Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives
Abstract
1. Introduction
2. Scope and Methods
3. Placental Lactogen in Animal Models (In Vitro and In Vivo Studies)
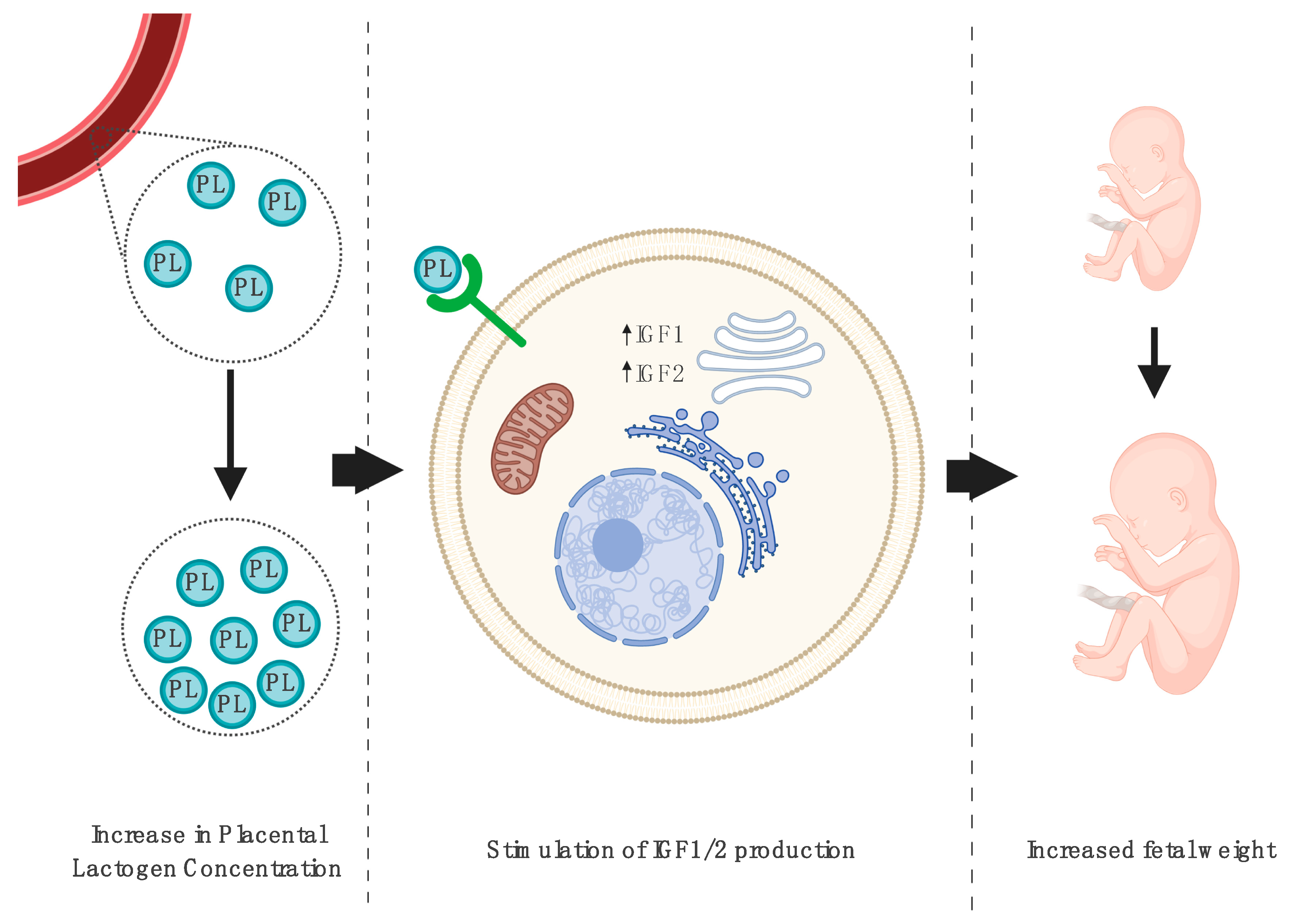
3.1. Impact on Fetal Growth
3.2. Placental Lactogen and Metabolic Changes
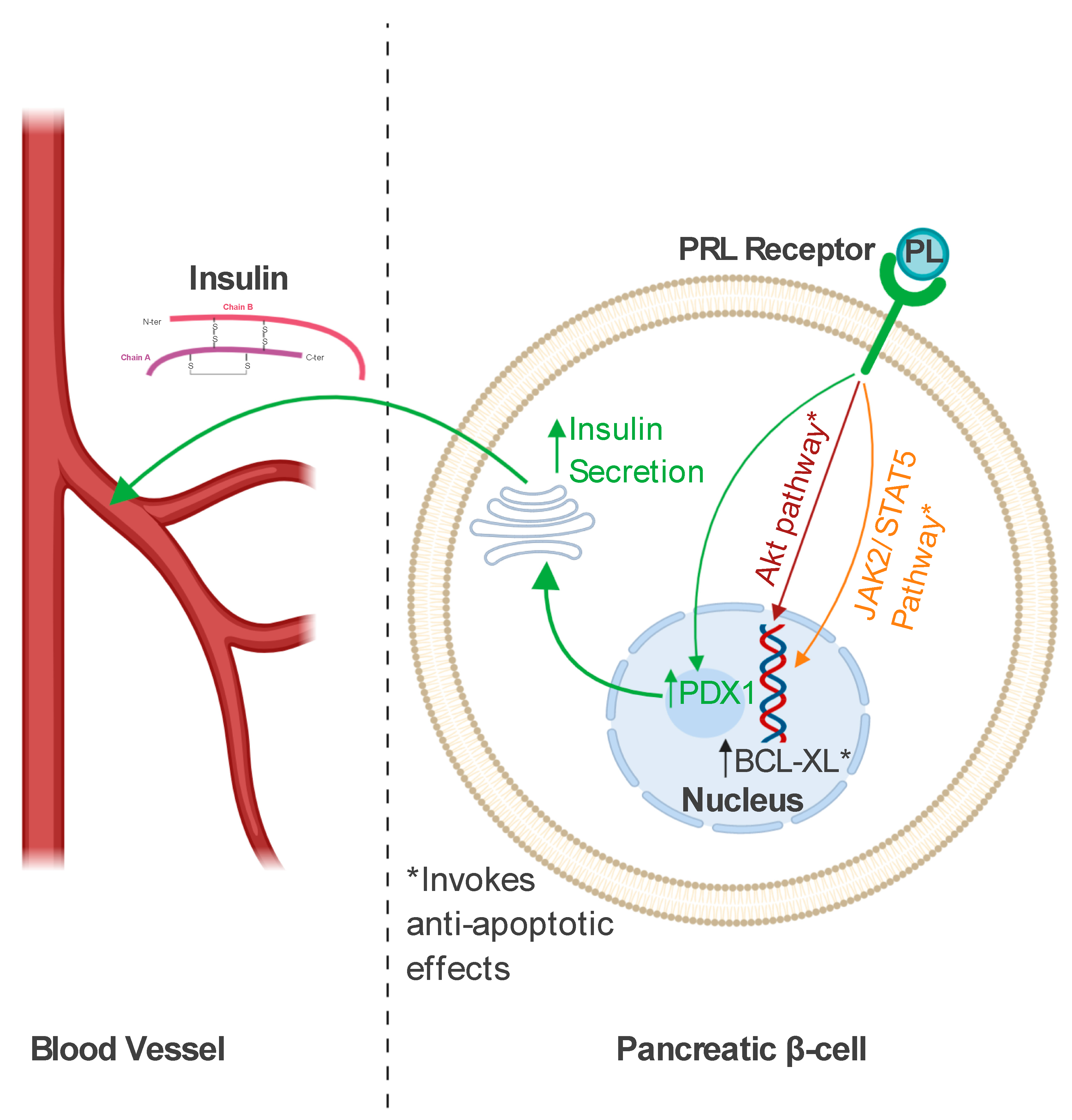
3.3. Role in Pancreatic Beta Cells
4. Clinical Utilities of Placental Lactogen
4.1. Maternal Applications: In Vitro and In Vivo Studies
4.1.1. General Information
4.1.2. Maternal Obesity and Food Intake
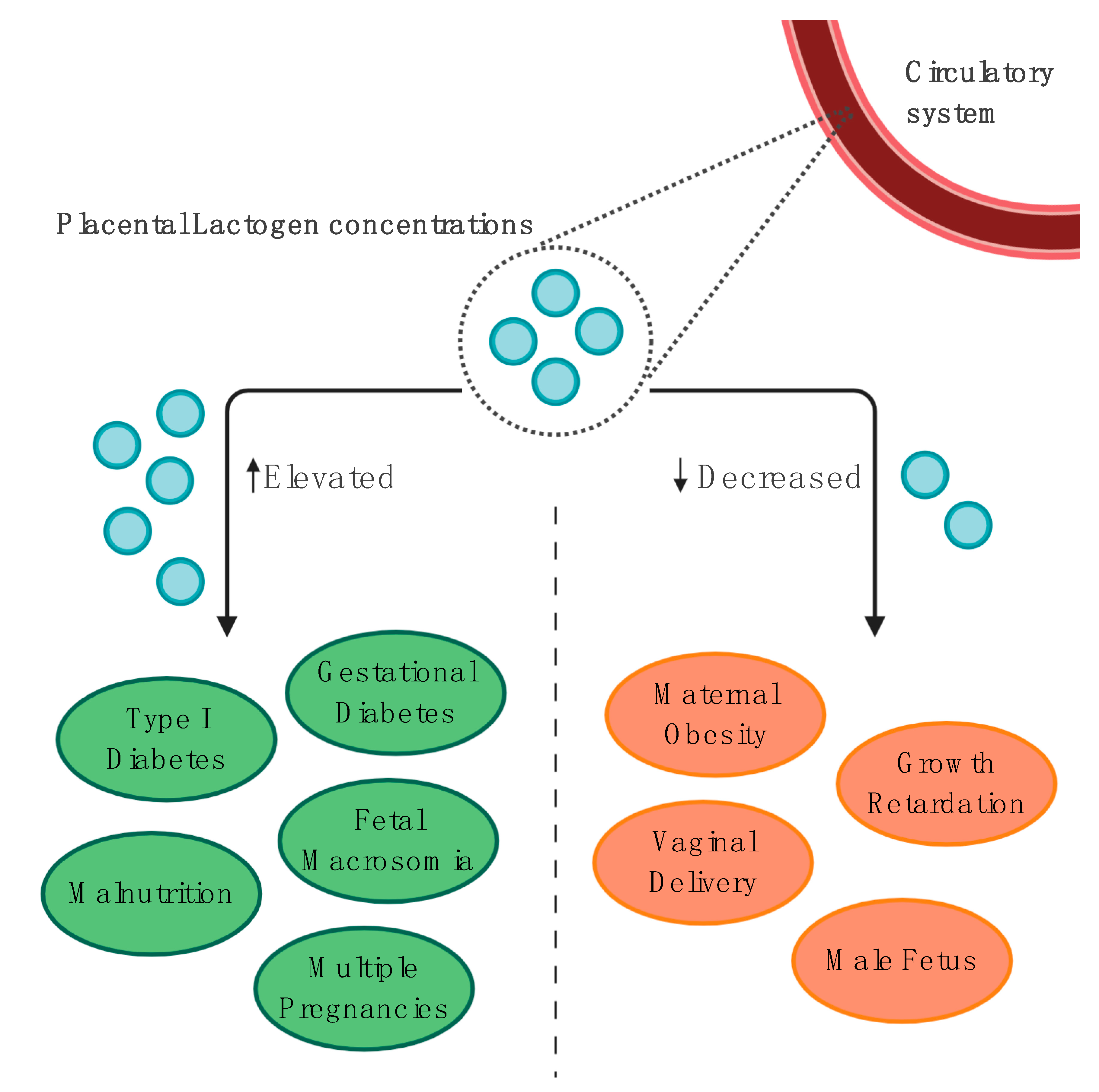
4.1.3. Gestational and Pregestational Diabetes Mellitus
Molecular Aspects
Placental Lactogen Concentrations throughout Pregnancy
Placental Lactogen as a GDM Screening Tool
Diabetes Complications
Long-Term Implications
4.2. Fetal Growth and Perinatal Outcomes
4.2.1. Association with Fetal Growth
4.2.2. Human Placental Lactogen as a Gestational Marker of Fetal Development
4.2.3. How Could Maternal Malnutrition Affect Fetal Development and PL Secretion?
4.2.4. Additional Contributions to the Regulation of Fetal Growth
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017, 288, 1–8. [Google Scholar]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356. [Google Scholar] [CrossRef]
- Huda, S.S.; Brodie, L.E.; Sattar, N. Obesity in pregnancy: Prevalence and metabolic consequences. Semin. Fetal Neonatal Med. 2010, 15, 70–76. [Google Scholar] [CrossRef]
- Vasudevan, C.; Renfrew, M.; McGuire, W. Fetal and perinatal consequences of maternal obesity. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F378–F382. [Google Scholar] [CrossRef]
- Garne, E.; Loane, M.; Dolk, H.; Barisic, I.; Addor, M.C.; Arriola, L.; Bakker, M.; Calzolari, E.; Matias Dias, C.; Doray, B.; et al. Spectrum of congenital anomalies in pregnancies with pregestational diabetes. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 134–140. [Google Scholar] [CrossRef]
- Ali, S.; Dornhorst, A. Diabetes in pregnancy: Health risks and management. Postgrad. Med. J. 2011, 87, 417–427. [Google Scholar] [CrossRef]
- Vambergue, A.; Fajardy, I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J. Diabetes 2011, 2, 196. [Google Scholar] [CrossRef] [PubMed]
- Wender-Ozegowska, E.; Bomba-Opoń, D.; Brazert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef]
- Bernasko, J. Intensive insulin therapy in pregnancy: Strategies for successful implementation in pregestational diabetes mellitus. J. Matern. Neonatal Med. 2007, 20, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Eberhardt, N.L. Structure and evolution of the growth hormone gene family. Endocr. Rev. 1983, 4, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J. Placental control of metabolic adaptations in the mother for an optimal pregnancy outcome. What goes wrong in gestational diabetes? Placenta 2018, 69, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Faria, T.N.; Deb, S.; Kwok, S.C.M.; Talamantes, F.; Soares, M.J. Ontogeny of placental lactogen-I and placental lactogen-II expression in the developing rat placenta. Dev. Biol. 1990, 141, 279–291. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ogren, L.; Endo, H.; Thordarson, G.; Bigsby, R.M.; Talamantes, F. Production of mouse placental lactogen-I and placental lactogen-II by the same giant cell. Endocrinology 1992, 131, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.C.; Bolifraud, P.; Durieux, D.; Pauloin, A.; Vidaud, M.; Kann, G. Placental growth hormone and lactogen production by perifused ovine placental explants: Regulation by growth hormone-releasing hormone and glucose. Biol. Reprod. 2002, 66, 555–561. [Google Scholar] [CrossRef][Green Version]
- Alvarez-Oxiley, A.V.; de Sousa, N.M.; Beckers, J.-F. Native and recombinant bovine placental lactogens. Reprod. Biol. 2008, 8, 85–106. [Google Scholar] [CrossRef]
