Evaluation of the French National Program on Home Return of Patients with Chronic Heart Failure (PRADO-IC): Pilot Study of 91 Patients During Its Deployment in the Bas Rhin Area
Abstract
1. Introduction
2. Patients and Method
2.1. Objective
2.2. The PRADO IC Program
- (1)
- Initialization of medical monitoring, in order to respond to the patient’s request by allowing him or her to return home as soon as hospitalization is no longer necessary;
- (2)
- Supporting the patient and strengthening post-hospitalization follow-up;
- (3)
- The duration of PRADO-IC support varies according to severity as assessed by the New York Heart Association (NYHA) classification [10]. A patient in NYHA class I or II receives home support for 2 months. Patients in NYHA class III and IV HF receive home support for a maximum duration of 6 months (renewable for 2-month periods).
- (1)
- A consultation with the attending physician within 8 days after returning home and another consultation within 2 months;
- (2)
- A weekly monitoring and education visit by the nurse during 2 months for all patients, and a visit every 2 weeks for 4 months for patients in class III and IV;
- (3)
- A consultation during the 2nd month with the cardiologist [10].
2.3. Type of Study
- (1)
- The internal medicine, diabetes and metabolic diseases service of Medical Clinic B at Strasbourg University Hospitals (Hôpitaux Universitaires de Strasbourg [(HUS], Strasbourg, France);
- (2)
- The cardiology service of the New Civil Hospital through the functional unit dedicated to HF patients at HUS;
- (3)
- The cardiology service of Haguenau Hospital Center (Centre Hospitalier de Haguenau [CHH], Haguenau, France).
2.4. Study Population and Inclusion Criteria
2.5. Outcome Criteria
2.5.1. Main Outcome Criterion
2.5.2. Secondary Outcome Criteria
2.6. Data Collection
2.7. Administrative Documents
2.8. Statistical Analysis
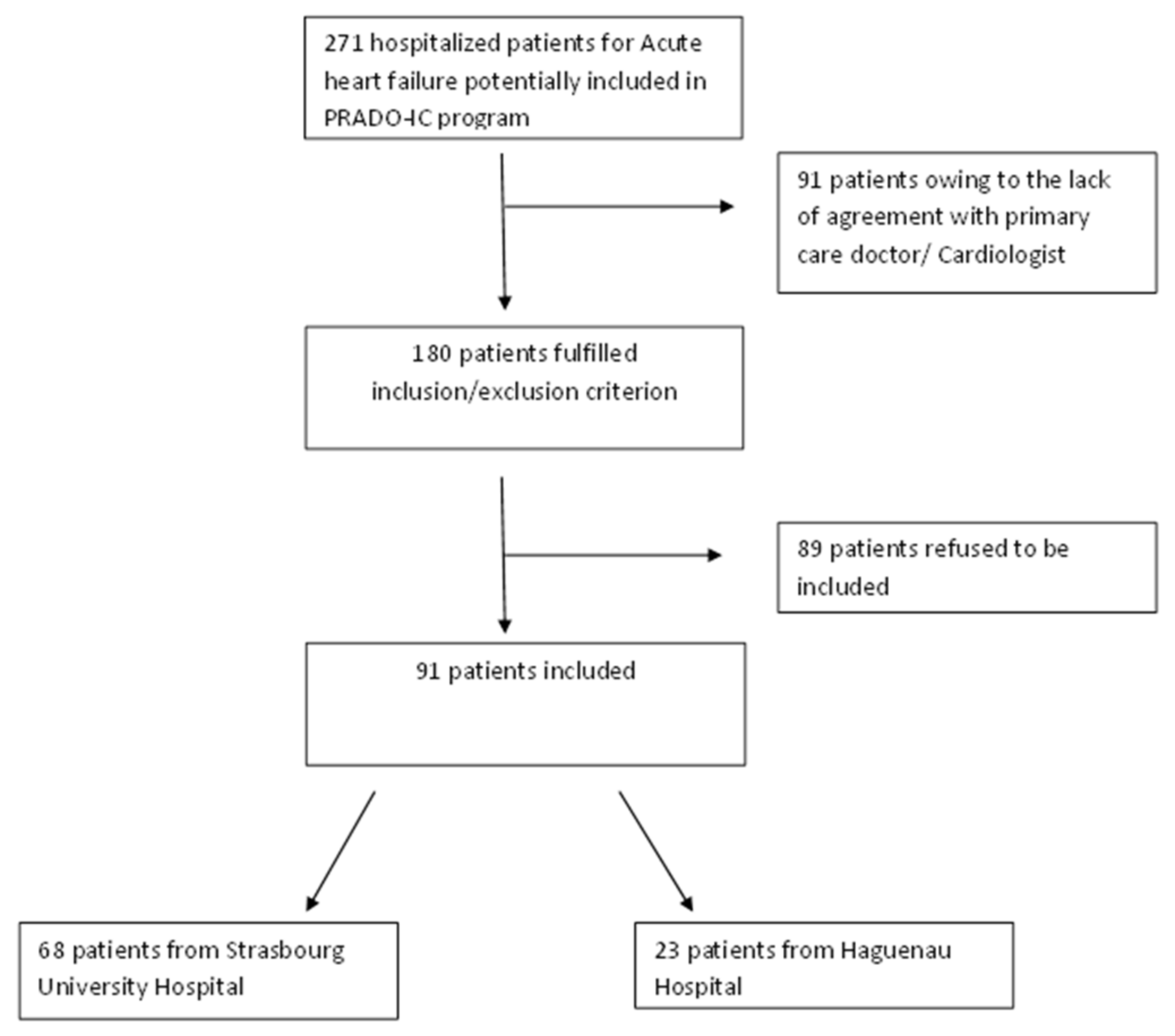
3. Results
3.1. Characteristics of the Population
