The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial
Abstract
1. Introduction
2. Experimental Section
2.1. Measurement of Biomarkers
2.2. Statistical Analyses
3. Results
3.1. Usefulness of Serum Biomarkers for Monitoring Neurodysfunction
3.2. Usefulness of Serum Biomarkers to Monitoring Structural Changes
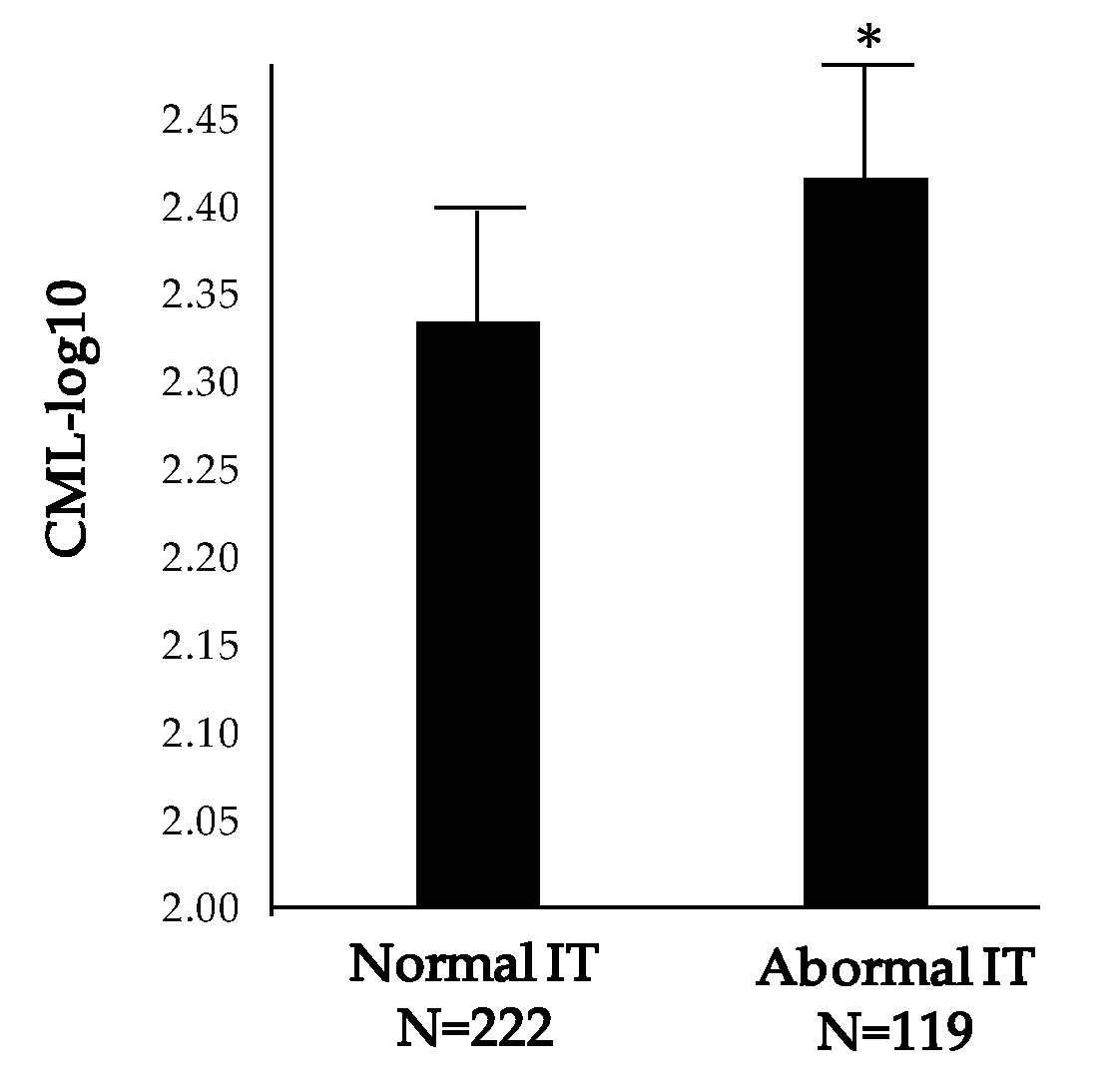
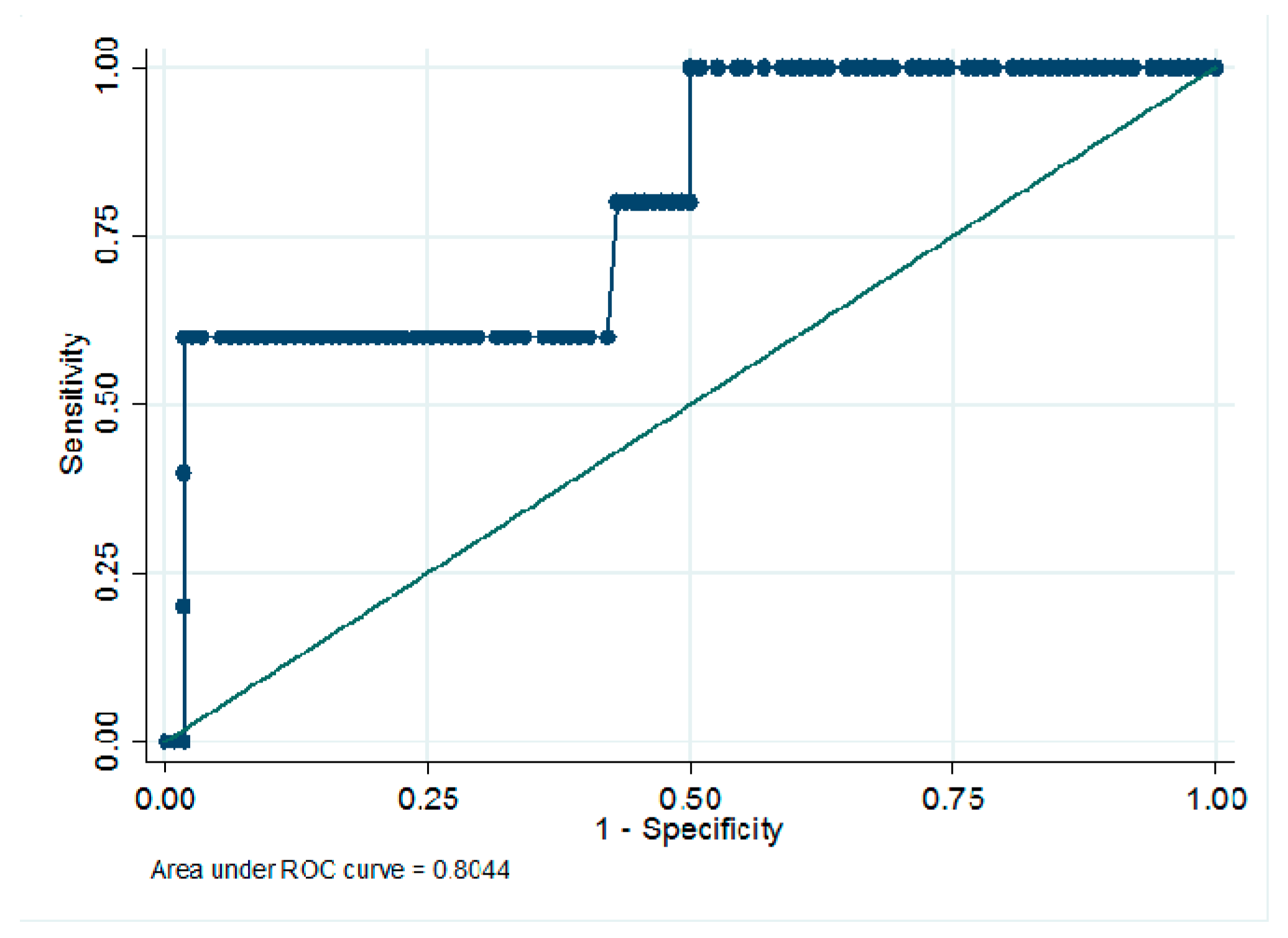
3.3. Usefulness of Serum Biomarkers in Monitoring Early Microvascular Changes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- El Hindy, N.; Ong, J.M. Asymmetric diabetic retinopathy. J. Diabetes 2010, 2, 125–126. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Prim. 2016, 2, 16013. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.J.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Simó, R.; Ballarini, S.; Cunha-Vaz, J.; Ji, L.; Haller, H.; Zimmet, P.Z.; Wong, T.Y. Non-Traditional Systemic Treatments for Diabetic Retinopathy: An Evidence-Based Review. Curr. Med. Chem. 2015, 22, 2580–2589. [Google Scholar] [CrossRef]
- Keenan, H.A.; Costacou, T.; Sun, J.K.; Doria, A.; Cavallerano, J.; Coney, J.; Orchard, T.; Aiello, L.P.; King, G.L. Clinical Factors Associated With Resistance to Microvascular Complications in Diabetic Patients of Extreme Disease Duration: The 50-year Medalist Study. Diabetes Care 2007, 30, 1995–1997. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Hernández, C.; Simó, R. Genetics in Diabetic Retinopathy: Current Concepts and New Insights. Curr. Genom. 2013, 14, 289–299. [Google Scholar] [CrossRef]
- Porta, M.; Toppila, I.; Sandholm, N.; Hosseini, M.; Forsblom, C.; Hietala, K.; Borio, L.; Harjutsalo, V.; Klein, B.E.; Klein, R.; et al. Variation in SLC19A3 and Protection From Microvascular Damage in Type 1 Diabetes. Diabetes 2015, 65, 1022–1030. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Simó, R.; Hernández, C. Circulating Biomarkers of Diabetic Retinopathy: An Overview Based on Physiopathology. J. Diabetes Res. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Vujosevic, S.; Simó, R. Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Investig. Opthalmol. Vis. Sci. 2017, 58, 68. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.; Ribeiro, L.; Costa, M.; Simó, R. Diabetic Retinopathy Phenotypes of Progression to Macular Edema: Pooled Analysis From Independent Longitudinal Studies of up to 2 Years’ Duration. Investig. Opthalmol. Vis. Sci. 2017, 58, 206. [Google Scholar] [CrossRef] [PubMed]