- Walker, W.H.; Fitzpatrick, S.L.; Saunders, G.F.; Barrera-Saldaña, H.A.; Resendez-Perez, D. The human placental lactogen genes: Structure, function, evolution and transcriptional regulation. Endocr. Rev. 1991, 12, 316–328. [Google Scholar] [CrossRef]
- Handwerger, S. Clinical counterpoint: The physiology of placental lactogen in human pregnancy. Endocr. Rev. 1991, 12, 329–336. [Google Scholar] [CrossRef]
- Untergasser, G.; Hermann, M.; Rumpold, H.; Pfister, G.; Berger, P. An unusual member of the human growth hormone/placental lactogen (GH/PL) family, the testicular alternative splicing variant hPL-A2: Recombinant expression revealed a membrane-associated growth factor molecule. Mol. Cell. Endocrinol. 2000, 167, 117–125. [Google Scholar] [CrossRef]
- Handwerger, S.; Freemark, M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J. Pediatr. Endocrinol. Metab. 2000, 13, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Freemark, M.; Strain, A.J.; Handwerger, S.; Milner, R.D.G. Placental lactogen and growth hormone receptors in human fetal tissues: Relationship to fetal plasma human placental lactogen concentrations and fetal growth. J. Clin. Endocrinol. Metab. 1988, 66, 1283–1290. [Google Scholar] [CrossRef]
- Colosi, P.; Talamantes, F.; Linzer, D.I.H. Molecular cloning and expression of mouse placental lactogen i complementary deoxyribonucleic acid. Mol. Endocrinol. 1987, 1, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Colosi, P.; Marr, G.; Lopez, J.; Haro, L.; Ogren, L.; Talamantes, F. Isolation, purification, and characterization of mouse placental lactogen. Proc. Natl. Acad. Sci. USA 1982, 79, 771–775. [Google Scholar] [CrossRef]
- Harigaya, T.; Smith, W.C.; Talamantes, F. Hepatic placental lactogen receptors during pregnancy in the mouse. Endocrinology 1988, 122, 1366–1372. [Google Scholar] [CrossRef]
- Kastrup, K.W.; Andersen, H.J.; Lebech, P. Somatomedin in newborns and the relationship to human chorionic somatotropin and fetal growth. Acta Pædiatrica 1978, 67, 757–762. [Google Scholar] [CrossRef]
- Newbern, D.; Freemark, M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 409–416. [Google Scholar] [CrossRef]
- Ryan, E.A.; Enns, L. Role of gestational hormones in the induction of insulin resistance. J. Clin. Endocrinol. Metab. 1988, 67, 341–347. [Google Scholar] [CrossRef]
- Barbour, L.A.; Shao, J.; Qiao, L.; Pulawa, L.K.; Jensen, D.R.; Bartke, A.; Garrity, M.; Draznin, B.; Friedman, J.E. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am. J. Obstet. Gynecol. 2002, 186, 512–517. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Augustine, R.A.; Grattan, D.R. Hormone interactions regulating energy balance during pregnancy. J. Neuroendocrinol. 2010, 22, 805–817. [Google Scholar] [CrossRef]
- Baker, C.M.; Goetzmann, L.N.; Cantlon, J.D.; Jeckel, K.M.; Winger, Q.A.; Anthony, R.V. Development of ovine chorionic somatomammotropin hormone-deficient pregnancies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R837–R846. [Google Scholar] [CrossRef] [PubMed]
- Jeckel, K.M.; Boyarko, A.C.; Bouma, G.J.; Winger, Q.A.; Anthony, R.V. Chorionic somatomammotropin impacts early fetal growth and placental gene expression. J. Endocrinol. 2018, 237, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.K.; Layfield, R.; Pratten, M.K. Growth promoting effects of human placental lactogen during early organogenesis: A link to insulin-like growth factors. J. Anat. 2001, 198, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, D.; Oden, J.; Kelly, P.A.; Mohan, S.; Alliouachene, S.; Pende, M.; Wentz, S.; Kerr, J.; Freemark, M. Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: Studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology 2005, 146, 103–112. [Google Scholar] [CrossRef][Green Version]