3.1.1. Main Features
3.1.2. Data Concerning Heart Failure
3.1.3. Data Concerning Heart Failure Treatment
3.2. Outcomes Criteria
3.2.1. Main Outcome Criterion
3.2.2. Secondary Outcomes Criteria
4. Discussion
- (1)
- Anticipation and organization of patients returning home after hospitalization;
- (2)
- Establishment of a care pathway based on urban health professionals and their awareness;
- (3)
- At-least-weekly monitoring of patients, allowing early detection of the warning signs of heart decompensation;
- (4)
5. Conclusions
6. Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Peretti, C.; Pérel, C.; Tuppin, P.; Iliou, M.C.; Juillière, Y.; Gabet, A.; Olié, V.; S Danet, S.; Danchin, N. Prévalences et statut fonctionnel des cardiopathies ischémiques et de l’insuffisance cardiaque dans la population adulte en France: Apports des enquêtes déclaratives Handicap-Santé. Bull. Epidémiol. Hebd. 2014, 9–10, 172–181. [Google Scholar]
- Franzin-Garrec, M. Insuffisance cardiaque. Une maladie chronique en augmentation alarmante. Soins Rev. Ref. Infirm. 2013, 774, 25. [Google Scholar]
- Tuppin, P.; Cuerq, A.; de Peretti, C.; Fagot-Campagna, A.; Danchin, N.; Juillière, Y.; Alla, F.; Allemand, H.; Bauters, C.; Drici, M.D.; et al. First hospitalization for heart failure in France in 2009: Patient characteristics and 30-day follow-up. Arch. Cardiovasc. Dis. 2013, 106, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Pérel, C.; Chin, F.; Tuppin, P.; Danchin, N.; Alla, F.; Juilliere, Y.; de Peretti, C. Taux de patients hospitalisés pour insuffisance cardiaque en 2008 et évolutions en 2002–2008, France. BEH 2012, 41, 466–470. [Google Scholar]
- CNAM Caisse Nationale d’Assurance Maladie. Caractéristiques et trajet de soins des insuffisants cardiaques du Régime Général. Point de Repères 2012, 38, 1–14. [Google Scholar]
- Loi n° 2004-806 du 9 Août 2004 Relative à la Politique de Santé Publique. Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000787078 (accessed on 9 February 2020).
