A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People?
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Collection
2.2. Laboratory Measurements
2.3. Redox Assays
2.3.1. Antioxidant Barrier
2.3.2. Oxidative Stress Products
2.3.3. Statistical Analysis
3. Results
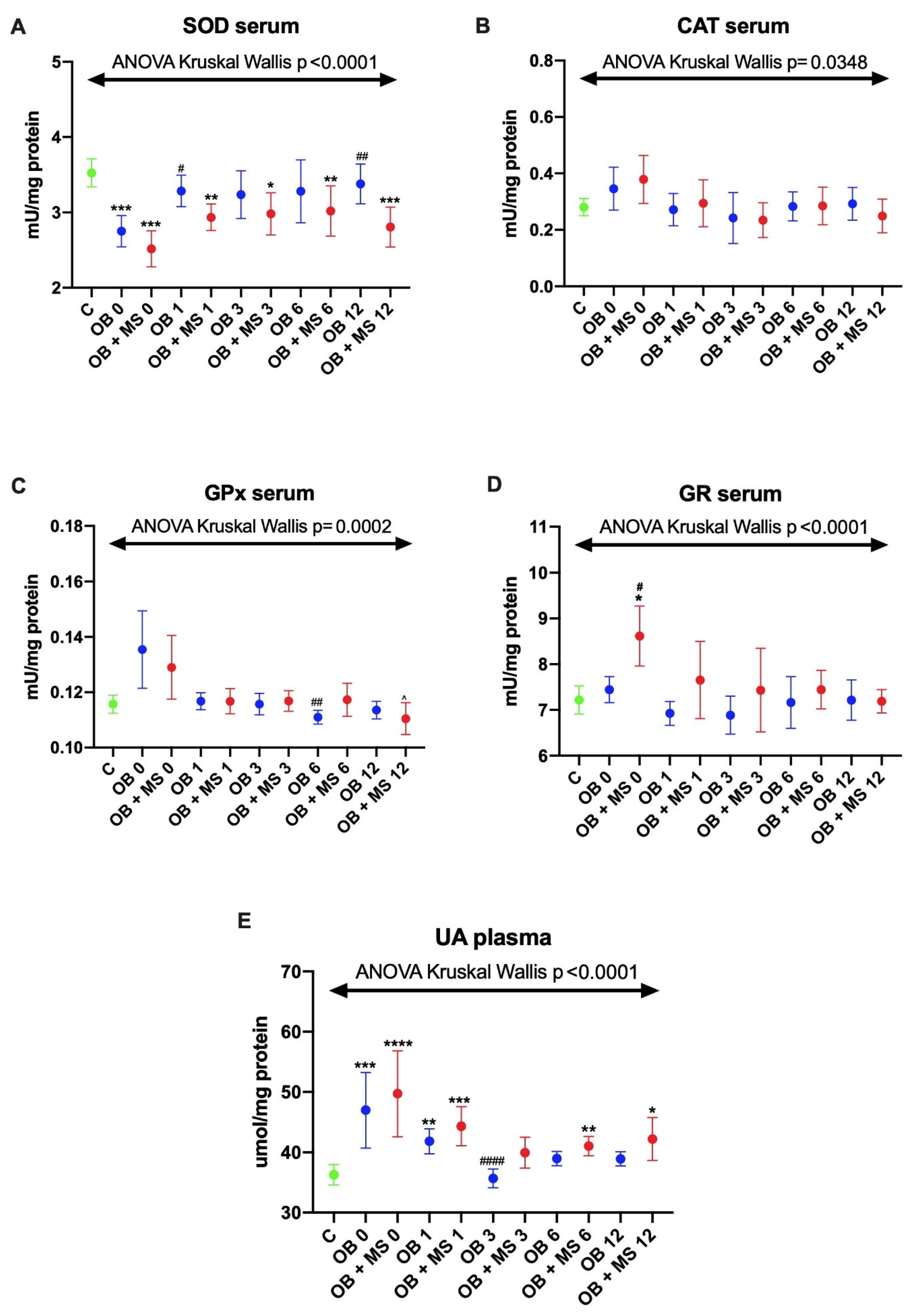
3.1. Antioxidant Barrier
3.1.1. Superoxide Dismutase (SOD)
3.1.2. Catalase (CAT) and Glutathione Peroxidase (GPx)
3.1.3. Glutathione Reductase (GR)
3.1.4. Uric Acid (UA)
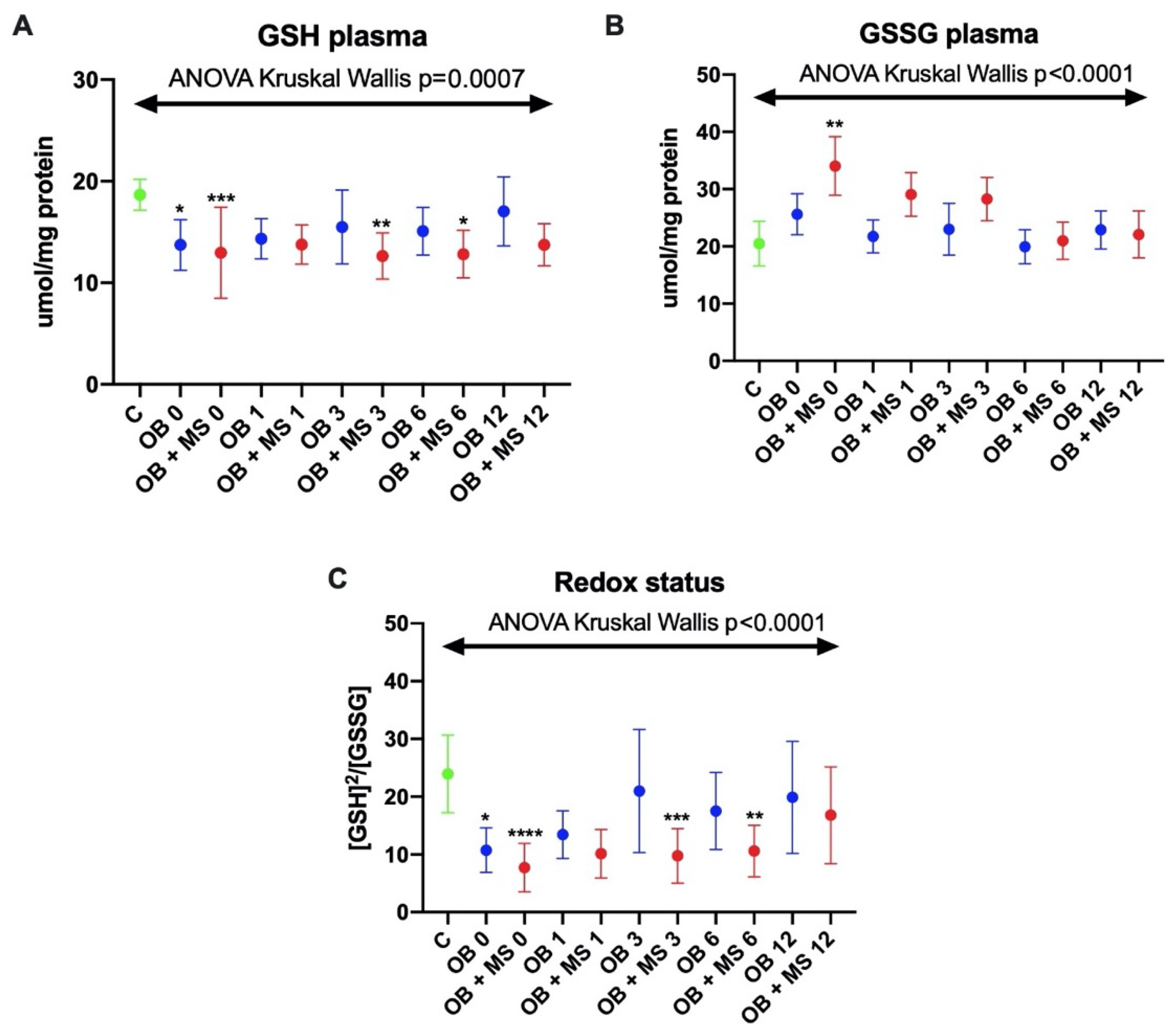
3.1.5. Reduced Glutathione (GSH)
3.1.6. Glutathione Disulfide (GSSG)
3.1.7. Redox Status
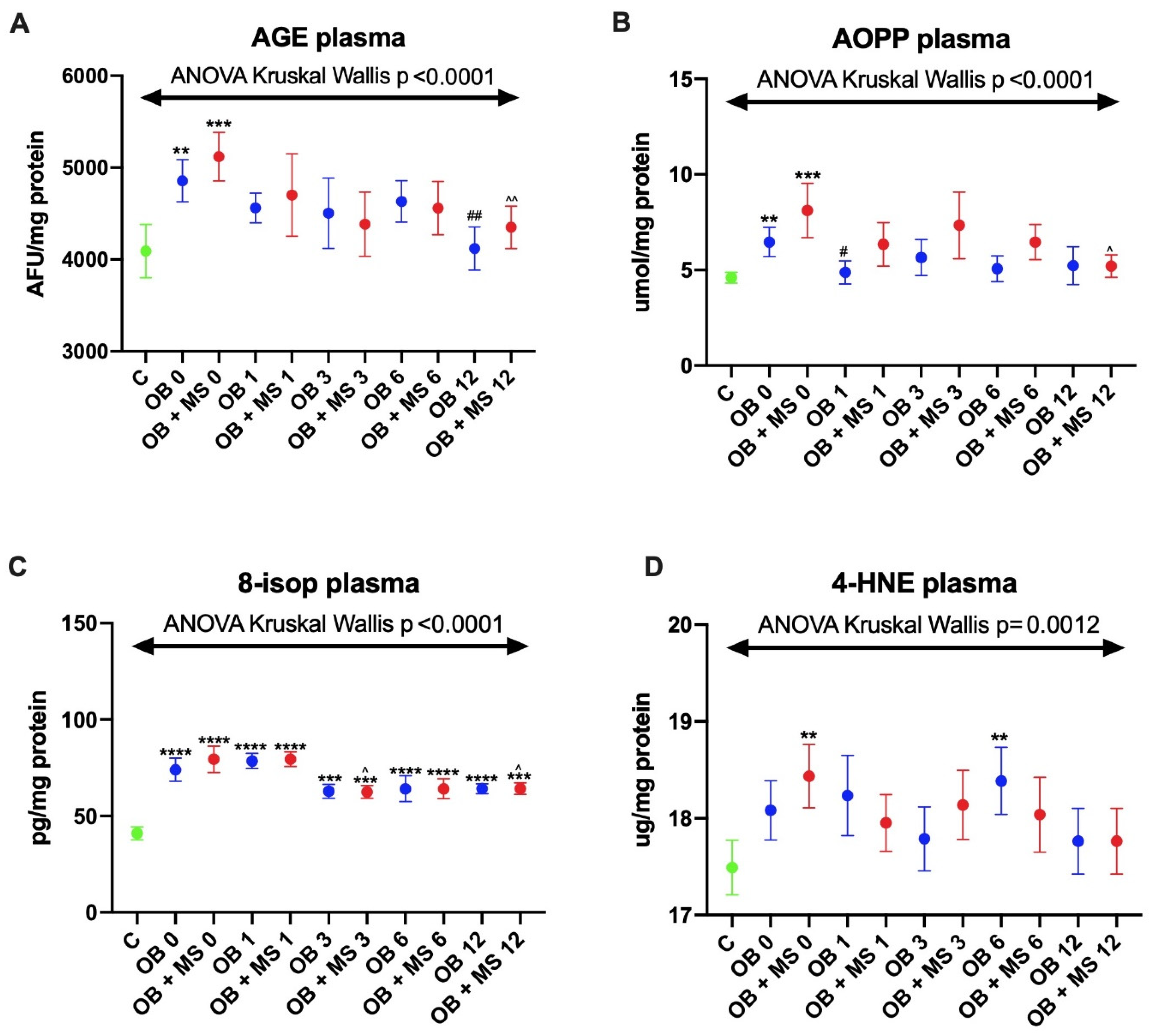
3.2. Oxidative Damage Products
3.2.1. Advanced Glycation End Products (AGE)
3.2.2. Advanced Oxidation Protein Products (AOPP)
3.2.3. 8-Isoprostanes (8-isop)
3.2.4. 4-Hydroxynoneal Protein Adducts (4-HNE)
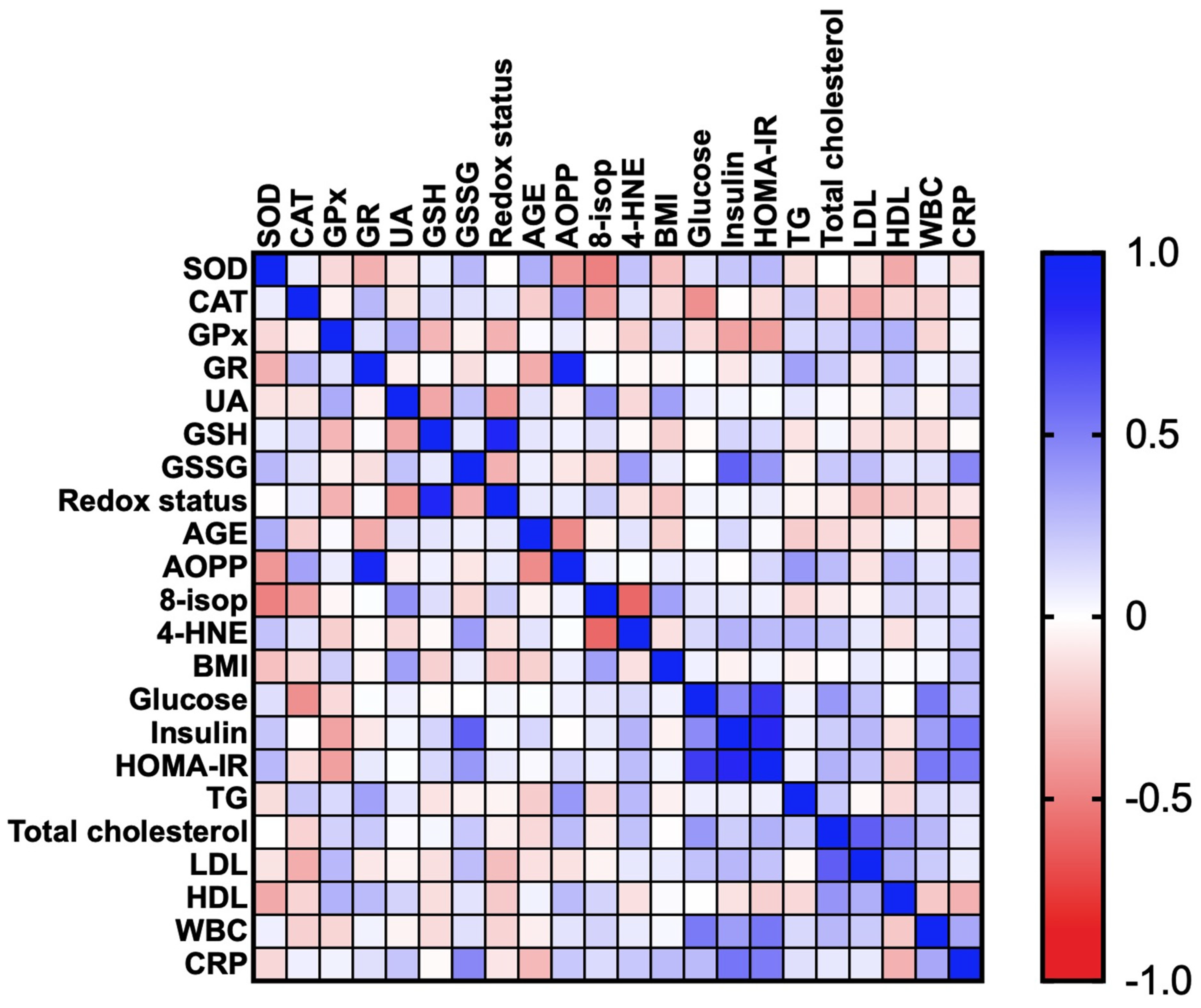
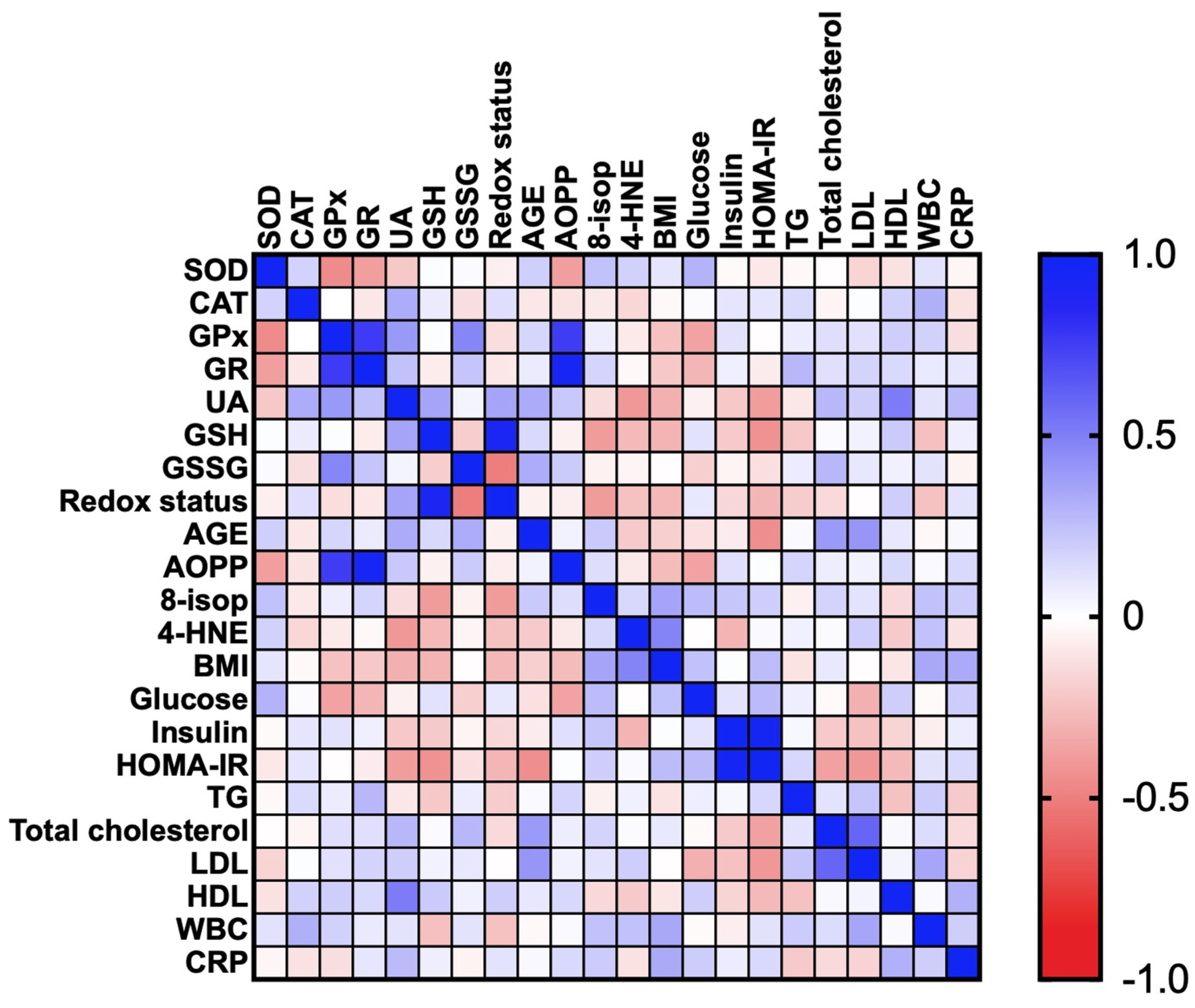
3.2.5. Correlations
3.2.6. ROC Analysis
4. Discussion
4.1. Antioxidant Barrier
4.2. Oxidative Damage to Proteins and Lipids
4.3. Diagnostic Significance
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, leptin and breast cancer: Epidemiological evidence and proposed mechanisms. Cancers (Basel) 2019, 11, E62. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type−2 diabetes. J. Physiol. Pharmacol. 2018, 69, 30683819. [Google Scholar]
- Korybalska, K.; Luczak, J.; Swora-Cwynar, E.; Kanikowska, A.; Czepulis, N.; Kanikowska, D.; Skalisz, H.; Breborowicz, A.; Grzymislawski, M.; Witowski, J. Weight loss-dependent and -independent effects of moderate calorie restriction on endothelial cell markers in obesity. J. Physiol. Pharmacol. 2017, 68, 597–608. [Google Scholar]
- Choromańska, B.; Myśliwiec, P.; Razak Hady, H.; Dadan, J.; Myśliwiec, H.; Chabowski, A.; Mikłosz, A. Metabolic Syndrome is Associated with Ceramide Accumulation in Visceral Adipose Tissue of Women with Morbid Obesity. Obesity 2019. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, B.; Mysliwiec, P.; Hady, H.R.; Dadan, J.; Mysliwiec, H.; Bonda, T.; Chabowski, A.; Miklosz, A. The implication of adipocyte ATP-binding cassette A1 and G1 transporters in metabolic complications of obesity. J. Physiol. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin resistance in obesity: An overview of fundamental alterations. Eat. Weight Disord. 2018, 23, 149–157. [Google Scholar] [CrossRef]