- Frydkjaer-Olsen, U.; Hansen, R.S.; Simo, R.; Cunha-Vaz, J.; Peto, T.; Grauslund, J.; Eurocondor, O.B.O.T. Correlation between Retinal Vessel Calibre and Neurodegeneration in Patients with Type 2 Diabetes Mellitus in the European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Ophthalmic Res. 2016, 56, 10–16. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; Garcia-Arumi, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2018, 68, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tsilibary, E.C. Microvascular basement membranes in diabetes mellitus. J. Pathol. 2003, 200, 537–546. [Google Scholar] [CrossRef]
- Roy, S.; Ha, J.; Trudeau, K.; Beglova, E. Vascular Basement Membrane Thickening in Diabetic Retinopathy. Curr. Eye Res. 2010, 35, 1045–1056. [Google Scholar] [CrossRef]
- Stitt, A.W. AGEs and Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2010, 51, 4867–4874. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Giardino, I.; Edelstein, D.; Brownlee, M. Changes in diabetic retinal matrix protein mRNA levels in a common transgenic mouse strain. Curr. Eye Res. 2000, 21, 581–587. [Google Scholar] [CrossRef]
- Masmiquel, L.; Segura, R.M.; Mateo, C.; Calatayud, M.; Marti, R.; Mesa, J.; Simó, R. Serum laminin as a marker of diabetic retinopathy development: A 4-year follow-up study. Am. J. Ophthalmol. 2000, 129, 347–352. [Google Scholar] [CrossRef]
- Hammes, H.-P.; Alt, A.; Niwa, T.; Clausen, J.T.; Bretzel, R.G.; Brownlee, M.; Schleicher, E.D. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia 1999, 42, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Z.; Luo, D.; Huang, Y.; Zhu, J.; Wang, X.; Hu, H.; Patrick, C. Exogenous advanced glycosylation end products induce diabetes-like vascular dysfunction in normal rats: A factor in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.-P.; Weiss, A.; Hess, S.; Araki, N.; Horiuchi, S.; Brownlee, M.; Preissner, K.T. Modification of vitronectin by advanced glycation alters functional properties in vitro and in the diabetic retina. Lab. Investig. 1996, 75, 325–338. [Google Scholar] [PubMed]