- Arumugam, R.; Fleenor, D.; Freemark, M. Effects of lactogen resistance and GH deficiency on mouse metabolism: Pancreatic hormones, adipocytokines, and expression of adiponectin and insulin receptors: Lactogen resistance and GH deficiency in mice. Endocrine 2007, 32, 182–191. [Google Scholar] [CrossRef]
- Fielder, P.J.; Talamantes, F. The insulin-like effects of mouse growth hormone on adipose tissue from virgin and pregnant mice. Metab. Clin. Exp. 1992, 41, 415–419. [Google Scholar] [CrossRef]
- Leturque, A.; Hauguel, S.; Sutter Dub, M.T.; Maulard, P.; Girard, J. Effects of placental lactogen and progesterone on insulin stimulated glucose metabolism in rat muscles in vitro. Diabete Metab. 1989, 15, 176–181. [Google Scholar]
- Houseknecht, K.L.; Bauman, D.E.; Vernon, R.G.; Byatt, J.C.; Collier, R.J. Insulin-like growth factors-I and -II, somatotropin, prolactin, and placental lactogen are not acute effectors of lipolysis in ruminants. Domest. Anim. Endocrinol. 1996, 13, 239–249. [Google Scholar] [CrossRef]
- Campbell, R.M.; Kostyo, J.L.; Scanes, C.G. Lipolytic and antilipolytic effects of human growth hormone, its 20-kilodalton variant, a reduced and carboxymethylated derivative, and human placental lactogen on chicken adipose tissue in vitro. Proc. Soc. Exp. Biol. Med. 1990, 193, 269–273. [Google Scholar] [CrossRef]
- Chen, H.; Kleinberger, J.W.; Takane, K.K.; Salim, F.; Fiaschi-Taesch, N.; Pappas, K.; Parsons, R.; Jiang, J.; Zhang, Y.; Liu, H.; et al. Augmented STAT5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells. Diabetes 2015, 64, 3784–3797. [Google Scholar] [CrossRef]
- Banerjee, R.R.; Cyphert, H.A.; Walker, E.M.; Chakravarthy, H.; Peiris, H.; Gu, X.; Liu, Y.; Conrad, E.; Goodrich, L.; Stein, R.W.; et al. Gestational diabetes mellitus from inactivation of prolactin receptor and MafB in islet β-cells. Diabetes 2016, 65, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Nalla, A.; Ringholm, L.; Søstrup, B.; Højrup, P.; Thim, L.; Levery, S.B.; Vakhrushev, S.Y.; Billestrup, N.; Mathiesen, E.R.; Damm, P.; et al. Implications for the offspring of circulating factors involved in beta cell adaptation in pregnancy. Acta Obstet. Gynecol. Scand. 2014, 93, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.F.; De Angelis, F.; Bova, L.; Bartolini, B.; Bertuzzi, F.; Nano, R.; Capuani, B.; Lauro, R.; Federici, M.; Lauro, D.; et al. Human placental lactogen (hPL-A) activates signaling pathways linked to cell survival and improves insulin secretion in human pancreatic islets. Islets 2011, 3, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Offield, M.F.; Jetton, T.L.; Labosky, P.A.; Ray, M.; Stein, R.W.; Magnuson, M.A.; Hogan, B.L.M.; Wright, C.V.E. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996, 122, 983–995. [Google Scholar] [PubMed]
- Kondegowda, N.G.; Mozar, A.; Chin, C.; Otero, A.; Garcia-Ocaña, A.; Vasavada, R.C. Lactogens protect rodent and human beta cells against glucolipotoxicity- induced cell death through Janus kinase-2 (JAK2)/signal transducer and activator of transcription-5 (STAT5) signalling. Diabetologia 2012, 55, 1721–1732. [Google Scholar] [CrossRef]
- Fujinaka, Y.; Takane, K.; Yamashita, H.; Vasavada, R.C. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J. Biol. Chem. 2007, 282, 30707–30717. [Google Scholar] [CrossRef]
- Linnemann, K.; Malek, A.; Sager, R.; Blum, W.F.; Schneider, H.; Fusch, C. Leptin production and release in the dually in vitro perfused human placenta. J. Clin. Endocrinol. Metab. 2000, 85, 4298–4301. [Google Scholar]
- Sarandakou, A.; Kassanos, D.; Phocas, I.; Kontoravdis, A.; Chryssicopoulos, A.; Zourlas, P.A. Amniotic fluid hormone profiles during normal and abnormal pregnancy. Clin. Exp. Obs. Gynecol. 1992, 19, 180–188. [Google Scholar]
- Hercz, P.; Siklos, P.; Ungár, L.; Farquharson, R.G.; Mohári, K.; Kocsár, L. Change of serum HPL level in maternal vein, umbilical cord vein and artery in mature and premature labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 1987, 24, 189–193. [Google Scholar] [CrossRef]
