Brain Circuit Alterations and Cognitive Disability in Late-Onset Cobalamin D Disorder
Abstract
1. Introduction
2. Experimental Section
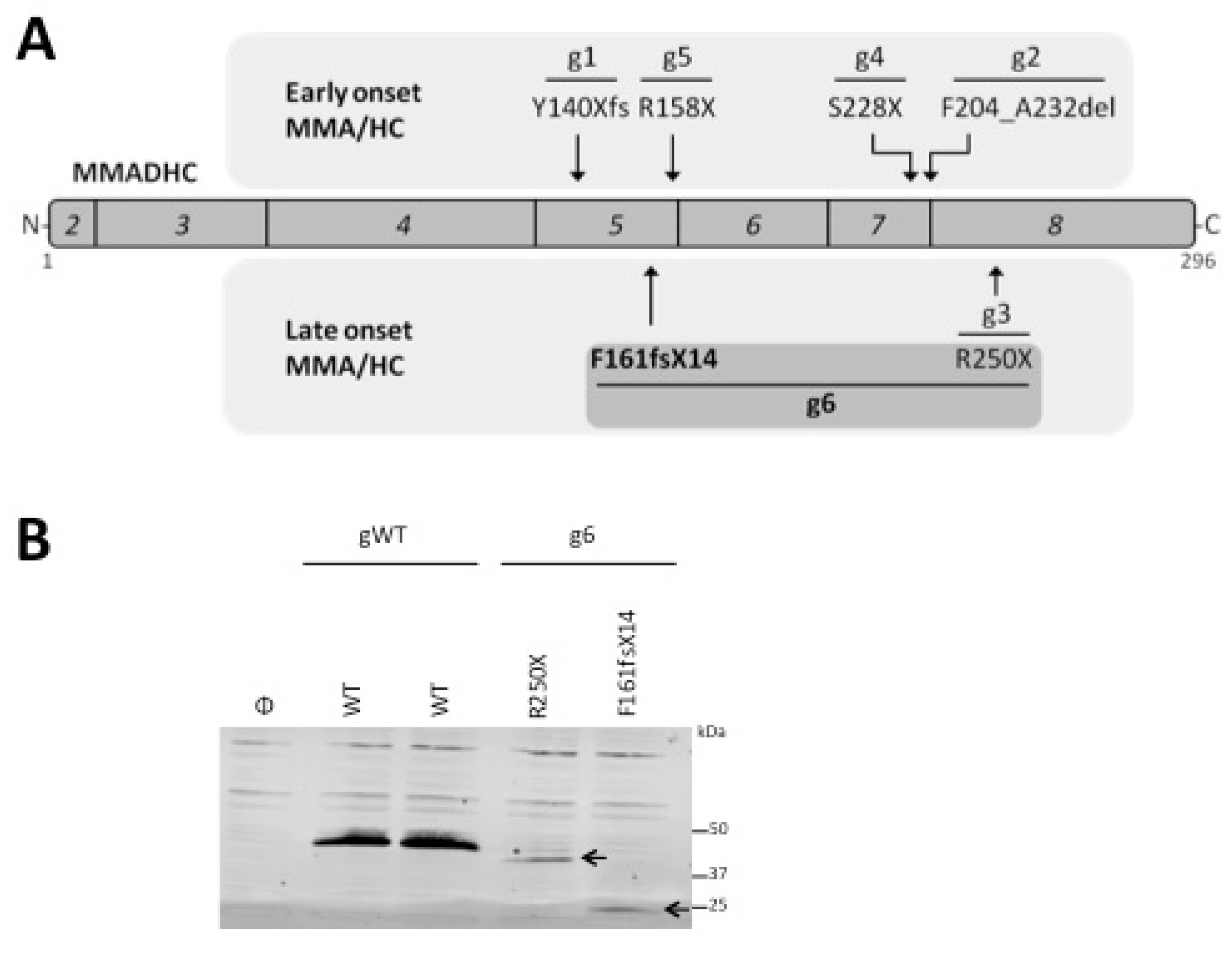
2.1. In Vitro Analysis of the MMADHC Variants
2.2. Magnetic Resonance Imaging
2.2.1. Imaging Acquisition
2.2.2. Imaging Analyses
2.3. Neuropsychological Evaluation
2.4. Ethical Considerations
3. Results
3.1. Clinical Characterization
3.2. Genotype Characterization
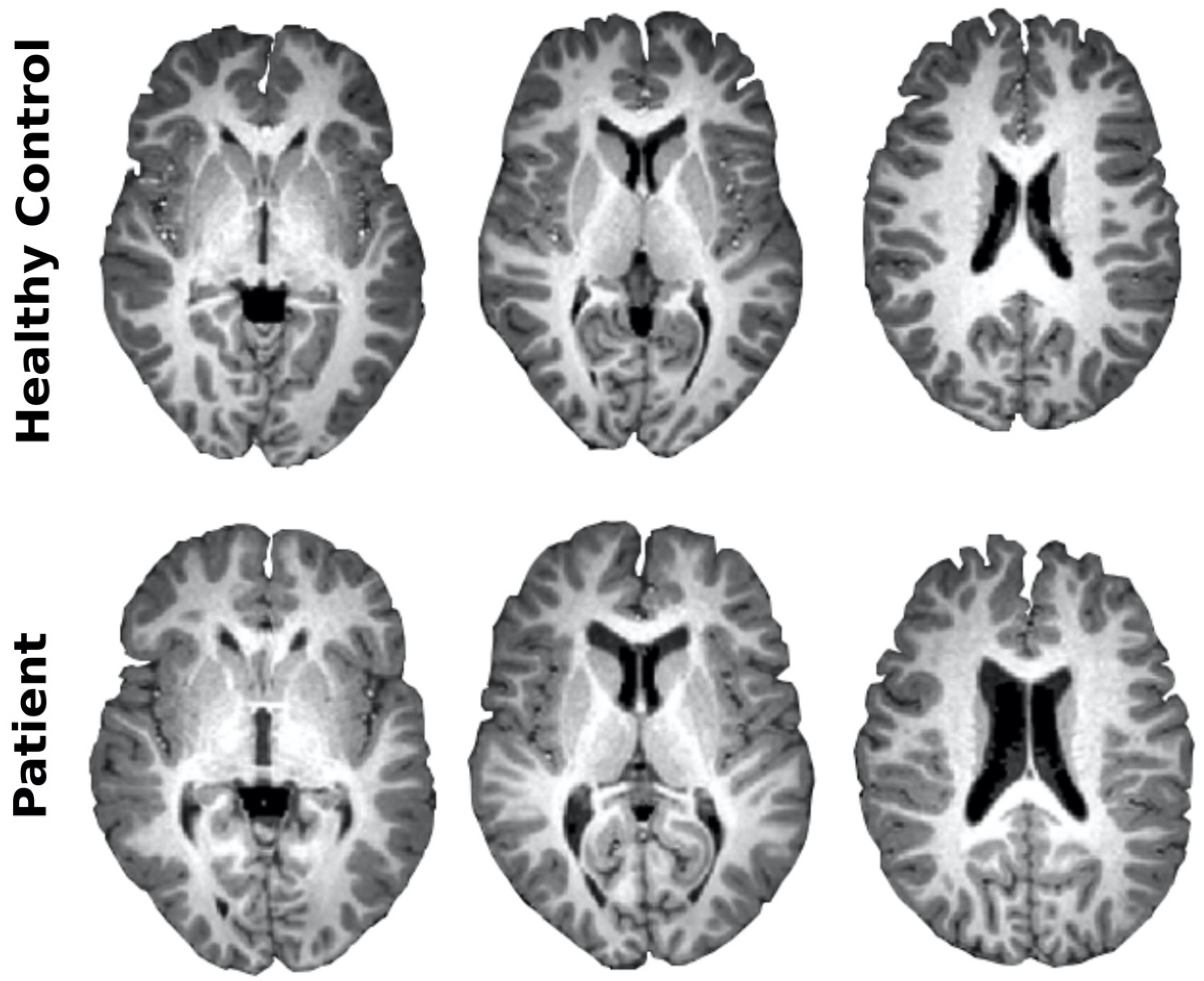
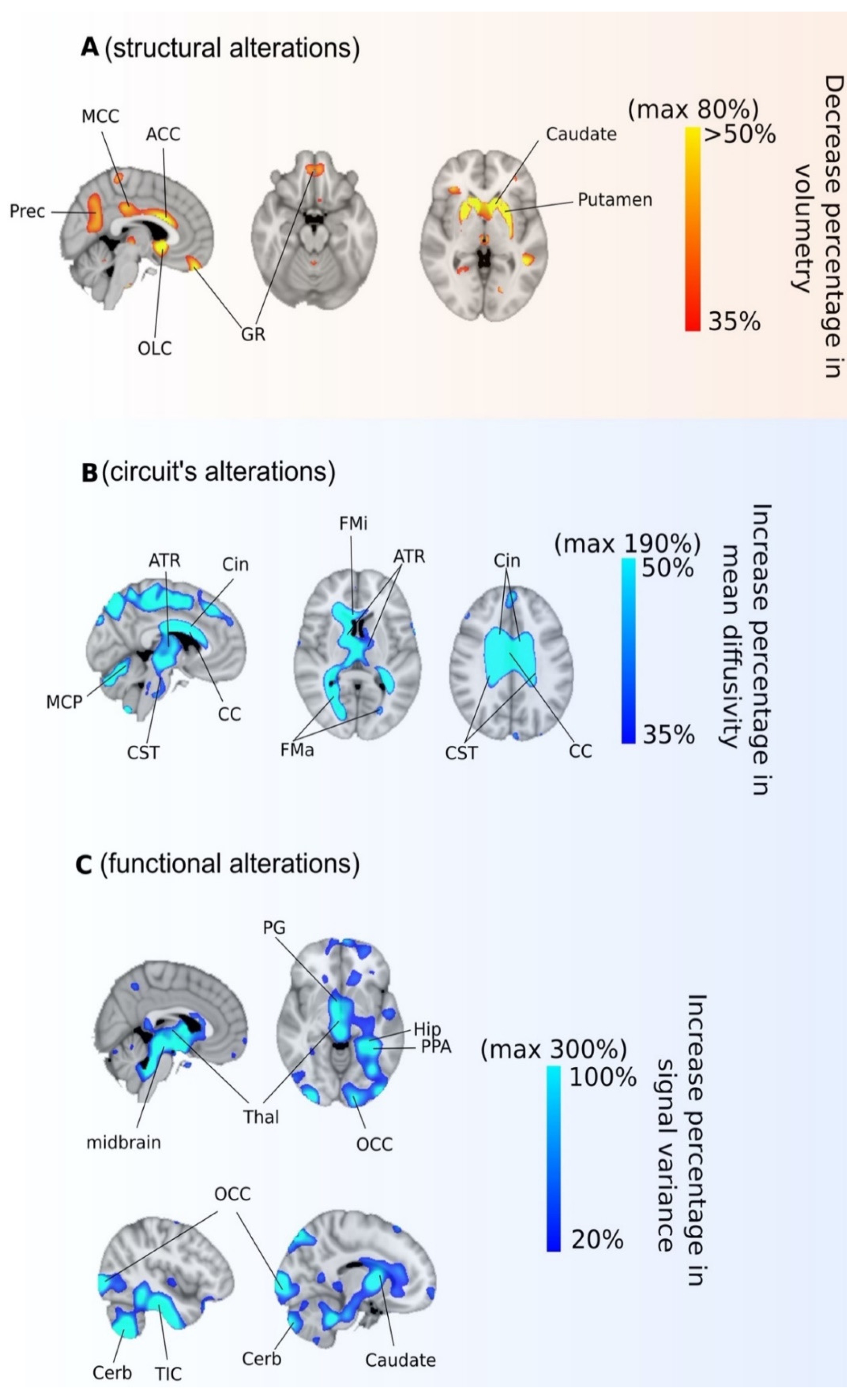
3.3. Imaging Characterization
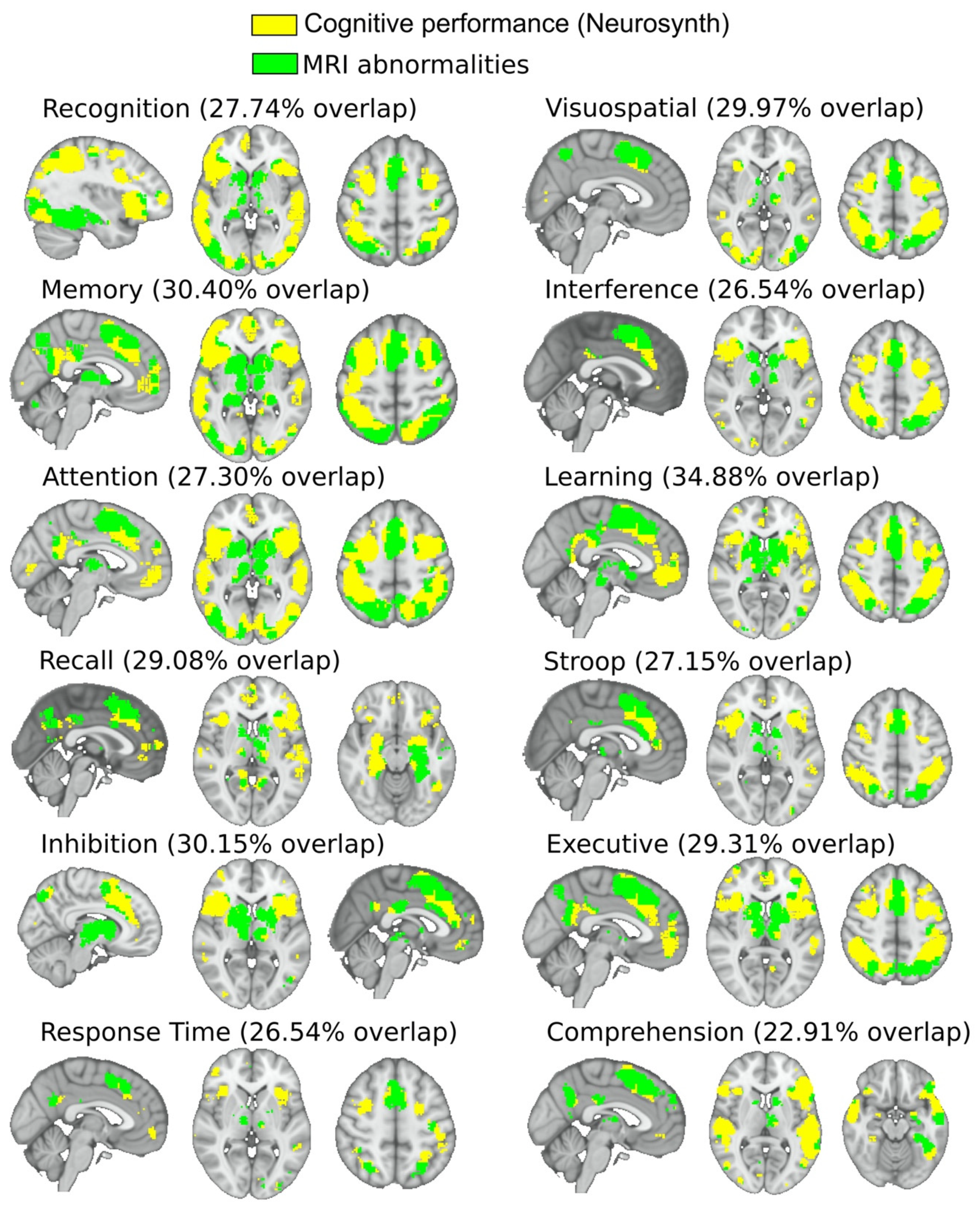
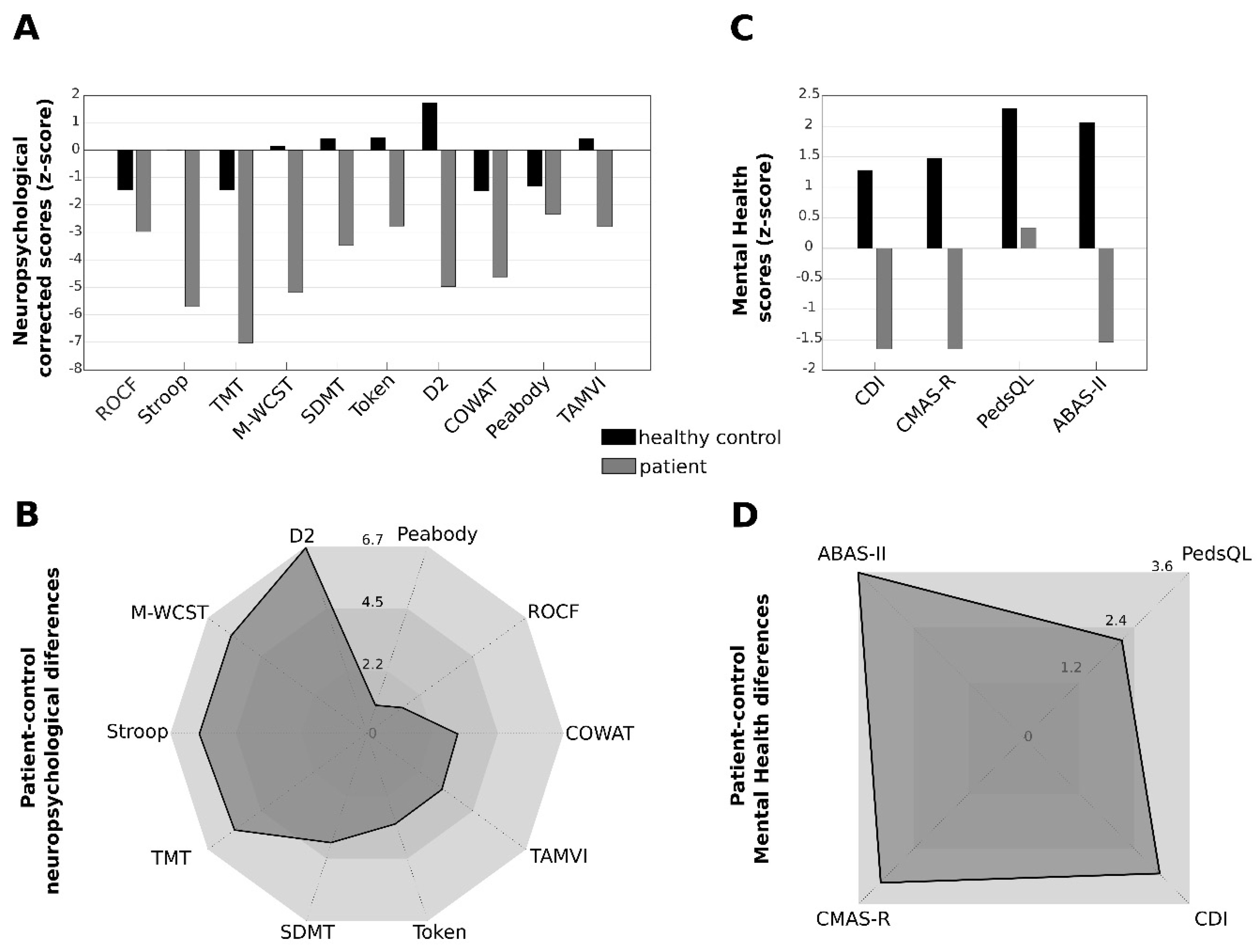
3.4. Neuropsychological Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huemer, M.; Baumgartner, M.R. The clinical presentation of cobalamin-related disorders: From acquired deficiencies to inborn errors of absorption and intracellular pathways. J. Inherit. Metab. Dis. 2019, 42, 686–705. [Google Scholar] [CrossRef]
- Huemer, M.; Diodato, D.; Schwahn, B.; Schiff, M.; Bandeira, A.; Benoist, J.-F.; Burlina, A.; Cerone, R.; Couce, M.L.; Garcia-Cazorla, A.; et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J. Inherit. Metab. Dis. 2017, 40, 21–48. [Google Scholar] [CrossRef]
- Stabler, S.P. Vitamin B 12 Deficiency. N. Engl. J. Med. 2013, 368, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.; Suormala, T.; Stucki, M.; Lerner-Ellis, J.P.; Rosenblatt, D.S.; Newbold, R.F.; Baumgartner, M.R.; Fowler, B. Gene Identification for the cblD Defect of Vitamin B 12 Metabolism. N. Engl. J. Med. 2008, 358, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.; Kim, J.C.; Miousse, I.R.; Fung, S.; du Moulin, M.; Buers, I.; Suormala, T.; Burda, P.; Frapolli, M.; Stucki, M.; et al. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat. Genet. 2012, 44, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Rosenblatt, D.S. Inborn errors of cobalamin absorption and metabolism. Am. J. Med. Genet. 2011, 157, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Suormala, T.; Baumgartner, M.R.; Coelho, D.; Zavadakova, P.; Kožich, V.; Koch, H.G.; Berghaüser, M.; Wraith, J.E.; Burlina, A.; Sewell, A.; et al. The cblD Defect Causes Either Isolated or Combined Deficiency of Methylcobalamin and Adenosylcobalamin Synthesis. J. Biol. Chem. 2004, 279, 42742–42749. [Google Scholar] [CrossRef]
