Can an Observational Gait Scale Produce a Result Consistent with Symmetry Indexes Obtained from 3-Dimensional Gait Analysis?: A Concurrent Validity Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Participants
2.3. Outcome Measures
2.3.1. Primary Outcome Measure: Spatiotemporal and Kinematic Parameters of Gait
2.3.2. Secondary Outcomes
2.4. Data Analyses
3. Results
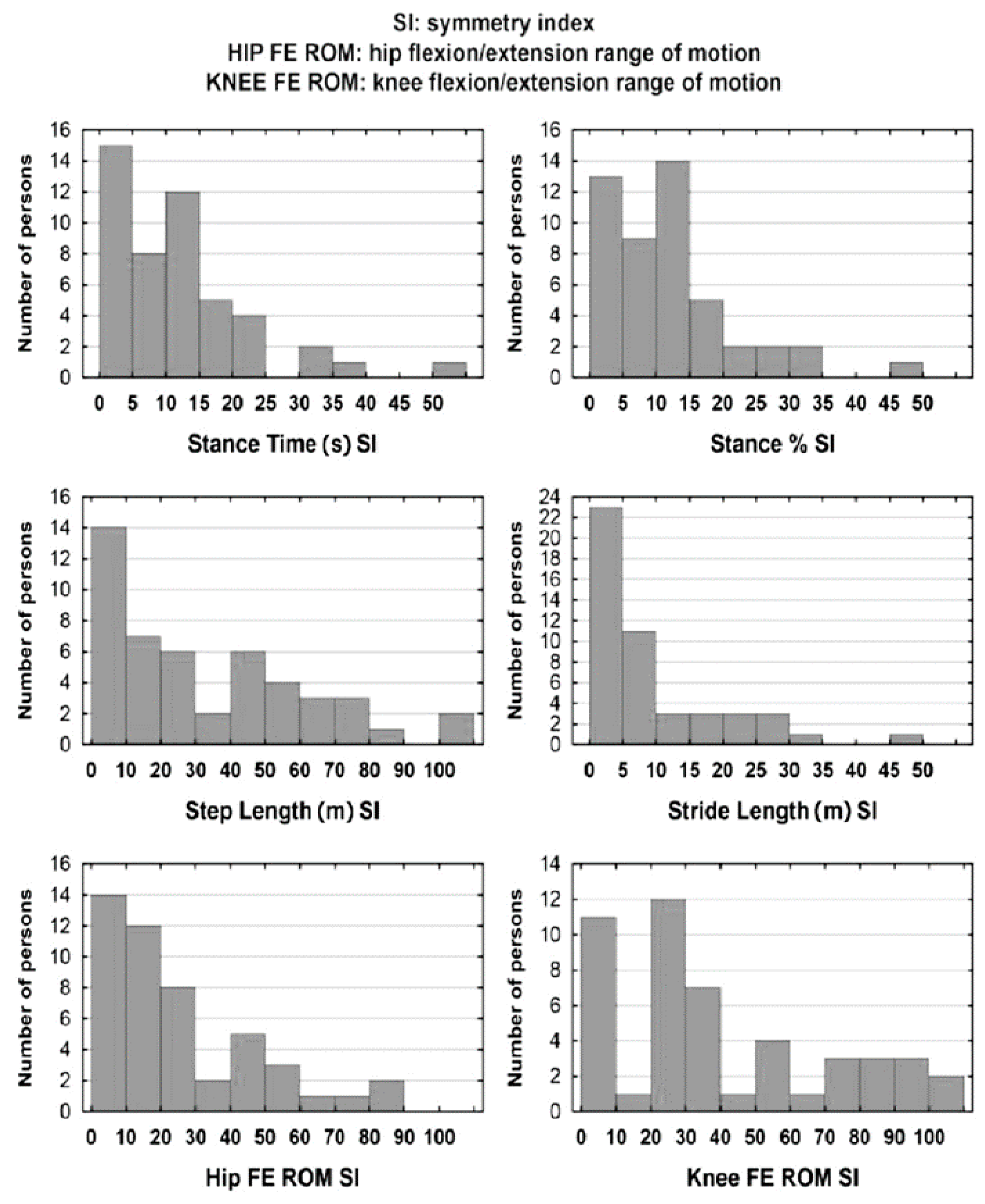
3.1. Gait Symmetry Indexes
3.2. Correlations Between Symmetry Indexes and Scores in the WGS
3.3. Regression Models Describing 3D Symmetry Indexes Using WGS Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems: Highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, A.A.; Black, P.O.; Kile, K.A.; Sherman, J.; Stellberg, B.; McCormick, J.; Roszkowski, J.; Swiggum, E. Gait training efficacy using a home-based practice model in chronic hemiplegia. Arch. Phys. Med. Rehabil. 1996, 77, 801–805. [Google Scholar] [CrossRef]
- Estrada-Barranco, C.; Cano-de-la-Cuerda, R.; Molina-Rueda, F. Construct validity of the Wisconsin Gait Scale in acute, subacute and chronic stroke. Gait Posture 2019, 68, 363–368. [Google Scholar] [CrossRef]
- Guzik, A.; Drużbicki, M.; Wolan-Nieroda, A.; Przysada, G.; Kwolek, A. The Wisconsin gait scale—The minimal clinically important difference. Gait Posture 2019, 68, 453–457. [Google Scholar] [CrossRef]
- Pizzi, A.; Carlucci, G.; Falsini, C.; Lunghi, F.; Verdesca, S.; Grippo, A. Gait in hemiplegia: Evaluation of clinical features with the Wisconsin Gait Scale. J. Rehabil. Med. 2007, 39, 170–174. [Google Scholar] [CrossRef]
- Yaliman, A.; Kesiktas, N.; Ozkaya, M.; Eskiyurt, N.; Erkan, O.; Yilmaz, E. Evaluation of intrarater and interrater reliability of the Wisconsin Gait Scale with using the video taped stroke patients in a Turkish sample. NeuroRehabilitation 2014, 34, 253–258. [Google Scholar] [CrossRef]
- Wellmon, R.; Degano, A.; Rubertone, J.A.; Campbell, S.; Russo, K.A. Interrater and intrarater reliability and minimal detectable change of the Wisconsin Gait Scale when used to examine videotaped gait in individuals post-stroke. Arch. Physiother. 2015, 5, 11. [Google Scholar] [CrossRef]
- Lu, X.; Hu, N.; Deng, S.; Li, J.; Qi, S.; Bi, S. The reliability, validity and correlation of two observational gait scales assessed by video tape for Chinese subjects with hemiplegia. J. Phys. Ther. Sci. 2015, 27, 3717–3721. [Google Scholar] [CrossRef]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Viteckova, S.; Kutilek, P.; Svoboda, Z.; Krupicka, R.; Kauler, J.; Szabo, Z. Gait symmetry measures: A review of current and prospective methods. Biomed. Signal. Proces. 2018, 42, 89–100. [Google Scholar] [CrossRef]