- Lebech, P.E.; Borggaard, B. Serum levels of human chorionic somatomammotropin (HCS) in normal and abnormal pregnancies. Acta Endocrinol. 1974, 77 (Suppl. S3), S35–S43. [Google Scholar] [CrossRef]
- Jin, Y.; Vakili, H.; Yan Liu, S.; Menticoglou, S.; Bock, M.E.; Cattini, P.A. Chromosomal architecture and placental expression of the human growth hormone gene family are targeted by pre-pregnancy maternal obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Vakili, H.; Jin, Y.; Menticoglou, S.; Cattini, P.A. CCAAT-enhancer-binding protein β (C/EBPβ) and downstream human placental growth hormone genes are targets for dysregulation in pregnancies complicated by maternal obesity. J. Biol. Chem. 2013, 288, 22849–22861. [Google Scholar] [CrossRef]
- Williams, C.; Coltart, T.M. Adipose tissue metabolism in pregnancy: The lipolytic effect of human placental lactogen. BJOG Int. J. Obstet. Gynaecol. 1978, 85, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Toro, A.; Vilariño-García, T.; Maymó, J.; Guadix, P.; Dueñas, J.L.; Fernández-Sánchez, M.; Varone, C.; Sánchez-Margalet, V. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2018, 22, 716–727. [Google Scholar] [CrossRef]
- Tessier, D.R.; Ferraro, Z.M.; Gruslin, A. Role of leptin in pregnancy: Consequences of maternal obesity. Placenta 2013, 34, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Page-Wilson, G.; Reitman-Ivashkov, E.; Meece, K.; White, A.; Rosenbaum, M.; Smiley, R.M.; Wardlaw, S.L. Cerebrospinal fluid levels of leptin, proopiomelanocortin, and agouti-related protein in human pregnancy: Evidence for leptin resistance. J. Clin. Endocrinol. Metab. 2013, 98, 264–271. [Google Scholar] [CrossRef]
- Coya, R.; Martul, P.; Algorta, J.; Aniel-Quiroga, M.A.; Busturia, M.A.; Señarís, R. Progesterone and human placental lactogen inhibit leptin secretion on cultured trophoblast cells from human placentas at term. Gynecol. Endocrinol. 2005, 21, 27–32. [Google Scholar] [CrossRef]
- Coya, R.; Martul, P.; Algorta, J.; Aniel-Quiroga, M.A.; Busturia, M.A.; Señarís, R. Effect of leptin on the regulation of placental hormone secretion in cultured human placental cells. Gynecol. Endocrinol. 2006, 22, 620–626. [Google Scholar] [CrossRef]
- Donadel, G.; Pastore, D.; Della-Morte, D.; Capuani, B.; Lombardo, M.F.; Pacifici, F.; Bugliani, M.; Grieco, F.A.; Marchetti, P.; Lauro, D. FGF-2b and h-PL transform duct and non-endocrine human pancreatic cells into endocrine insulin secreting cells by modulating differentiating genes. Int. J. Mol. Sci. 2017, 18, 2234. [Google Scholar] [CrossRef]
- Le, T.N.; Elsea, S.H.; Romero, R.; Chaiworapongsa, T.; Francis, G.L. Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet. Test. Mol. Biomarkers 2013, 17, 567–571. [Google Scholar] [CrossRef]
- Ngala, R.A.; Fondjo, L.A.; Gmagna, P.; Ghartey, F.N.; Awe, M.A. Placental peptides metabolism and maternal factors as predictors of risk of gestational diabetes in pregnant women. A case-control study. PLoS ONE 2017, 12, e0181613. [Google Scholar] [CrossRef]
- Lolis, D.; Tzingounis, V.; Kaskarelis, D. Maternal serum and amniotic fluid levels of human placental lactogen in gestational diabetes. Eur. J. Clin. Invest. 1978, 8, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Ye, C.; Kramer, C.K.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B. Evaluation of circulating determinants of beta-cell function in women with and without gestational diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.C.; Gyves, M.T.; Ilan, J. Comparisons of human placental lactogen mRNA levels from placentas of diabetics and normal term. Mol. Cell. Endocrinol. 1985, 39, 61–69. [Google Scholar] [CrossRef]
- Soler, N.G.; Nicholson, H.O.; Malins, J.M. Serial determinations of human placental lactogen in the management of diabetic pregnancy. Lancet 1975, 306, 54–57. [Google Scholar] [CrossRef]