- Fejfer, K.; Buczko, P.; Niczyporuk, M.; Ładny, J.R.; Hady, H.R.; Knaś, M.; Waszkiel, D.; Klimiuk, A.; Zalewska, A.; Maciejczyk, M. Oxidative Modification of Biomolecules in the Nonstimulated and Stimulated Saliva of Patients with Morbid Obesity Treated with Bariatric Surgery. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Heropolitanska-Pliszka, E.; Pietrucha, B.; Sawicka-Powierza, J.; Bernatowska, E.; Wolska-Kusnierz, B.; Pac, M.; Car, H.; Zalewska, A.; Mikoluc, B. Antioxidant Defense, Redox Homeostasis, and Oxidative Damage in Children With Ataxia Telangiectasia and Nijmegen Breakage Syndrome. Front. Immunol. 2019, 10, 2322. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.R.; Ładny, J.R.; Morawska, K.; Zalewska, A. Glutathione Metabolism, Mitochondria Activity, and Nitrosative Stress in Patients Treated for Mandible Fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef]
- Knaś, M.; Maciejczyk, M.; Sawicka, K.; Hady, H.R.; Niczyporuk, M.; Ładny, J.R.; Matczuk, J.; Waszkiel, D.; Żendzian-Piotrowska, M.; Zalewska, A. Impact of morbid obesity and bariatric surgery on antioxidant/oxidant balance of the unstimulated and stimulated human saliva. J. Oral Pathol. Med. 2016, 45, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, A.; le Roux, C.; Pournaras, D. The metabolic benefits of different bariatric operations: What procedure to choose? Endocr. Connect. 2020, 9, R28–R35. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes. Facts 2018, 28, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. J. Am. Med. Assoc. 2014. [Google Scholar] [CrossRef] [PubMed]
- Al Assal, K.; Prifti, E.; Belda, E.; Sala, P.; Clément, K.; Dao, M.C.; Doré, J.; Levenez, F.; Taddei, C.R.; Fonseca, D.C.; et al. Gut microbiota profile of obese diabetic women submitted to Roux-en-Y gastric bypass and its association with food intake and postoperative diabetes remission. Nutrients 2020, 278. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.; Pappan, K.L.; Gandhi, A.V.; Saxena, S.; Kang, C.Y.; McMahon, H.; Chipitsyna, G.I.; Tichansky, D.S.; Arafat, H.A. Conserved Metabolic Changes in Nondiabetic and Type 2 Diabetic Bariatric Surgery Patients: Global Metabolomic Pilot Study. J. Diabetes Res. 2016. [Google Scholar] [CrossRef]