- Clements, R.S.; Robison, W.G.; Cohen, M.P. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/dbmice. J. Diabetes Complicat. 1998, 12, 28–33. [Google Scholar] [CrossRef]
- Schmidt, K.-G.; Bergert, H.; Funk, R.H.W. Neurodegenerative Diseases of the Retina and Potential for Protection and Recovery. Curr. Neuropharmacol. 2008, 6, 164–178. [Google Scholar] [CrossRef]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M.; DCCT Skin Collagen Ancillary Study Group. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar]
- Sydow, K.; Münzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Malecki, M.T.; Undas, A.; Cyganek, K.; Mirkiewicz-Sieradzka, B.; Wołkow, P.; Osmenda, G.; Walus-Miarka, M.; Guzik, T.J.; Sieradzki, J. Plasma asymmetric dimethylarginine (ADMA) is associated with retinopathy in type 2 diabetes. Diabetes Care 2007, 30, 2899–2901. [Google Scholar] [CrossRef] [PubMed]
- Abhary, S.; Kasmeridis, N.; Burdon, K.P.; Kuot, A.; Whiting, M.J.; Yew, W.P.; Petrovsky, N.; Craig, J.E. Diabetic Retinopathy Is Associated With Elevated Serum Asymmetric and Symmetric Dimethylarginines. Diabetes Care 2009, 32, 2084–2086. [Google Scholar] [CrossRef] [PubMed]
- Simão, S.; Costa, M.Â.; Sun, J.K.; Cunha-Vaz, J.; Simó, R.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Development of a Normative Database for Multifocal Electroretinography in the Context of a Multicenter Clinical Trial. Ophthalmic Res. 2017, 57, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.; Santos, A.R.B.M.; Nunes, S.; Lobo, C.; Cunha-Vaz, J. Subclinical Macular Edema as a Predictor of Progression to Clinically Significant Macular Edema in Type 2 Diabetes. Ophthalmol. 2013, 230, 201–206. [Google Scholar] [CrossRef]
- Wautier, M.P.; Massin, P.; Guillausseau, P.J.; Huijberts, M.; Levy, B.; Boulanger, E.; Laloi-Michelin, M.; Wautier, M. N(carboxymethyl)lysine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Metab. 2003, 29, 44–52. [Google Scholar] [CrossRef]