- Ursell, W.; Brudenell, M.; Chard, T. Placental Lactogen Levels in Diabetic Pregnancy. Br. Med. J. 1973, 2, 80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muralimanoharan, S.; Maloyan, A.; Myatt, L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: Role of miR-143. Clin. Sci. 2016, 130, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Botta, R.M.; Donatelli, M.; Bucalo, M.L.; Bellomonte, M.L.; Bompiani, G.D. Placental lactogen, progesterone, total estriol and prolactin plasma levels in pregnant women with insulin-dependent diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 1984, 16, 393–401. [Google Scholar] [CrossRef]
- Luthman, M.; Stock, S.; Werner, S.; Bremme, K. Growth hormone-binding protein in plasma is inverselycorrelated to placental lactogen and augmented with increasing body mass index in healthy pregnant women and women with gestational diabetes mellitus. Gynecol. Obstet. Invest. 1994, 38, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinoza, I.; Smith, R.F.; Gillmer, M.; Schidlmeir, A.; Hockaday, T.D. High levels of growth hormone and human placental lactogen in pregnancy complicated by diabetes. Diabetes Res. 1986, 3, 119–125. [Google Scholar]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetesd2019. Diabetes Care 2019, 41 (Suppl. S1), S13–S27. [Google Scholar]
- Henderson, C.E.; Divon, M.Y. Combining human placental lactogen with routine glucose challenge tests. Prim. Care Update Ob. Gyns. 1998, 5, 189–190. [Google Scholar] [CrossRef]
- Daskalakis, G.; Marinopoulos, S.; Krielesi, V.; Papapanagiotou, A.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Placental pathology in women with gestational diabetes. Acta Obstet. Gynecol. Scand. 2008, 87, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W. Distal villous immaturity. Diagnostic Histopathol. 2012, 18, 189–194. [Google Scholar] [CrossRef]
- Greco, M.A.; Kamat, B.R.; Demopoulos, R.I. Placental protein distribution in maternal diabetes mellitus: An immunocytochemical study. Fetal Pediatr. Pathol. 1989, 9, 679–690. [Google Scholar] [CrossRef]
- Persson, B.; Hansson, U. Hypoglycaemia in pregnancy. Baillieres. Clin. Endocrinol. Metab. 1993, 7, 731–739. [Google Scholar] [CrossRef]
- Björklund, A.O.; Adamson, U.K.C.; Carlström, K.A.M.; Hennen, G.; Igout, A.; Lins, P.E.S.; Westgren, L.M.R. Placental hormones during induced hypoglycaemia in pregnant women with insulin-dependent diabetes mellitus: Evidence of an active role for placenta in hormonal counter-regulation. BJOG Int. J. Obstet. Gynaecol. 1998, 105, 649–655. [Google Scholar] [CrossRef]
- Larinkari, J.; Laatikainen, L.; Ranta, T.; Mörönen, P.; Pesonen, K.; Laatikainen, T. Metabolic control and serum hormone levels in relation to retinopathy in diabetic pregnancy. Diabetologia 1982, 22, 327–332. [Google Scholar] [CrossRef]
- Bermea, K.C.; Rodríguez-García, A.; Tsin, A.; Barrera-Saldaña, H.A. Somatolactogens and diabetic retinopathy. Growth Horm. IGF Res. 2018, 41, 42–47. [Google Scholar] [CrossRef]
- Holmes, D. Falling insulin requirements—a red flag for pre-eclampsia. Nat. Rev. Endocrinol. 2017, 13, 563. [Google Scholar] [CrossRef]
- Ram, M.; Feinmesser, L.; Shinar, S.; Maslovitz, S. The importance of declining insulin requirements during pregnancy in patients with pre-gestational gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 148–152. [Google Scholar] [CrossRef]