- Miousse, I.R.; Watkins, D.; Coelho, D.; Rupar, T.; Crombez, E.A.; Vilain, E.; Bernstein, J.A.; Cowan, T.; Lee-Messer, C.; Enns, G.M.; et al. Clinical and Molecular Heterogeneity in Patients with the CblD Inborn Error of Cobalamin Metabolism. J. Pediatr. 2009, 154, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Scholl-Bürgi, S.; Hadaya, K.; Kern, I.; Beer, R.; Seppi, K.; Fowler, B.; Baumgartner, M.R.; Karall, D. Three new cases of late-onset cblC defect and review of the literature illustrating when to consider inborn errors of metabolism beyond infancy. Orphanet. J. Rare. Dis. 2014, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Polvikoski, T.; Kivipelto, M.; Tanskanen, M.; Myllykangas, L.; Erkinjuntti, T.; Mäkelä, M.; Oinas, M.; Paetau, A.; Scheltens, P.; et al. Plasma homocysteine, Alzheimer and cerebrovascular pathology: A population-based autopsy study. Brain 2013, 136, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Carmel, R.; Melnyk, S.; James, S.J. Cobalamin deficiency with and without neurologic abnormalities: Differences in homocysteine and methionine metabolism. Blood 2003, 101, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Chien Tu, M.; Ping Lo, C.; Yuan Chen, C. Vitamin B12 deficiency: Cognitive impairment and neuroimaging correlates. Neuropsychiatry 2016, 6. [Google Scholar] [CrossRef]
- Pulido, R. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998, 17, 7337–7350. [Google Scholar] [CrossRef]
- Mingo, J.; Erramuzpe, A.; Luna, S.; Aurtenetxe, O.; Amo, L.; Diez, I.; Schepens, J.T.G.; Hendriks, W.J.A.J.; Cortés, J.M.; Pulido, R. One-Tube-Only Standardized Site-Directed Mutagenesis: An Alternative Approach to Generate Amino Acid Substitution Collections. PLoS ONE 2016, 11, e0160972. [Google Scholar] [CrossRef]
- Mingo, J.; Luna, S.; Gaafar, A.; Nunes-Xavier, C.E.; Torices, L.; Mosteiro, L.; Ruiz, R.; Guerra, I.; Llarena, R.; Angulo, J.C.; et al. Precise definition of PTEN C-terminal epitopes and its implications in clinical oncology. NPJ Precis Onc. 2019, 3, 11. [Google Scholar] [CrossRef]
- Douaud, G.; Smith, S.; Jenkinson, M.; Behrens, T.; Johansen-Berg, H.; Vickers, J.; James, S.; Voets, N.; Watkins, K.; Matthews, P.M.; et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007, 130, 2375–2386. [Google Scholar] [CrossRef]
- Rasero, J.; Alonso-Montes, C.; Diez, I.; Olabarrieta-Landa, L.; Remaki, L.; Escudero, I.; Mateos, B.; Bonifazi, P.; Fernandez, M.; Arango-Lasprilla, J.C.; et al. Group-Level Progressive Alterations in Brain Connectivity Patterns Revealed by Diffusion-Tensor Brain Networks across Severity Stages in Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef]