- Feltner, C.; Jones, C.D.; Cené, C.W.; Zheng, Z.-J.; Sueta, C.A.; Coker-Schwimmer, E.J.; Arvanitis, M.; Lohr, K.N.; Middleton, J.C.; Jonas, D.E. Transitional care interventions to prevent readmissions for people with heart failure. Ann. Intern Med. 2014, 160, 774–784. [Google Scholar] [CrossRef]
- Takeda, A.; Taylor, S.J.; Taylor, R.S.; Khan, F.; Krum, H.; Underwood, M. Clinical service organisation for heart failure. Cochrane Database Syst. Rev. 2012, 9, CD002752. [Google Scholar] [CrossRef]
- Vedel, I.; Khanassov, V. Transitional care for patients with congestive heart failure: A systematic review and meta-analysis. Ann. Fam. Med. 2015, 13, 562–571. [Google Scholar] [CrossRef]
- PRADO, le Programme de Retour à Domicile. Insuffisance Cardiaque. Available online: http://www.cpam-bordeaux.fr/prado/PRADO_IC_9et10avril2013-2.pdf (accessed on 16 April 2018).
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Holland, R.; Battersby, J.; Harvey, I.; Lenaghan, E.; Smith, J.; Hay, L. Systematic review of multidisciplinary interventions in heart failure. Heart Br. Card. Soc. 2005, 91, 899–906. [Google Scholar] [CrossRef]
- Institut de Veille Sanitaire. Maladies Cardio-Vasculaires. L’insuffisance Cardiaque. Available online: http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-chroniques-et-traumatismes/Maladies-cardio-neuro-vasculaires/L-insuffisance-cardiaque (accessed on 6 February 2018).
- McAlister, F.A.; Stewart, S.; Ferrua, S.; McMurray, J.J.J.V. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J. Am. Coll. Cardiol. 2004, 44, 810–819. [Google Scholar] [PubMed]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.; de Boer, R.A.; Drexel, H.; Ben, G.T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef] [PubMed]
- Juillière, Y.; Suty-Selton, C.; Riant, E.; Darracq, J.-P.; Dellinger, A.; Labarre, J.-P.; Druelle, J.; Mulak, G.; Danchin, N.; Jourdain, P. Prescription of cardiovascular drugs in the French ODIN cohort of heart failure patients according to age and type of chronic heart failure. Arch. Cardiovasc. Dis. 2014, 107, 21–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zannad, F.; Mebazaa, A.; Juillière, Y.; Cohen-Solal, A.; Guize, L.; Alla, F.; Rougé, P.; Blin, P.; Barlet, M.H.; Paolozzi, L.; et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur. J. Heart Fail. 2006, 8, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé. Guide du Parcours de Soins. Insuffisance Cardiaque; HAS: Saint-Denis La Plaine, France, 2014; Available online: https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-04/guide_parcours_de_soins_ic_web.pdf (accessed on 5 February 2018).
- Hughes, C.M. Medication non-adherence in the elderly: How big is the problem? Drugs Aging 2004, 21, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, M.H.L.; Jaarsma, T. Adherence in heart failure in the elderly: Problem and possible solutions. Int. J. Cardiol. 2008, 125, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Juillière, Y.; Jourdain, P.; Suty-Selton, C.; Béard, T.; Berder, V.; Maître, B.; Trochu, J.N.; Drouet, E.; Pace, B.; Mulak, G.; et al. Therapeutic patient education and all-cause mortality in patients with chronic heart failure: A propensity analysis. Int. J. Cardiol. 2013, 168, 388–395. [Google Scholar] [CrossRef]
- Gonseth, J.; Guallar-Castillón, P.; Banegas, J.R.; Rodríguez-Artalejo, F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: A systematic review and meta-analysis of published reports. Eur. Heart J. 2004, 25, 1570–1595. [Google Scholar] [CrossRef]
- Jourdain, P.; Juillière, Y.; Steering and Working Group Committee Members of the French Task Force on Therapeutic Education in Heart Failure. Therapeutic education in patients with chronic heart failure: Proposal for a multiprofessional structured programme, by a French Task Force under the auspices of the French Society of Cardiology. Arch. Cardiovasc. Dis. 2011, 104, 189–201. [Google Scholar]
- Hernandez, A.F.; Greiner, M.A.; Fonarow, G.C.; Hammill, B.G.; Heidenreich, P.A.; Yancy, C.W.; Peterson, E.D.; Curtis, L.H. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010, 303, 1716–1722. [Google Scholar] [CrossRef]
- Nyweide, D.J.; Anthony, D.L.; Bynum, J.P.W.; Strawderman, R.L.; Weeks, W.B.; Casalino, L.P.; Fisher, E.S. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013, 173, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- ICARLIM. Présentation du Réseau—Santé-Limousin v.2. Available online: http://www.sante-limousin.fr/professionnels/reseaux-de-sante/icarlim/presentation (accessed on 9 November 2018).