- International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. IDF Commun. 2006, 1–23. [Google Scholar]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Sinauer Associates, Inc.: Sunderland, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Mize, C.E.; Longdon, R. Hepatic glutathione reductase. I. Purification and general kinetic properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar]
- Moron, M.; Depierre, J.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Zalewska, A.; Maciejczyk, M.; Szulimowska, J.; Imierska, M.; Błachnio-Zabielska, A. High-Fat Diet Affects Ceramide Content, Disturbs Mitochondrial Redox Balance, and Induces Apoptosis in the Submandibular Glands of Mice. Biomolecules 2019, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Ziembicka, D.; Żendzian-Piotrowska, M.; Maciejczyk, M. The Impact of High-Fat Diet on Mitochondrial Function, Free Radical Production, and Nitrosative Stress in the Salivary Glands of Wistar Rats. Oxid. Med. Cell. Longev. 2019, 2019, 2606120. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar]
- Maciejczyk, M.; Skutnik-Radziszewska, A.; Zieniewska, I.; Matczuk, J.; Domel, E.; Waszkiel, D.; Żendzian-Piotrowska, M.; Szarmach, I.; Zalewska, A. Antioxidant Defense, Oxidative Modification, and Salivary Gland Function in an Early Phase of Cerulein Pancreatitis. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Tian, X.; Yan, C.; Liu, M.; Zhang, Q.; Liu, D.; Liu, Y.; Li, S.; Han, Y. Obesity and insulin resistance. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2017; Volume 960, pp. 277–304. [Google Scholar]
- Illán-Gómez, F.; Gonzálvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragón-Alonso, A.; Pascual-Díaz, M.; Pérez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012. [Google Scholar] [CrossRef]
- Molnár, D.; Decsi, T.; Koletzko, B. Reduced antioxidant status in obese children with multimetabolic syndrome. Int. J. Obes. 2004. [Google Scholar] [CrossRef] [PubMed]