- Padmanabhan, S.; McLean, M.; Cheung, N.W. Falling insulin requirements are associated with adverse obstetric outcomes in women with preexisting diabetes. Diabetes Care 2014, 37, 2685–2692. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Lee, V.W.; McLean, M.; Athayde, N.; Lanzarone, V.; Khoshnow, Q.; Peek, M.J.; Cheung, N.W. The association of falling insulin requirements with maternal biomarkers and placental dysfunction: A prospective study of women with preexisting diabetes in pregnancy. Diabetes Care 2017, 40, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Caufriez, A.; Frankenne, F.; Hennen, G.; Copinschi, G. Regulation of maternal IGF-I by placental GH in normal and abnormal human pregnancies. Am. J. Physiol. Endocrinol. Metab. 1993, 265, E572–E577. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Ye, C.; Kramer, C.K.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B. Maternal serum prolactin and prediction of postpartum b-cell function and risk of prediabetes/diabetes. Diabetes Care 2016, 39, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Bergelin, R.O.; Wahl, P.W.; Walden, C.E. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes 1985, 34 (Suppl. S2), 71–77. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.J.; Shackleton, P.; Obiekwe, B.C.; Chard, T. Relationship of maternal and fetal levels of human placental lactogen to the weight and sex of the fetus. Placenta 1984, 5, 455–458. [Google Scholar] [CrossRef]
- Männik, J.; Vaas, P.; Rull, K.; Teesalu, P.; Rebane, T.; Laan, M. Differential expression profile of Growth Hormone/Chorionic Somatomammotropin genes in placenta of small- and large-for-gestational-age newborns. J. Clin. Endocrinol. Metab. 2010, 95, 2433–2442. [Google Scholar] [CrossRef]
- Small, M.; Cameron, A.; Lunan, C.B.; MacCuish, A.C. Macrosomia in Pregnancy Complicated by Insulin-Dependent Diabetes Mellitus. Diabetes Care 1987, 10, 594. [Google Scholar] [CrossRef]
- Gardner, M.O. Maternal serum concentrations of human placental lactogen, estradiol and pregnancy specific β1-glycoprotein and fetal growth retardation. Acta Obstet. Gynecol. Scand. Suppl. 1997, 165, 56–58. [Google Scholar] [CrossRef]
- Markestad, T. Prediction of fetal growth based on maternal serum concentrations of human chorionic gonadotropin, human placental lactogen and estriol. Acta Obstet. Gynecol. Scand. Suppl. 1997, 165, 50–55. [Google Scholar] [PubMed]
- Pedersen, J.F.; Sørensen, S.; Ruge, S. Human placental lactogen and pregnancy-associated plasma protein A in first trimester and subsequent fetal growth. Acta Obstet. Gynecol. Scand. 1995, 74, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Dutton, P.J.; Warrander, L.K.; Roberts, S.A.; Bernatavicius, G.; Byrd, L.M.; Gaze, D.; Kroll, J.; Jones, R.L.; Sibley, C.P.; Frøen, J.F.; et al. Predictors of poor perinatal outcome following maternal perception of reduced fetal movements—A prospective cohort study. PLoS ONE 2012, 7, e39784. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, P.G.; Aspillaga, M.O.; Lind, T. Accurate assessment of early gestational age in normal and diabetic women by serum human placental lactogen concentration. Lancet 1983, 322, 304–306. [Google Scholar] [CrossRef]
- Lassarre, C.; Hardouin, S.; Daffos, F.; Forestier, F.; Frankenne, F.; Binoux, M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr. Res. 1991, 29, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. North Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal undernutrition influences placental-fetal development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Singh, S.; Shah, P.; Gupta, N.; Kochupillai, N. Effect of maternal malnutrition and anemia on the endocrine regulation of fetal growth. Endocr. Res. 2004, 30, 189–203. [Google Scholar] [CrossRef]
- Tyson, J.E.; Austin, K.; Farinholt, J.; Fiedler, J. Endocrine-metabolic response to acute starvation in human gestation. Am. J. Obstet. Gynecol. 1976, 125, 1073–1084. [Google Scholar] [CrossRef]