- Patterson, K.K.; Gage, W.H.; Broooks, D.; Black, S.E.; Mcilroy, W.E. Changes in gait symmetry and velocity after stroke: A cross-sectional study from weeks to years after stroke. Neurorehabil. Neural. Repair. 2010, 24, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Horton, M.G.; Wikholm, J.B. Importance of four variables of walking to patients with stroke. Int. J. Rehab. Res. 1991, 14, 246–250. [Google Scholar] [CrossRef]
- Goldie, P.A.; Matyas, T.A.; Evans, O.M. Gait after stroke: Initial deficit and changes in temporal patterns for each gait phase. Arch. Phys. Med. Rehabil. 2001, 82, 1057–1065. [Google Scholar] [CrossRef]
- Wall, J.; Turnbull, G. Gait asymmetries in residual hemiplegia. Arch. Phys. Med. Rehabil. 1986, 67, 550–553. [Google Scholar]
- von Schroeder, H.P.; Coutts, R.D.; Lyden, P.D.; Billings, E.Jr.; Nickel, V.L. Gait parameters following stroke: A practical assessment. J. Rehabil. Res. Dev. 1995, 32, 25–31. [Google Scholar]
- Bensoussan, L.; Mesure, S.; Viton, J.M.; Delarque, A. Kinematic and kinetic asymmetries in hemiplegic patients’ gait initiation patterns. J. Rehabil. Med. 2006, 38, 287–294. [Google Scholar] [CrossRef]
- Boudarham, J.; Roche, N.; Pradon, D.; Bonnyaud, C.; Bensmail, D.; Zory, R. Variations in Kinematics during Clinical Gait Analysis in Stroke Patients. PLoS ONE 2013, 8, e66421. [Google Scholar] [CrossRef]
- Friedman, P.J. Gait recovery after hemiplegic stroke. Int. Disabil. Stud. 1990, 12, 119–122. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Ferrarello, F.; Bianchi, V.A.; Baccini, M.; Rubbieri, G.; Mossello, E.; Cavallini, M.Ch.; Marchionni, N.; Di Bari, M. Tools for observational gait analysis in patients with stroke: A systematic review. Phys Ther. 2013, 93, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Drużbicki, M.; Przysada, G.; Kwolek, A.; Brzozowska-Magoń, A.; Wyszyńska, J.; Podgórska-Bednarz, J. Assessment of test-retest reliability and internal consistency of the Wisconsin Gait Scale in hemiparetic post-stroke patients. Postepy Rehabil. 2016, 30, 41–53. [Google Scholar] [CrossRef][Green Version]
- Robinson, R.O.; Herzog, W.; Nigg, B.M. Use of force platform variables to quantify the effects of chiropractic manipulation on gait symmetry. J. Manip. Physiol. Ther. 1987, 10, 172–176. [Google Scholar]
- Kim, C.M.; Eng, J.J. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture 2003, 18, 23–28. [Google Scholar] [CrossRef]
- Titianova, E.B.; Tarkka, I.M. Asymmetry in walking performance and postural sway in patients with chronic unilateral cerebral infarction. J. Rehabil. Res. Dev. 1995, 32, 236–244. [Google Scholar]
- Bovonsunthonchai, S.; Hiengkaew, V.; Vachalathiti, R.; Vongsirinavarat, M. Gait symmetrical indexes and their relationships to muscle tone, lower extremity function, and postural balance in mild to moderate stroke. J. Med. Assoc. Thai. 2011, 94, 476–484. [Google Scholar]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Mukaka, M.M. A guide to appropriate use of Correlation coefficient in medical research. Malaw. Med. J. 2012, 24, 69–71. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, Needham heights; Allyn & Bacon: Boston, MA, USA, 2001. [Google Scholar]