- Sfar, S.; Boussoffara, R.; Sfar, M.T.; Kerkeni, A. Antioxidant enzymes activities in obese Tunisian children. Nutr. J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Kossakowska, A.; Taranta-Janusz, K.; Zięba, S.; Fejfer, K.; Salamonowicz, M.; Kostecka-Sochoń, P.; Wasilewska, A.; Maciejczyk, M. Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. J. Clin. Med. 2020, 9, 548. [Google Scholar] [CrossRef]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Free Radic. Res. 2019. [Google Scholar] [CrossRef]
- Styskal, J.; Van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Karin, M. JNK1 and IKKβ: Molecular links between obesity and metabolic dysfunction. FASEB J. 2010. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mohseni, R.; Sadeghabadi, Z.A.; Goodarzi, M.T.; Teimouri, M.; Nourbakhsh, M.; Razzaghy Azar, M. Evaluation of Mn-superoxide dismutase and catalase gene expression in childhood obesity: Its association with insulin resistance. J. Pediatr. Endocrinol. Metab. 2018. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Ładny, J.R. Salivary Antioxidant Barrier, Redox Status, and Oxidative Damage to Proteins and Lipids in Healthy Children, Adults, and the Elderly. Oxid. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signalling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Bankoglu, E.E.; Seyfried, F.; Arnold, C.; Soliman, A.; Jurowich, C.; Germer, C.T.; Otto, C.; Stopper, H. Reduction of DNA damage in peripheral lymphocytes of obese patients after bariatric surgery-mediated weight loss. Mutagenesis 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, H.; Han, X.; Zhang, P.; Mao, Z. Uric acid level changes after bariatric surgery in obese subjects with type 2 diabetes mellitus. Ann. Transl. Med. 2019, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, S.; Yoo, J.; Kim, S.; Yoo, S.; Song, H.; Kim, Y.S.; Choi, E.; Kim, Y.K. The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2012. [Google Scholar] [CrossRef] [PubMed]
- Nejatinamini, S.; Ataie-Jafari, A.; Qorbani, M.; Nikoohemat, S.; Kelishadi, R.; Asayesh, H.; Hosseini, S. Association between serum uric acid level and metabolic syndrome components. J. Diabetes Metab. Disord. 2015. [Google Scholar] [CrossRef]