- Diez, I.; Bonifazi, P.; Escudero, I.; Mateos, B.; Muñoz, M.A.; Stramaglia, S.; Cortes, J.M. A novel brain partition highlights the modular skeleton shared by structure and function. Sci. Rep. 2015, 5, 10532. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, P.; Erramuzpe, A.; Diez, I.; Gabilondo, I.; Boisgontier, M.P.; Pauwels, L.; Stramaglia, S.; Swinnen, S.P.; Cortes, J.M. Structure–function multi-scale connectomics reveals a major role of the fronto-striato-thalamic circuit in brain aging. Hum. Brain Mapp. 2018, 39, 4663–4677. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Panesar, S.; Fernandes, D.; Meola, A.; Yoshino, M.; Fernandez-Miranda, J.C.; Vettel, J.M.; Verstynen, T. Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage 2018, 178, 57–68. [Google Scholar] [CrossRef]
- Oishi, K. MRI Atlas of Human White Matter, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-12-382081-5. [Google Scholar]
- Yan, C.-G.; Cheung, B.; Kelly, C.; Colcombe, S.; Craddock, R.C.; Di Martino, A.; Li, Q.; Zuo, X.-N.; Castellanos, F.X.; Milham, M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 2013, 76, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. L’examen psychologique dans les cas d’enc´ephalopathie traumatique. Arch. Psychol 1941, 28, 286–340. [Google Scholar]
- Golden, C.J. STROOP: Test de Colores y Palabras: Manual; TEA Ediciones S.A.: Madrid, Spain, 1994. [Google Scholar]
- Brickenkamp, R. Aufmerksamkeits-Belastungs-Test Handanweisung d2; C. J. Hogrefe: Toronto, ON, Canada, 1962. [Google Scholar]
- Grant, D.A.; Berg, E.A. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 1948, 38, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.E. A modified card sorting test sensitive to frontal lobe defects. Cortex 1976, 12, 313–324. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digits Modalities Test; Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- De Renzi, E.; Faglioni, P. Normative Data and Screening Power of a Shortened Version of the Token Test. Cortex 1978, 14, 41–49. [Google Scholar] [CrossRef]
- Benton, A.; Hamsher, K.S. Multilingual Aphasia Examination; University of Iowa: Iowa City, IA, USA, 1989. [Google Scholar]
- Dunn, L.M.; Dunn, L.M. Examiner’s Manual for the Peabody Picture Vocabulary Test Third Edition; American Guidance Service: Circle Pines, MN, USA, 1997. [Google Scholar]
- Rivera, D.; Olabarrieta-Landa, L.; Arango-Lasprilla, J.C. Diseño y creación del Test de Aprendizaje y Memoria Verbal Infantil (TAMVI-I) en población hispano hablante de 6 a 17 años de edad. In Neuropsicología Infantil; Arango-Lasprilla, J.C., Rivera, D., Olabarrieta-Landa, L., Eds.; Manual Moderno: Bogotá, Colombia, 2017; pp. 317–338. ISBN 958-8993-15-6. [Google Scholar]