- Hsu, A.L.; Tang, P.F.; Jan, M.H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F. Gait disturbances in patients with stroke. PM. R. 2014, 6, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Bastian, A.J. Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil. Neural. Repair. 2014, 28, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Nadkarni, N.K.; Black, S.E.; Mcilroy, W.E. Temporal gait symmetry and velocity differ in their relationship to age. Gait Posture 2012, 35, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Awad, L.N.; Palmer, J.A.; Pohlig, R.T.; Binder-Macleod, S.A.; Reisman, D.S. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil. Neural. Repair. 2015, 29, 416–423. [Google Scholar] [CrossRef] [PubMed]
| 3-Dimensional Gait Parameters | s | 95% C.I. | Med | Min | Max | |
|---|---|---|---|---|---|---|
| Paretic limb | ||||||
| Stance Time (s) | 1.11 | 0.33 | (1.01, 1.21) | 1.02 | 0.62 | 1.86 |
| Stance % | 0.66 | 0.06 | (0.64, 0.68) | 0.67 | 0.54 | 0.79 |
| Step Length (m) | 0.24 | 0.11 | (0.20, 0.27) | 0.21 | 0.07 | 0.56 |
| Stride Length (m) | 0.60 | 0.24 | (0.53, 0.67) | 0.55 | 0.22 | 1.17 |
| Hip FE ROM | 27.4 | 8.2 | (25.0, 29.8) | 26.3 | 10.9 | 47.7 |
| Knee FE ROM | 28.8 | 11.8 | (25.3, 32.2) | 27.5 | 9.6 | 48.2 |
| Non-paretic limb | ||||||
| Stance Time (s) | 1.24 | 0.40 | (1.12, 1.35) | 1.19 | 0.69 | 2.10 |
| Stance % | 0.74 | 0.07 | (0.72, 0.76) | 0.74 | 0.58 | 0.89 |
| Step Length (m) | 0.27 | 0.11 | (0.24, 0.30) | 0.27 | 0.09 | 0.59 |
| Stride Length (m) | 0.62 | 0.24 | (0.55, 0.69) | 0.59 | 0.20 | 1.22 |
| Hip FE ROM | 33.5 | 5.7 | (31.8, 35.1) | 33.6 | 18.2 | 45.0 |
| Knee FE ROM | 40.2 | 7.6 | (37.9, 42.4) | 39.4 | 26.6 | 60.6 |
| SI for Spatiotemporal and Kinematic Parameters | Med | s | Min | Max | A | |
|---|---|---|---|---|---|---|
| Stance Time (s) | 11.7 | 10.3 | 11.0 | 0.0 | 55.0 | 1.78 |
| Stance % | 11.9 | 10.6 | 10.1 | 0.3 | 46.9 | 1.33 |
| Step Length (m) | 32.8 | 23.6 | 28.9 | 0.8 | 107.5 | 0.85 |
| Stride Length (m) | 9.7 | 6.1 | 10.2 | 0.4 | 48.8 | 1.80 |
| Hip FE ROM | 25.1 | 16.3 | 22.0 | 0.3 | 84.9 | 1.23 |
| Knee FE ROM | 40.2 | 30.6 | 31.7 | 1.0 | 114.3 | 0.75 |
| WGS (items) | Symmetry Indexes Identified for 3D Gait Parameters | |||||
|---|---|---|---|---|---|---|
| Stance Time (s) | Stance % | Step Length (m) | Stride Length (m) | Hip FE ROM | Knee FE ROM | |
| Stance Phase Affected Leg | ||||||
| Use of hand-held gait aid | 0.12 | 0.22 | 0.00 | 0.13 | 0.15 | 0.37 ** |
| Stance time on impaired side | 0.80 *** | 0.91 *** | 0.14 | 0.43 ** | 0.43 ** | 0.49 *** |
| Step length of unaffected side | 0.23 | 0.36 * | 0.10 | 0.55 *** | 0.57 *** | 0.51 *** |