- Wei, F.; Chang, B.; Yang, X.; Wang, Y.; Chen, L.; Li, W.D. Serum Uric Acid Levels were Dynamically Coupled with Hemoglobin A1c in the Development of Type 2 Diabetes. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hori, O.; Brett, J.; Du Yan, S.; Wautier, J.L.; Stern, D. Cellular receptors for advanced glycation end products: Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. 1994, 14, 1521–1528. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Çakatay, U.; Kayali, R.; Uzun, H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin. Exp. Med. 2008. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–28. [Google Scholar] [CrossRef]
- Atabek, M.; Keskin, M.; Yazici, C.; Kendirci, M.; Hatipoglu, N.; Koklu, E.; Kurtoglu, S. Protein oxidation in obesity and insulin resistance. Eur. J. Pediatr. 2006, 165, 753–756. [Google Scholar] [CrossRef]
- Korkmaz, G.G.; Altinoglu, E.; Civelek, S.; Sozer, V.; Erdenen, F.; Tabak, O.; Uzun, H. The association of oxidative stress markers with conventional risk factors in the metabolic syndrome. Metabolism 2013. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Matczuk, J.; Żendzian-Piotrowska, M.; Niklińska, W.; Fejfer, K.; Szarmach, I.; Ładny, J.R.; Zieniewska, I.; Zalewska, A. Eight-Week Consumption of High-Sucrose Diet Has a Pro-Oxidant Effect and Alters the Function of the Salivary Glands of Rats. Nutrients 2018, 10, 1530. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Patryn, E.; Boehm, D.; Berdowska, I.; Zielinski, B.; Noczynska, A. Advanced oxidation protein products (AOPPs) in juvenile overweight and obesity prior to and following weight reduction. Clin. Biochem. 2008. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Wassel, C.L.; Stoddard, P.J.; Harris, T.B.; Cummings, S.R.; Kritchevsky, S.B.; Goodpaster, B.H.; Green, C.; Satterfield, S.; Gross, M.D. F 2-isoprostanes and adiposity in older adults. Obesity 2011. [Google Scholar] [CrossRef]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic. Biol. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Gerreth, K. Karolina Salivary Redox Biomarkers in Selected Neurodegenerative Diseases. J. Clin. Med. 2020, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Werbel, K.; Wasilewska, A.; Zalewska, A. Salivary FRAP as A Marker of Chronic Kidney Disease Progression in Children. Antioxidants 2019, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wiśniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Sawczuk, B.; Maciejczyk, M.; Sawczuk-Siemieniuk, M.; Posmyk, R.; Zalewska, A.; Car, H. Salivary Gland Function, Antioxidant Defence and Oxidative Damage in the Saliva of Patients with Breast Cancer: Does the BRCA1 Mutation Disturb the Salivary Redox Profile? Cancers (Basel) 2019, 11, 1501. [Google Scholar] [CrossRef]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary Antioxidants and Oxidative Stress in Psoriatic Patients: Can Salivary Total Oxidant Status and Oxidative Status Index Be a Plaque Psoriasis Biomarker? Oxid. Med. Cell. Longev. 2020, 2020, 9086024. [Google Scholar] [CrossRef] [PubMed]
- Klimiuk, A.; Zalewska, A.; Sawicki, R.; Knapp, M.; Maciejczyk, M. Salivary Oxidative Stress Increases With the Progression of Chronic Heart Failure. J. Clin. Med. 2020, 9, 769. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Taranta-Janusz, K.; Wasilewska, A.; Kossakowska, A.; Zalewska, A. A Case-Control Study of Salivary Redox Homeostasis in Hypertensive Children. Can Salivary Uric Acid be a Marker of Hypertension? J. Clin. Med. 2020, 9, 837. [Google Scholar] [CrossRef]

| C | OB 0 | OB + MS 0 | OB 1 | OB + MS 1 | OB 3 | OB + MS 3 | OB 6 | OB + MS 6 | OB 12 | OB + MS 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 42 (28–56) | 39 (28–52) | 49 (28–56) | - | - | - | - | - | - | - | - |
| Weight (kg) | 62 (55–72) | 118**** (99–169) | 125**** (94–170) | 107.5**** (90–156) | 113.5**** (84–160) | 95.5****## (80–124) | 102**** (77–138) | 85**#### (72–113) | 92.5****^^^^ (70–125) | 78#### (63–104) | 85*^^^^ (64–112) |
| BMI (kg/m2) | 23.04 (21.93–24.80) | 44.87**** (40.16–61.34) | 46.52**** (40.03–60.96) | 40.39**** (30.34–58.01) | 41.62**** (32.81–56.68) | 36.19****## (28.34–46.86) | 39.30**** (29.05–49.91) | 32.03**#### (26.15–42.06) | 34.41****^^^^ (26.21–47.62) | 28.73#### (24.09–37.65) | 31.23*^^^^ (22.67–40.15) |