- Kovacs, M. Rating scales to assess depression in school-aged children. Acta. Paedopsychiatr. 1981, 46, 305–315. [Google Scholar]
- Reynolds, C.R.; Richmond, B.O. Escala de Ansiedad Manifiesta en Niños CMAS-R: Lo que Pienso y Siento. Cuestionarios; Manual Moderno: México City, Mexico, 1997. [Google Scholar]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef]
- Oakland, T.; Harrison, P. Adaptive Behavior Assessment System (ABAS-II); The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Arango-Lasprilla, J.C.; Rivera, D.; Nicholls, E.; Aguayo Arelis, A.; García de la Cadena, C.; Peñalver Guia, A.I.; Vergara-Moragues, E.; Rodriguez-Lorenzana, A.; Marín-Morales, A.; Soto-Añari, M.; et al. Modified Wisconsin Card Sorting Test (M-WCST): Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 617–626. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Rivera, D.; Ertl, M.M.; Muñoz Mancilla, J.M.; García-Guerrero, C.E.; Rodriguez-Irizarry, W.; Aguayo Arelis, A.; Rodríguez-Agudelo, Y.; Barrios Nevado, M.D.; Vélez-Coto, M.; et al. Rey–Osterrieth Complex Figure—Copy and immediate recall (3 minutes): Normative data for Spanish-speaking pediatric populations. NeuroRehabilitation 2017, 41, 593–603. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Rivera, D.; Trapp, S.; Jiménez-Pérez, C.; Hernández Carrillo, C.L.; Pohlenz Amador, S.; Vergara-Moragues, E.; Rodríguez-Agudelo, Y.; Rodriguez-Irizarry, W.; García de la Cadena, C.; et al. Symbol Digit Modalities Test: Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Rivera, D.; Ramos-Usuga, D.; Vergara-Moragues, E.; Montero-López, E.; Adana Díaz, L.A.; Aguayo Arelis, A.; García-Guerrero, C.E.; García de la Cadena, C.; Llerena Espezúa, X.; et al. Trail Making Test: Normative data for the Latin American Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Olabarrieta-Landa, L.; Rivera, D.; Ibáñez-Alfonso, J.A.; Albaladejo-Blázquez, N.; Martín-Lobo, P.; Delgado-Mejía, I.D.; Lara, L.; Rabago Barajas, B.V.; Rodriguez Salgado, A.M.; Paredes Quispe, L.A.; et al. Peabody Picture Vocabulary Test-III: Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Olabarrieta-Landa, L.; Rivera, D.; Rodríguez-Lorenzana, A.; Pohlenz Amador, S.; García-Guerrero, C.E.; Padilla-López, A.; Sánchez-SanSegundo, M.; Velázquez-Cardoso, J.; Díaz Marante, J.P.; Caparros-Gonzalez, R.A.; et al. Shortened Version of the Token Test: Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 649–659. [Google Scholar] [CrossRef]