- Le Reseau D’aide a Domicile de L’insuffisance Cardiaque (RADIC). Available online: http://2.chu-poitiers.fr/16e66b26-b174-4dc5-bd80-e5f6e8a8d553.aspx (accessed on 9 November 2018).
- RESIC38. RESIC38 Home Page. Available online: http://www.resic38.org/ (accessed on 9 November 2018).
- ICALOR. ICALOR Home Page. Available online: http://www.icalor.org/ (accessed on 9 November 2018).
- RESICARD. RESICARD Home Page. Available online: http://www.resicard.com/ (accessed on 9 November 2018).
- Andrès, E.; Talha, S.; Zulfiqar, A.A.; Hajjam, M.; Ervé, S.; Hajjam, J.; Gény, B.; Hajjam El Hassani, A. Current researches and new perspectives of telemedicine in chronic heart failure. J. Clin. Med. 2018, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Gabet, A.; Lamarche-Vadel, A.; Chin, F.; Juillière, Y.; de Peretti, C.; Olié, V. Mortalité due à l’insuffisance cardiaque en France, évolutions 2000–2010. Bull. Epidémiol. Hebd. 2014, 21–22, 386–394. [Google Scholar]
- Tuppin, P.; Cuerq, A.; de Peretti, C.; Fagot-Campagna, A.; Danchin, N.; Juillière, Y.; Alla, F.; Allemand, H.; Bauters, C.; Drici, M.D.; et al. Two-year outcome of patients after a first hospitalization for heart failure: A national observational study. Arch. Cardiovasc. Dis. 2014, 107, 158–168. [Google Scholar] [CrossRef]
- Jourdain, P.; Baudouin, C.; Groshens, S.; Gueneau, P.; Rousseau, M.; Saura, M.; Hryschyschyn, N.; Dagorn, J.; Funck, F. Analyse médico-économique de l’impact d’une prise en charge de l’insuffisance cardiaque chronique en unité thérapeutique d’insuffisance cardiaque versus prise en charge habituelle de ville. Ann. Cardiol. Angéiologie. 2015, 64, 318–324. [Google Scholar] [CrossRef]
| Variable | n = 91 | % |
|---|---|---|
| Medical antecedents | ||
| High blood pressure | 84 | 92.3 |
| Dyslipidemia | 72 | 79.1 |
| Atrial Fibrillation | 57 | 62.6 |
| Chronic renal failure | 56 | 61.5 |
| Coronary disease | 43 | 47.3 |
| Diabetes | 41 | 45.1 |
| COPD | 31 | 34.1 |
| Obesity | 23 | 25.3 |
| Proven vascular disease | 23 | 25.3 |
| Pacemaker | 19 | 20.9 |
| Cancer in progression (>5 years) | 14 | 15.4 |
| Automatic defibrillator | 12 | 13.2 |
| NYHA Classification | ||
| NYHA class II | 48 | 52.7 |
| NYHA class III | 34 | 37.4 |
| NYHA class IV | 9 | 9.9 |
| Heart failure etiology | ||
| Ischemic heart disease | 41 | 45.1 |
| Hypertension | 13 | 14.3 |
| Valvular heart disease | 10 | 11 |
| Arrhythmia | 12 | 13.2 |
| Toxic | 8 | 8.8 |
| Primary | 2 | 2.2 |
| Unknown etiology | 5 | 5.5 |
| LVEF | ||
| <30% | 35 | 38.5 |
| 35–50% | 30 | 32.9 |
| >50% | 26 | 27.5 |
| Precipitating factor | ||
| Chronic anemia | 54 | 59.3 |
| Infection | 21 | 23.1 |
| Arrhythmia | 17 | 18.7 |
| Acute myocardial ischemia | 9 | 9.9 |
| Inappropriate sodium diet | 9 | 9.9 |
| Acute renal failure | 8 | 8.8 |
| Uncontrolled hypertension | 8 | 8.8 |
| Non-compliance with therapy | 7 | 7.7 |
| Acute anemia | 4 | 4.4 |
| No identified | 8 | 8.8 |
| Treatment | n = 91% | |
|---|---|---|
| Loop diuretic | 82 | 90.1 |
| β-blocker | 77 | 84.6 |
| ACEI | 52 | 57.1 |