| WHR | 0.71 (0.64 – 0.74) | 0.95**** (0.81–1.04) | 0.97**** (0.84–1.15) | 0.95**** (0.73–1.01) | 0.98**** (0.87–1.15) | 0.93**** (0.80–1.01) | 0.98**** (0.83–1.12) | 0.92*** (0.81–0.98) | 0.96**** (0.86–1.13) | 0.90*# (0.80–0.98) | 0.93***^^ (0.84–0.99) |
| Waist circumference (cm) | 73 (66–86) | 132.5 (110–149) | 140.5 (120–161) | 125 (100–135) | 134.5 (115–152) | 112 (90–129) | 121.5 (101–139) | 101.5 (82–116) | 110 (92–130) | 92 (78–108) | 100 (82–116) |
| CRP (mg/L) | 5.5 (5.1–6.5) | 8.8 (1.5–27.6) | 11.7**** (5.3–18.2) | 5.2 (0.5–26) | 8.35 (1.5–13.6) | 6.1 (0.3–16.5) | 7.1 (0.6–16.5) | 4.76 (0.3–19.5) | 6.05^^ (0.5–11.05) | 4.85## (0.2–7.8) | 5.5^^^ (1.2–16.1) |
| Glucose (mg/dL) | 76 (67–92) | 98**** (76–122) | 106*** (89–189) | 94**** (69–115) | 99**** (75–147) | 88 (60–142) | 97**** (79–116) | 84.5# (68–109) | 95**** (81–157) | 84## (73–111) | 90**^^^ (77–99) |
| Insulin (uIU/mL) | 7.5 (7–9.4) | 17.85**** (8.2–38.6) | 22.25*** (9.3–40.7) | 11.6**# (4–28) | 15.15**** (5.8–35.3) | 8.15#### (2.7–16.2) | 10.5*^^^ (3.6–43.8) | 8.9#### (4.1–12.3) | 8.75^^^^ (4.5–17.1) | 8.1#### (3–14.5) | 8.5^^^^ (4.5–9.9) |
| HOMA-IR | 1.44 (1.19–1.86) | 4.15**** (1.78–9.34) | 5.64*** (3.05–8.1) | 2.77***# (0.73–6.56) | 3.82**** (1.25–8.62) | 1.94#### (0.46–3.8) | 2.47****^^ (0.95–10.16) | 1.88#### (0.75–2.91) | 1.98*^^^^ (1.1–3.84) | 1.67#### (0.58–3.50) | 1.91^^^^ (1.07–2.17) |
| ALT (IU/L) | 23 (16–35) | 24 (6–93) | 27 (12–54) | 23.5 (6–52) | 27.5 (14–56) | 20 (6–43) | 24 (13–59) | 17 (6–51) | 21 (12–31) | 18 (8–37) | 19 (8–43) |
| AST (IU/L) | 22 (16–36) | 19 (13–44) | 21 (14–50) | 23.5 (14–98) | 27 (14–85) | 19 (12–36) | 21 (13–43) | 17 (9–48) | 20 (12–43) | 18 (12–52) | 19 (12–37.2) |
| Cholesterol (mg/dL) | 175 (159–189) | 195 (147–231) | 211**** (167–268) | 170.5 (124–210) | 192* (126–235) | 176 (120–217) | 181^ (120–237) | 178 (114–245) | 188 (148–264) | 176 (114–231) | 174^^^ (138–295) |
| LDL (mg/dL) | 119 (115–123) | 124.5 (100–159) | 144** (122–181) | 110 (83–153) | 122^^ (66–183) | 106 (69–159) | 116^^^ (50–189) | 109 (61–166) | 110^^^^ (83–157) | 113# (59–134) | 102^^^^ (64–164) |