- Olabarrieta-Landa, L.; Rivera, D.; Lara, L.; Rute-Pérez, S.; Rodríguez-Lorenzana, A.; Galarza-del-Angel, J.; Peñalver Guia, A.I.; Ferrer-Cascales, R.; Velázquez-Cardoso, J.; Campos Varillas, A.I.; et al. Verbal fluency tests: Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 673–686. [Google Scholar] [CrossRef]
- Rivera, D.; Salinas, C.; Ramos-Usuga, D.; Delgado-Mejía, I.D.; Vasallo Key, Y.; Hernández Agurcia, G.P.; Valencia Vásquez, J.; García-Guerrero, C.E.; García de la Cadena, C.; Rabago Barajas, B.V.; et al. Concentration Endurance Test (d2): Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 661–671. [Google Scholar] [CrossRef]
- Rivera, D.; Olabarrieta-Landa, L.; Rabago Barajas, B.V.; Irías Escher, M.J.; Saracostti Schwartzman, M.; Ferrer-Cascales, R.; Martín-Lobo, P.; Calderón, J.A.; Aguilar Uriarte, M.A.; Peñalver Guia, A.I.; et al. Newly developed Learning and Verbal Memory Test (TAMV-I): Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 695–706. [Google Scholar] [CrossRef]
- Rivera, D.; Morlett-Paredes, A.; Peñalver Guia, A.I.; Irías Escher, M.J.; Soto-Añari, M.; Aguayo Arelis, A.; Rute-Pérez, S.; Rodríguez-Lorenzana, A.; Rodríguez-Agudelo, Y.; Albaladejo-Blázquez, N.; et al. Stroop Color-Word Interference Test: Normative data for Spanish-speaking pediatric population. NeuroRehabilitation 2017, 41, 605–616. [Google Scholar] [CrossRef]
- De las Heras, J.; Aldámiz-Echevarría, L.; Cabrera, A. Frontoparietal Subdural Hematoma in a Child with Mental Regression. JAMA Neurol. 2018, 75, 759. [Google Scholar] [CrossRef]
- Abu-El-Haija, A.; Mendelsohn, B.A.; Duncan, J.L.; Moore, A.T.; Glenn, O.A.; Weisiger, K.; Gallagher, R.C. Cobalamin D Deficiency Identified Through Newborn Screening. In JIMD Reports, Volume 44; Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 44, pp. 73–77. ISBN 978-3-662-58616-7. [Google Scholar]
- Soylu Ustkoyuncu, P.; Kendirci, M.; Kardas, F.; Gokay, S.; Per, H.; Kacar Bayram, A. Neutropenia and Increased Mean Corpuscular Volume (MCV) With Abnormal Neurologic Findings: A Case of Cobalamin D Deficiency. J. Pediatr. Hematol. Oncol. 2019, 41, e54–e56. [Google Scholar] [CrossRef]
- Stucki, M.; Coelho, D.; Suormala, T.; Burda, P.; Fowler, B.; Baumgartner, M.R. Molecular mechanisms leading to three different phenotypes in the cblD defect of intracellular cobalamin metabolism. Hum. Mol. Genet. 2012, 21, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Cancho García, E.; Geán, E.; Oliver Tormo, B.; Torrents, A.; Esteban Durán, E. Mutation of the MMADHC gene in adult-onset cobalamin D deficiency: A report of 2 potentially treatable cases. Neurología (Engl. Ed.) 2019, 34, 419–421. [Google Scholar] [CrossRef]