- Braun, T.; Husar, A.; Challis, J.R.G.; Dudenhausen, J.W.; Henrich, W.; Plagemann, A.; Sloboda, D.M. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta 2013, 34, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Li, S.; Moss, T.J.M.; Newnham, J.P.; Challis, J.R.G.; Gluckman, P.D.; Sloboda, D.M. Maternal betamethasone administration reduces binucleate cell number and placental lactogen in sheep. J. Endocrinol. 2007, 194, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.B.; Tunster, S.J.; Heazell, A.E.P.; John, R.M. Placental PHLDA2 expression is increased in cases of fetal growth restriction following reduced fetal movements. BMC Med. Genet. 2016, 17, 17. [Google Scholar] [CrossRef] [PubMed]
| Study Title [Reference] | Number of Participants | Clinical Characteristics | Placental Lactogen Measurement, Method | Main Findings | First Author (Year of Study) |
|---|---|---|---|---|---|
| Placental peptides metabolism and maternal factors as predictors of risk of gestational diabetes in pregnant women. A case-control study. [61] | 200 | 150 healthy participants—12 developed GDM, 50 controls with T1D | I and II trimester of pregnancy, Sandwich Elisa in maternal serum | I trimester: PL levels significantly higher in patients with T1D, II trimester: No difference in PL between 12 patients who developed GDM and 138 healthy controls, PL levels were significantly higher in patients with T1D compared with those with GDM | Ngala et al. (2017) |
| Maternal serum and amniotic fluid levels of human placental lactogen in gestational diabetes. [62] | 46 | 16 patients with GDM, 30 healthy controls | Between the 37th and 39th weeks of gestation, radioimmunoassay in maternal serum and amniotic fluid | No difference in PL serum levels, significantly higher concentrations of amniotic fluid PL in patients with diabetes | Lolis et al. (1978) |
| Evaluation of circulating determinants of beta-cell function in women with and without gestational diabetes. [63] | 395 | 105 patients with GDM, 290 healthy controls | PL was measured “in the late second trimester”, ELISA #20-HPLHU-E01 (Alpco) in maternal serum | No differences in PL levels between diabetic and non-patients with diabetes | Retnakaran et al. (2016) |
| Serial determinations of human placental lactogen in the management of diabetic pregnancy. [65] | 138 | 98 patients with diabetes—White classes: A—14, B—36, C—28, D + F—20, 40 normal controls | PL concentrations were determined at the 27–29, 30–31, 32–34, and 35–37 weeks of gestation. Radioimmunoassay in maternal serum | PL concentrations after 32 weeks’ gestation were significantly higher in patients with diabetes compared with controls. No differences in PL levels between various White classes | Soler et al. (1975) |
| Placental Lactogen Levels in Diabetic Pregnancy. [66] | 34 | “patients with abnormal glucose tolerance tests during pregnancy”, 29 insulin-dependent | A total of 219 measurements in 34 patients. At each visit from 20 weeks’ gestation, and weekly from 32 weeks until delivery. Radioimmunoassay in maternal serum | PL serum levels higher than those in a normal population | Ursell et al. (1973) |
| Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: Role of miR-143. [67] | 18 | 6 patients with A1GDM, 6 patients with A2GDM, 6 patients that were healthy controls | Placental tissue collected at term after C-section, Sandwich Elisa (Genway)—in placental homogenate | PL significantly increased in A2GDM patients compared with those with A1GDM and controls | Muralimanoharan et al. (2016) |
| Placental lactogen, progesterone, total estriol and prolactin plasma levels in pregnant women with insulin-dependent diabetes mellitus. [68] | 25 | 15 insulin-dependent patients (White’s class B–C), 10 healthy controls | PL measured every 4 weeks from the 12th to 36th week of gestation. Radioimmunoassay in maternal serum (Biodata kit) | PL significantly lower in patients with diabetes at the 12th, 20th, 24th, 32nd, and 36th weeks of gestation | Botta et al. (1984) |
| Study Title [Reference] | Clinical Characteristics | Analyzed Parameters | Main Findings | First Author (Year of Study) |
|---|---|---|---|---|