| TG (mg/dL) | 135 (119–145) | 128.5 (62–197) | 150 (104–289) | 108.5 (66–209) | 136 (75–367) | 103.5** (46–182) | 137 (78–240) | 96****## (48–182) | 130 (89–214) | 98****## (36–182) | 102**^^^^ (59–146) |
| HDL (mg/dL) | 60 (45–68) | 50** (33–62) | 46**** (31–72) | 47**** (30–106) | 45**** (31–68) | 47*** (27–141) | 49*** (35–69) | 50** (35–72) | 52.5 (35–86) | 53 (38–81) | 56^^ (43–70) |
| WBC (103/μL) | 7.5 (4.4–9.1) | 8.295* (5.26–12.15) | 9.53* (5.85–12.91) | 6.53## (4.63–9.64) | 7.16 (4.95–12.7) | 5.78*#### (4.61–10.54) | 6.9 (3.9–11.54) | 5.98### (4.75–9.27) | 7.195 (4.4–10.94) | 5.92*#### (4.07–8.52) | 6.96^^^ (4.21–10.26) |
| RBC (106/μL) | 4.6 (3.9–5.2) | 4.7 (3.51–5.78) | 4.6 (4–5.46) | 4.79 (4.11–6.03) | 4.77 (4.31–6.01) | 4.655 (4.11–5.13) | 4.75 (4.11–5.45) | 4.65 (3.98–5.69) | 4.75 (4.02–5.66) | 4.62 (4.03–5.4) | 4.50 (3.33–5.65) |
| HGB (g/dL) | 13.8 (11.5–14.7) | 13.1 (11–15.2) | 13.35 (12.1–16.2) | 13.2 (10–15.9) | 13.6 (11.2–16.6) | 13.1 (11.5–15.7) | 13.2 (10.9–16.3) | 13.4 (9.7–15.4) | 13.4 (10.6–16.3) | 13.65 (9.1–15.8) | 14.15 (8.7–16.2) |
| PLT (103/μL) | 289 (265–315) | 264.5 (141–417 | 258 (183–418) | 261 (121–412) | 229* (128–405) | 265.5 (130–312) | 247 (131–345) | 273.5 (188–425) | 251.5 (167–375) | 214.5*** (130–345) | 224** (164–378) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2020, 9, 976. https://doi.org/10.3390/jcm9040976
Choromańska B, Myśliwiec P, Łuba M, Wojskowicz P, Dadan J, Myśliwiec H, Choromańska K, Zalewska A, Maciejczyk M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? Journal of Clinical Medicine. 2020; 9(4):976. https://doi.org/10.3390/jcm9040976
Chicago/Turabian StyleChoromańska, Barbara, Piotr Myśliwiec, Magdalena Łuba, Piotr Wojskowicz, Jacek Dadan, Hanna Myśliwiec, Katarzyna Choromańska, Anna Zalewska, and Mateusz Maciejczyk. 2020. "A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People?" Journal of Clinical Medicine 9, no. 4: 976. https://doi.org/10.3390/jcm9040976
APA StyleChoromańska, B., Myśliwiec, P., Łuba, M., Wojskowicz, P., Dadan, J., Myśliwiec, H., Choromańska, K., Zalewska, A., & Maciejczyk, M. (2020). A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? Journal of Clinical Medicine, 9(4), 976. https://doi.org/10.3390/jcm9040976