- Yeh, H.-R.; Park, H.-K.; Kim, H.-J.; Ko, T.-S.; Won, H.-S.; Lee, M.-Y.; Shim, J.-Y.; Yum, M.-S. Neurodevelopmental outcomes in children with prenatally diagnosed corpus callosal abnormalities. Brain Dev. 2018, 40, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Malavolti, A.M.; Chau, V.; Brown-Lum, M.; Poskitt, K.J.; Brant, R.; Synnes, A.; Grunau, R.E.; Miller, S.P. Association between corpus callosum development on magnetic resonance imaging and diffusion tensor imaging, and neurodevelopmental outcome in neonates born very preterm. Dev. Med. Child. Neurol. 2017, 59, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Crossley, N.A.; Mechelli, A.; Scott, J.; Carletti, F.; Fox, P.T.; McGuire, P.; Bullmore, E.T. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014, 137, 2382–2395. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. (Regul. Ed.) 2013, 17, 683–696. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gupta, R.K.; Garg, R.K.; Rai, Y.; Roy, B.; Pandey, C.M.; Malhotra, H.S.; Narayana, P.A. DTI Correlates of Cognition in Conventional MRI of Normal-Appearing Brain in Patients with Clinical Features of Subacute Combined Degeneration and Biochemically Proven Vitamin B12 Deficiency. AJNR Am. 2014, 35, 872–877. [Google Scholar] [CrossRef]
- Gupta, P.K.; Garg, R.K.; Gupta, R.K.; Malhotra, H.S.; Paliwal, V.K.; Rathore, R.K.S.; Verma, R.; Singh, M.K.; Rai, Y.; Pandey, C.M. Diffusion tensor tractography and neuropsychological assessment in patients with vitamin B12 deficiency. Neuroradiology 2014, 56, 97–106. [Google Scholar] [CrossRef]
- de Rezende Pinto, W.B.V.; de Souza, P.V.S.; Rogério, R.M.; Pedroso, J.L.; Barsottini, O.G.P. Vitamin B12 deficiency mimicking neuroimaging features of motor neuron disease. Arq. Neuropsiquiatr. 2014, 72, 85. [Google Scholar] [CrossRef]
- Roy, B.; Trivedi, R.; Garg, R.K.; Gupta, P.K.; Tyagi, R.; Gupta, R.K. Assessment of functional and structural damage in brain parenchyma in patients with vitamin B12 deficiency: A longitudinal perfusion and diffusion tensor imaging study. Magn. Reson. Imaging 2015, 33, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Gupta, R.K.; Gupta, P.K.; Malhotra, H.S.; Saha, I.; Garg, R.K. Assessment of brain cognitive functions in patients with vitamin B12 deficiency using resting state functional MRI: A longitudinal study. Magn. Reson. Imaging 2016, 34, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Miousse, I.R.; Watkins, D.; Rosenblatt, D.S.; Raiman, J.A.J. Clinical, Biochemical, and Molecular Presentation in a Patient with the cblD-Homocystinuria Inborn Error of Cobalamin Metabolism. In JIMD Reports; Zschocke, J., Gibson, K.M., Brown, G., Morava, E., Peters, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 17, pp. 77–81. ISBN 978-3-662-44577-8. [Google Scholar]