- Boehm, B.O.; Schilling, S.; Rösinger, S.; Lang, G.; Lang, G.; Kientsch-Engel, R.; Stahl, P. Elevated serum levels of N?-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia 2004, 47, 1376–1379. [Google Scholar] [CrossRef]
- Ghanem, A.; Elewa, A.; Arafa, L.F. Pentosidine and N-carboxymethyl-lysine: Biomarkers for type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2010, 21, 48–54. [Google Scholar] [CrossRef]
- Hirata, K.; Kubo, K. Relationship between blood levels of N-carboxymethyl-lysine and pentosidine and the severity of microangiopathy in type 2 diabetes. Endocr. J. 2004, 51, 537–544. [Google Scholar] [CrossRef]
- Grossin, N.; Wautier, M.-P.; Meas, T.; Guillausseau, P.J.; Massin, P.; Wautier, J. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab. 2008, 34, 392–395. [Google Scholar] [CrossRef]
- Choudhuri, S.; Dutta, D.; Sen, A.; Chowdhury, I.H.; Mitra, B.; Mondal, L.K.; Saha, A.; Bhadhuri, G.; Bhattacharya, B. Role of N–epsilon- carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol. Vis. 2013, 19, 100–113. [Google Scholar]
- Prasad, K. AGE–RAGE stress: A changing landscape in pathology and treatment of Alzheimer’s disease. Mol. Cell. Biochem. 2019, 459, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Perrotte, M.; Landri, S.; Lepage, A.; Fülöp, T.; Ramassamy, C. Circulating and Extracellular Vesicles Levels of N-(1-Carboxymethyl)-L-Lysine (CML) Differentiate Early to Moderate Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 69, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Masmiquel, L.L.; Burgos, R.; Mateo, C.; Marti, R.; Segura, R.M.; Simó, R. Effect of panretinal photocoagulation on serum levels of laminin in patients with diabetes: A prospective study. Br. J. Ophthalmol. 1999, 83, 1056–1059. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Sensitivity | Intra-assay coefficient | Inter-assay coefficient | |
|---|---|---|---|
| CML | 2.25 ng/mL | <10% | <12% |
| Lam-P1 | 5.00 ng/mL | <8% | <12% |
| ADMA | 4.34 ng/mL | <8% | <12% |
| Placebo | Brimonidine | Somatostatin | |
|---|---|---|---|
| n = 123 | n = 97 | n = 120 | |
| Age (years) | 62.4 ± 7.1 | 63.7 ± 6.0 | 62.6 ± 6.6 |
| Gender (% males) | 66.1 | 66.0 | 65.0 |
| BMI (Kg/m2) | 30.8 ± 5.6 | 30.8 ± 5.3 | 31.1 ± 5.4 |