| ARABs | 21 | 23.1 |
| Aldosterone Antagonists | 38 | 41.8 |
| ARABsI+ β-blocker | 63 | 69.2 |
| ARABs+ β-blocker+ Aldosterone Antagonists | 30 | 32.9 |
| Amiodarone | 25 | 27.5 |
| Dihydropyridine | 14 | 15.4 |
| Digitalis | 11 | 12.1 |
| Nitrates | 9 | 9.9 |
| Ivabradine | 7 | 8.7 |
| Antiplatelet | 56 | 61.5 |
| Anticoagulant | 55 | 60.4 |
| Length | Hospitalizations for Heart Failure | Other causes of Hospitalizations | ||||
|---|---|---|---|---|---|---|
| Before Inclusion | After Inclusion | P | Before Inclusion | After Inclusion | P | |
| 30 days | 0.18 (0.42) | 0.15 (0.36) | 0.56 | 0.16 (0.37) | 0.02 (0.15) | <0.001 |
| 6 months | 0.98 (1.04) | 0.53 (0.81) | <0,001 | 0.52 (0.72) | 0.28 (0.59) | 0.006 |
| 1 year | 1.04 (1.14) | 1.04 (1.05) | <0.001 | 0.92 (1.04) | 0.48 (0.78) | 0.002 |
| Length | Readmissions other than HF Decompensation | ||
|---|---|---|---|
| Before Inclusion | After Inclusion | p | |
| 30 days | 0.02 ± 0.15 | 0.16 ± (−0.37) | <0.001 |
| 6 months | 0.52 ± 0.72 | 0.28 ± 0.59 | 0.006 |
| 1 year | 0.92 ± 1.04 | 0.48 ± 0.78 | 0.002 |
| Length of hospitalization(days) for HF | |||
| Before Inclusion | After Inclusion | p | |
| 30 days | 11.67 ± 4.34 | 9.77 ± 3.92 | 0.31 |
| 6 months | 18.02 ± 7.78 | 14.28 ± 11.57 | 0.006 |
| 1 year | 22,07 ± 10.33 | 16.39 ± 15.94 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radreau, M.; Lorenzo-Villalba, N.; Talha, S.; Von Hunolstein, J.-J.; Hanssen, M.; Koenig, A.; Couppie, P.; Geny, B.; Severac, F.; Roul, G.; et al. Evaluation of the French National Program on Home Return of Patients with Chronic Heart Failure (PRADO-IC): Pilot Study of 91 Patients During Its Deployment in the Bas Rhin Area. J. Clin. Med. 2020, 9, 1222. https://doi.org/10.3390/jcm9041222
Radreau M, Lorenzo-Villalba N, Talha S, Von Hunolstein J-J, Hanssen M, Koenig A, Couppie P, Geny B, Severac F, Roul G, et al. Evaluation of the French National Program on Home Return of Patients with Chronic Heart Failure (PRADO-IC): Pilot Study of 91 Patients During Its Deployment in the Bas Rhin Area. Journal of Clinical Medicine. 2020; 9(4):1222. https://doi.org/10.3390/jcm9041222
Chicago/Turabian StyleRadreau, Mylène, Noel Lorenzo-Villalba, Samy Talha, Jean-Jacques Von Hunolstein, Michel Hanssen, Anne Koenig, Philippe Couppie, Bernard Geny, Francois Severac, Gérald Roul, and et al. 2020. "Evaluation of the French National Program on Home Return of Patients with Chronic Heart Failure (PRADO-IC): Pilot Study of 91 Patients During Its Deployment in the Bas Rhin Area" Journal of Clinical Medicine 9, no. 4: 1222. https://doi.org/10.3390/jcm9041222
APA StyleRadreau, M., Lorenzo-Villalba, N., Talha, S., Von Hunolstein, J.-J., Hanssen, M., Koenig, A., Couppie, P., Geny, B., Severac, F., Roul, G., Zulfiqar, A.-A., & Andrès, E. (2020). Evaluation of the French National Program on Home Return of Patients with Chronic Heart Failure (PRADO-IC): Pilot Study of 91 Patients During Its Deployment in the Bas Rhin Area. Journal of Clinical Medicine, 9(4), 1222. https://doi.org/10.3390/jcm9041222