- Goodman, S.I.; Moe, P.G.; Hammond, K.B.; Mudd, S.H.; Uhlendorf, B.W. Homocystinuria with methylmalonic aciduria: Two cases in a sibship. Biochem. Med. 1970, 4, 500–515. [Google Scholar] [CrossRef]
- Morris, M.S. The role of B vitamins in preventing and treating cognitive impairment and decline. Adv. Nutr. 2012, 3, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Doets, E.L.; van Wijngaarden, J.P.; Szczecińska, A.; Dullemeijer, C.; Souverein, O.W.; Dhonukshe-Rutten, R.A.M.; Cavelaars, A.E.J.M.; van’t Veer, P.; Brzozowska, A.; de Groot, L.C.P.G.M. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol. Rev. 2013, 35, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kåreholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Mühlmann, M.; Ertl-Wagner, B.; et al. Association of Vitamin B12, Folate, and Sulfur Amino Acids With Brain Magnetic Resonance Imaging Measures in Older Adults: A Longitudinal Population-Based Study. JAMA Psychiatr. 2016, 73, 606–613. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Refsum, H.; Johnston, C.; Smith, S.M.; Bradley, K.M.; de Jager, C.; Budge, M.M.; Smith, A.D. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008, 71, 826–832. [Google Scholar] [CrossRef]
- Kloppenborg, R.P.; Geerlings, M.I.; Visseren, F.L.; Mali, W.P.T.M.; Vermeulen, M.; van der Graaf, Y.; Nederkoorn, P.J.; SMART Study Group. Homocysteine and progression of generalized small-vessel disease: The SMART-MR Study. Neurology 2014, 82, 777–783. [Google Scholar] [CrossRef]
- Fjell, A.M.; Sneve, M.H.; Grydeland, H.; Storsve, A.B.; Walhovd, K.B. The Disconnected Brain and Executive Function Decline in Aging. Cereb. Cortex 2017, 27, 2303–2317. [Google Scholar] [CrossRef]
- Goh, J.O.; Beason-Held, L.L.; An, Y.; Kraut, M.A.; Resnick, S.M. Frontal function and executive processing in older adults: Process and region specific age-related longitudinal functional changes. Neuroimage 2013, 69, 43–50. [Google Scholar] [CrossRef]
| Variables | Basal | Study Visit |
|---|---|---|
| Age (years) | 11.4 | 11.8 |
| Plasma homocysteine (µmol/L) (normal range: 5–15) | 144 | 30.6 |
| Serum methylmalonic acid (µmol/L) (normal range: 0.08 to 0.58) | 67.8 | 5.3 |