| Diabetes duration (years) | 11.6 ± 5.8 | 11.1 ± 5.5 | 11.4 ± 5.5 |
| Diabetes treatment (%) | |||
| Diet | 4.8 | 2.1 | 4.2 |
| Oral agents | 65.3 | 76.3 | 73.3 |
| Oral agents + insulin | 24.2 | 21.6 | 20.8 |
| Insulin | 5.6 | 0.0 | 1.7 |
| HbA1C (%) | 7.21 ± 0.97 | 7.22 ± 1.09 | 7.11 ± 0.92 |
| Hypertension (%) | 71.0 | 73.2 | 71.7 |
| Dyslipidemia (%) | 69.4 | 67.0 | 67.5 |
| Microalbuminuria (%) | 19.3 | 22.7 | 19.1 |
| Cardiovascular disease (%) | 19.4 | 14.4 | 21.7 |
| ETDRS 10/20-35(%) | 42.7/57.3 | 38.1/61.9 | 43.3/56.7 |
| BCVA letter score | 85.9 ± 5.2 | 86.1 ± 5.2 | 85.7 ± 4.6 |
| ETDRS 10 | ETDRS 20-35 | ||
|---|---|---|---|
| n = 140 | n = 201 | p | |
| CML (log-transformed) | 2.35 ± 0.28 | 2.36 ± 0.26 | n.s. |
| Lam-P1 (log-transformed) | 2.46 ± 0.47 | 2.41 ± 0.47 | n.s. |
| ADMA (log-transformed) | 1.91 ± 0.27 | 1.90 ± 0.30 | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; Gibson, J.; et al. The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. J. Clin. Med. 2020, 9, 1233. https://doi.org/10.3390/jcm9041233
Hernández C, Porta M, Bandello F, Grauslund J, Harding SP, Aldington SJ, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, et al. The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Journal of Clinical Medicine. 2020; 9(4):1233. https://doi.org/10.3390/jcm9041233
Chicago/Turabian StyleHernández, Cristina, Massimo Porta, Francesco Bandello, Jakob Grauslund, Simon P. Harding, Stephen J. Aldington, Catherine Egan, Ulrik Frydkjaer-Olsen, José García-Arumí, Jonathan Gibson, and et al. 2020. "The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial" Journal of Clinical Medicine 9, no. 4: 1233. https://doi.org/10.3390/jcm9041233
APA StyleHernández, C., Porta, M., Bandello, F., Grauslund, J., Harding, S. P., Aldington, S. J., Egan, C., Frydkjaer-Olsen, U., García-Arumí, J., Gibson, J., Lang, G. E., Lattanzio, R., Massin, P., Midena, E., Ponsati, B., Ribeiro, L., Scanlon, P., Cunha-Vaz, J., & Simó, R., for the European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). (2020). The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Journal of Clinical Medicine, 9(4), 1233. https://doi.org/10.3390/jcm9041233