| Somatomedin in newborns and the relationship to human chorionic somatotropin and fetal growth. [26] | 22 pregnant patients | PL levels in the maternal serum during the III trimester of pregnancy and cord blood at term | No correlation between PL levels, and birth weight and length | Kastrup et al. (1978) |
| Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. [86] | 273 patients in singelton pregnancies | PL concentrations in maternal blood measured at 36 weeks of gestation | Positive correlation between maternal blood PL concentrations, birth weight, and birth length | Knopp et al. (1985) |
| Relationship of maternal and fetal levels of human placental lactogen to the weight and sex of the fetus. [87] | 101 pregnant patients | PL levels in the maternal serum at 38–42 weeks of gestation, cord artery, and cord vein collected at term | Positive correlation between maternal serum PL and birth weight, with no correlation in the case of umbilical cord blood | Houghton et al. (1984) |
| Differential expression profile of Growth Hormone/Chorionic Somatomammotropin genes in placenta of small- and large-for-gestational-age newborns. [88] | 72 patients in uncomplicated singelton pregnancies | CSH1 and CSH2 gene mRNA in term placental tissue | CSH1 and CSH2 gene transcript levels were significantly higher in LGA newborns compared with SGA and AGA neonates | Männik et al. (2010) |
| Macrosomia in Pregnancy Complicated by Insulin-Dependent Diabetes Mellitus. [89] | 83 patients with insulin-dependent diabetes | PL maternal serum concentrations during the III trimester of pregnancy | Mothers of macrosomic infants have significantly higher concentrations of serum PL | Small et al. (1987) |
| Maternal serum concentrations of human placental lactogen, estradiol and pregnancy specific β1-glycoprotein and fetal growth retardation. [90] | 200 multiparous women with fetal growth retardation risk factors | PL maternal serum levels measured at a mean of 18 weeks’ gestational age | Higher maternal levels of PL are associated with a decreased prevalence of fetal growth retardation | Gardner (1997) |
| Prediction of fetal growth based on maternal serum concentrations of human chorionic gonadotropin, human placental lactogen and estriol. [91] | 214 patients, mothers of 102 SGA infants and 112 non-SGA neonates | PL levels in maternal serum were measured serially at 17, 25, 33, and 37 weeks of gestation | Significant differences in PL measured at 17, 33, and 37 weeks of pregnancy in mothers of SGA and non-SGA infants | Markestad et al. (1997) |
| Human placental lactogen and pregnancy-associated plasma protein A in first trimester and subsequent fetal growth. [92] | 93 patients with uncomplicated singelton pregnancies | Maternal PL serum concentrations measured between the 8th and 14th week of pregnancy | PL is negatively correlated with gestational age at delivery | Pedersen et al. (1995) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibiak, R.; Jankowski, M.; Gutaj, P.; Mozdziak, P.; Kempisty, B.; Wender-Ożegowska, E. Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives. J. Clin. Med. 2020, 9, 1142. https://doi.org/10.3390/jcm9041142
Sibiak R, Jankowski M, Gutaj P, Mozdziak P, Kempisty B, Wender-Ożegowska E. Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives. Journal of Clinical Medicine. 2020; 9(4):1142. https://doi.org/10.3390/jcm9041142
Chicago/Turabian StyleSibiak, Rafał, Maurycy Jankowski, Paweł Gutaj, Paul Mozdziak, Bartosz Kempisty, and Ewa Wender-Ożegowska. 2020. "Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives" Journal of Clinical Medicine 9, no. 4: 1142. https://doi.org/10.3390/jcm9041142
APA StyleSibiak, R., Jankowski, M., Gutaj, P., Mozdziak, P., Kempisty, B., & Wender-Ożegowska, E. (2020). Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives. Journal of Clinical Medicine, 9(4), 1142. https://doi.org/10.3390/jcm9041142