| Plasma methionine (µmol/L) (normal range: 7 to 47) | 18 | 36 |
| Plasma propionylcarnitine (µmol/L) (normal range: 0.05 to 0.5) | 6.1 | 1.7 |
| Serum cobalamin (pg/mL) (normal range: 220–980) | 312 | >2000 |
| Patient Phenotype | Patient Genotype 1 | Onset | Alelle 1 | Protein Change Alelle 1 3 | Gene Location | Alelle 2 | Protein Change Alelle 2 | Gene Location | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MMA/HC | g1 (1) | Early | c.419dupA | Y140Xfs | Exon 5 | Y140Xfs | Y140Xfs | Exon 5 | [4] |
| MMA/HC | g5 (1) | Early | c.472C > T | R158X | Exon 5 | c.472C > T | R158X | Exon 5 | [50] |
| MMA/HC | g4 (1) | Early | c.683C > G | S228X | Exon 7 | c.683C > G | S228X | Exon 7 | [8] |
| MMA/HC | g2 (1) | Early | c.696 + 1_4delGTGA | F204_A232del (skip exon 7) | Intron 7 | c.696 + 1_4delGTGA | F204_A232del (skip exon 7) | Intron 7 | [4] |
| MMA/HC | g3 (5) | Late | c.748C > T | R250X | Exon 8 | c.748C > T | R250X | Exon 8 | [4,51,52,53] |
| MMA/HC | g6 (1) 2 | Late | c.438_442delAGAGT | F161fsX14 | Exon 5 | c.748C > T | R250X | Exon 8 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Las Heras, J.; Diez, I.; Jimenez-Marin, A.; Cabrera, A.; Ramos-Usuga, D.; Diaz-Fernandez, M.V.; Torices, L.; Nunes-Xavier, C.E.; Pulido, R.; Arango-Lasprilla, J.C.; et al. Brain Circuit Alterations and Cognitive Disability in Late-Onset Cobalamin D Disorder. J. Clin. Med. 2020, 9, 990. https://doi.org/10.3390/jcm9040990
De Las Heras J, Diez I, Jimenez-Marin A, Cabrera A, Ramos-Usuga D, Diaz-Fernandez MV, Torices L, Nunes-Xavier CE, Pulido R, Arango-Lasprilla JC, et al. Brain Circuit Alterations and Cognitive Disability in Late-Onset Cobalamin D Disorder. Journal of Clinical Medicine. 2020; 9(4):990. https://doi.org/10.3390/jcm9040990
Chicago/Turabian StyleDe Las Heras, Javier, Ibai Diez, Antonio Jimenez-Marin, Alberto Cabrera, Daniela Ramos-Usuga, Marta Venecia Diaz-Fernandez, Leire Torices, Caroline E. Nunes-Xavier, Rafael Pulido, Juan Carlos Arango-Lasprilla, and et al. 2020. "Brain Circuit Alterations and Cognitive Disability in Late-Onset Cobalamin D Disorder" Journal of Clinical Medicine 9, no. 4: 990. https://doi.org/10.3390/jcm9040990
APA StyleDe Las Heras, J., Diez, I., Jimenez-Marin, A., Cabrera, A., Ramos-Usuga, D., Diaz-Fernandez, M. V., Torices, L., Nunes-Xavier, C. E., Pulido, R., Arango-Lasprilla, J. C., & Cortes, J. M. (2020). Brain Circuit Alterations and Cognitive Disability in Late-Onset Cobalamin D Disorder. Journal of Clinical Medicine, 9(4), 990. https://doi.org/10.3390/jcm9040990