| Weight shift to the affected side | −0.16 | −0.21 | 0.00 | 0.15 | 0.15 | 0.20 |
| Stance width | 0.04 | 0.21 | 0.11 | 0.25 | 0.00 | 0.20 |
| Toe Off Affected Leg | ||||||
| Guardedness (pause prior to advancing affected leg) | 0.24 | 0.38 ** | 0.36 * | 0.32 * | 0.38 ** | 0.47 *** |
| Hip extension of affected side | 0.44 ** | 0.50 *** | 0.03 | 0.42 ** | 0.90 *** | 0.63 *** |
| Swing Phase Affected Leg | ||||||
| External rotation during initial swing | 0.31 * | 0.26 | 0.06 | 0.12 | 0.24 | 0.50 *** |
| Circumduction at mid swing | 0.36 * | 0.40 ** | 0.20 | 0.11 | 0.25 | 0.48 *** |
| Hip hiking at mid swing | 0.23 | 0.36 * | 0.20 | 0.36 * | 0.74 *** | 0.64 *** |
| Knee flexion from toe off to mid swing | 0.46 ** | 0.54 *** | 0.37 ** | 0.47 *** | 0.57 *** | 0.94 *** |
| Toe clearance | 0.23 | 0.37 ** | 0.26 | 0.18 | 0.34 * | 0.34 * |
| Pelvic rotation at terminal swing | 0.13 | 0.25 | 0.31 * | 0.19 | 0.27 | 0.58 *** |
| Heel Strike Affected Leg | ||||||
| Initial foot contact | 0.29 * | 0.39 ** | 0.40 ** | 0.29 * | 0.51 *** | 0.60 *** |
| Total score | 0.43 ** | 0.57 *** | 0.29 * | 0.43 ** | 0.65 *** | 0.82 *** |
| Dependent Variable | R2 | Regression Model Formula |
|---|---|---|
| Stance Time (s) SI | 65.4% | −8.84 + 12.65·WGS2 |
| Stance % SI | 82.7% | −9.27 + 13.04·WGS2 |
| Step Length (m) SI | 23.7% | 13.58 − 12.58·WGS7 + 22.05·WGS14 |
| Stride Length (m) SI | 46.4% | −15.55 + 5.74·WGS2 + 6.66·WGS3 + 4.19·WGS4 |
| Hip FE ROM SI | 87.5% | −20.22 + 22.10·WGS7 − 4.37·WGS9 + 8.62·WGS10 |
| Knee FE ROM SI | 88.6% | −40.48+8.87·WGS1 + 10.22·WGS7 + 28.67·WGS11 − 6.01·WGS14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzik, A.; Drużbicki, M.; Perenc, L.; Podgórska-Bednarz, J. Can an Observational Gait Scale Produce a Result Consistent with Symmetry Indexes Obtained from 3-Dimensional Gait Analysis?: A Concurrent Validity Study. J. Clin. Med. 2020, 9, 926. https://doi.org/10.3390/jcm9040926
Guzik A, Drużbicki M, Perenc L, Podgórska-Bednarz J. Can an Observational Gait Scale Produce a Result Consistent with Symmetry Indexes Obtained from 3-Dimensional Gait Analysis?: A Concurrent Validity Study. Journal of Clinical Medicine. 2020; 9(4):926. https://doi.org/10.3390/jcm9040926
Chicago/Turabian StyleGuzik, Agnieszka, Mariusz Drużbicki, Lidia Perenc, and Justyna Podgórska-Bednarz. 2020. "Can an Observational Gait Scale Produce a Result Consistent with Symmetry Indexes Obtained from 3-Dimensional Gait Analysis?: A Concurrent Validity Study" Journal of Clinical Medicine 9, no. 4: 926. https://doi.org/10.3390/jcm9040926
APA StyleGuzik, A., Drużbicki, M., Perenc, L., & Podgórska-Bednarz, J. (2020). Can an Observational Gait Scale Produce a Result Consistent with Symmetry Indexes Obtained from 3-Dimensional Gait Analysis?: A Concurrent Validity Study. Journal of Clinical Medicine, 9(4), 926. https://doi.org/10.3390/jcm9040926






