, B.H.; Vestergaard, M.; Catov, J.M.; Olsen, J. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am. J. Obstet. Gynecol. 2009, 201, 269. [Google Scholar] [CrossRef]
- Backes, C.H.; Markham, K.; Moorehead, P.; Cordero, L.; Nankervis, C.A.; Giannone, P.J. Maternal Preeclampsia and Neonatal Outcomes. J. Pregnancy 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Nahum Sacks, K.; Friger, M.; Shoham-Vardi, I.; Spiegel, E.; Sergienko, R.; Landau, D.; Sheiner, E. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. 2018, 13, 181–186. [Google Scholar] [CrossRef]
- Hakim, J.; Senterman, M.K.; Hakim, A.M. Preeclampsia is a biomarker for vascular disease in both mother and child: The need for a medical alert system. Int. J. Pediatr. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Eriksson, J.G.; Osmond, C.; Thornburg, K.; Barker, D.J.P. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The Helsinki birth cohort study. Stroke 2009, 40, 1176–1180. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, 1–22. [Google Scholar] [CrossRef]
- Wainstock, T.; Walfisch, A.; Shoham-Vardi, I.; Segal, I.; Harlev, A.; Sergienko, R.; Sheiner, E.; Landau, D. Fertility treatments and pediatric neoplasms of the offspring: Results of a population-based cohort with a median follow-up of 10 years. Am. J. Obstet. Gynecol. 2017, 216, 314. [Google Scholar] [CrossRef]
- Gutbir, Y.; Wainstock, T.; Sheiner, E.; Segal, I.; Sergienko, R.; Landau, D.; Walfisch, A. Low Apgar score in term newborns and long-term infectious morbidity: A population-based cohort study with up to 18 years of follow-up. Eur. J. Pediatr. 2020. [Google Scholar] [CrossRef]
- Salem Yaniv, S.; Levy, A.; Wiznitzer, A.; Holcberg, G.; Mazor, M.; Sheiner, E. A significant linear association exists between advanced maternal age and adverse perinatal outcome. Arch. Gynecol. Obstet. 2011, 283, 755–759. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Sheiner, E.; Sherf, M.; Wiznitzer, A.; Sergienko, R.; Shoham-Vardi, I. Lack of prenatal care in a traditional community: Trends and perinatal outcomes. Arch. Gynecol. Obstet. 2012, 285, 1237–1242. [Google Scholar] [CrossRef]
- Sheiner, E.; Shoham-Vardi, I.; Weitzman, D.; Gohar, J.; Carmi, R. Decisions regarding pregnancy termination among Bedouin couples referred to third level ultrasound clinic. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 76, 141–146. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L. Epigenetic mechanisms in developmental programming of adult disease. Drug Discov. Today 2011, 16, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
| Maternal and Newborn Characteristic | No Preeclampsia n = 232,841 | Mild Preeclampsia n = 7279 | Severe Preeclampsia n = 2222 | p-Value 1 |
|---|---|---|---|---|
| Maternal age (years, mean ± SD) | 28.13 ± 5.79 | 28.66 ± 6.31 | 29.00 ± 6.94 | <0.001 |
| Ethnicity (%) | ||||
| Jewish | 109,751 (47.1%) | 4136 (56.8%) | 978 (44.0%) | <0.001 |
| Bedouins | 123,090 (52.9%) | 3143 (43.2%) | 1244 (56.0%) | |
| Parity (%) | ||||
| 1 | 53,348 (22.9%) | 2854 (39.2%) | 964 (43.4%) | <0.001 |
| 2–4 | 120,382 (51.7%) | 2903 (39.9%) | 657 (29.6%) | |
| 5+ | 59,060 (25.4%) | 1502 (20.9%) | 601 (27%) | |
| Gestational age (weeks, mean ± SD) | 39.17 ± 1.82 | 38.71 ± 1.8 | 36.66 ± 3.01 | <0.001 |
| Preterm (<37) | 14,150 (6.1%) | 701 (9.6%) | 965 (43.4%) | <0.001 |
| Preterm (<34) | 2212 (1.0%) | 55 (0.8%) | 328 (14.8%) | <0.001 |
| Low birth weight (<2500 g) | 13,668 (5.9%) | 770 (10.6%) | 1026 (46.2%) | <0.001 |
| Apgar scores <7 at 1 min (%) | 11,035 (4.7%) | 349 (4.8%) | 352 (15.8%) | <0.001 |
| Ophthalmic Morbidity | No Preeclampsia n = 232,841 | Mild Preeclampsia n = 7279 | Severe Preeclampsia n = 2222 | p-Value 1 |
|---|---|---|---|---|
| Vascular and others | 645 (0.3%) | 11 (0.2%) | 12 (0.5%) | 0.008 |
| Visual disturbance | 271 (0.1%) | 7 (0.1%) | 5 (0.2%) | 0.286 |
| Infection\inflammation | 1411 (0.6%) | 45 (0.6%) | 12 (0.5%) | 0.915 |
| Retinopathy of prematurity (ROP) | 3 (<0.01%) | 0 (-) | 1 (<0.01%) | <0.001 |
| Total ophthalmic hospitalizations | 2240 (1.0%) | 61 (0.8%) | 29 (1.3%) | 0.141 |
| Variables | Adjusted HR | 95% CI | p-Value 1 | |
|---|---|---|---|---|
| Low Limit | Up Limit | |||
| I-Total Ophthalmic Morbidity * | ||||
| No preeclampsia | 1 (ref) | |||
| Mild preeclampsia | 0.848 | 0.658–1.094 | 0.205 | |
| Severe preeclampsia or eclampsia | 1.358 | 0.941–1.959 | 0.102 | |
| Maternal age (years) | 0.995 | 0.988–1.003 | 0.206 | |
| Ethnicity | 0.811 | 0.746–0.881 | <0.001 | |
| II-Vascular and Other Ophthalmic Morbidity ** | ||||
| No preeclampsia | 1 (ref) | |||
| Mild preeclampsia | 0.535 | 0.295–0.970 | 0.040 | |
| Severe preeclampsia or eclampsia | 1.861 | 1.051–3.295 | 0.033 | |
| Maternal age (years) | 0.992 | 0.979–1.005 | 0.230 | |
| Ethnicity | 0.628 | 0.535–0.737 | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedar Sade, E.; Wainstock, T.; Tsumi, E.; Sheiner, E. Prenatal Exposure to Preeclampsia and Long-Term Ophthalmic Morbidity of the Offspring. J. Clin. Med. 2020, 9, 1271. https://doi.org/10.3390/jcm9051271
Kedar Sade E, Wainstock T, Tsumi E, Sheiner E. Prenatal Exposure to Preeclampsia and Long-Term Ophthalmic Morbidity of the Offspring. Journal of Clinical Medicine. 2020; 9(5):1271. https://doi.org/10.3390/jcm9051271
Chicago/Turabian StyleKedar Sade, Eliel, Tamar Wainstock, Erez Tsumi, and Eyal Sheiner. 2020. "Prenatal Exposure to Preeclampsia and Long-Term Ophthalmic Morbidity of the Offspring" Journal of Clinical Medicine 9, no. 5: 1271. https://doi.org/10.3390/jcm9051271
APA StyleKedar Sade, E., Wainstock, T., Tsumi, E., & Sheiner, E. (2020). Prenatal Exposure to Preeclampsia and Long-Term Ophthalmic Morbidity of the Offspring. Journal of Clinical Medicine, 9(5), 1271. https://doi.org/10.3390/jcm9051271






