Association of HSV-1 and Reduced Oral Bacteriota Diversity with Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Sampling
2.2. Autologous HSCT Procedures
2.3. Evaluation of Candida spp. and HSV in the Oral Cavity
2.4. Analysis of Bacterial Communities
2.5. Statistical Analysis
3. Results
3.1. OM Is Associated with the Detection of HSV-1
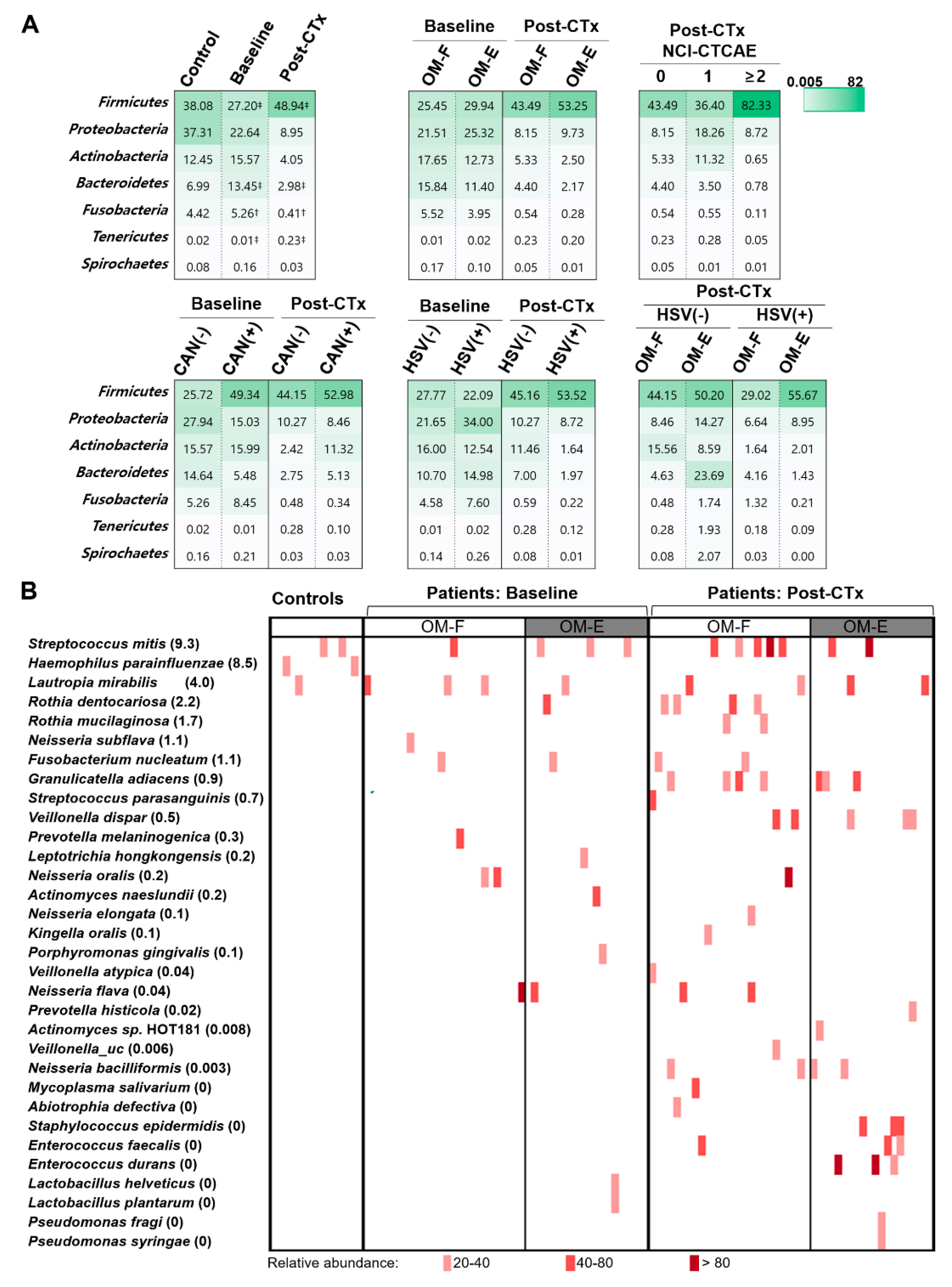
3.2. Chemotherapy-Accompanied Dysbiosis of Buccal Mucosal Bacteriota Is Associated with Severe OM
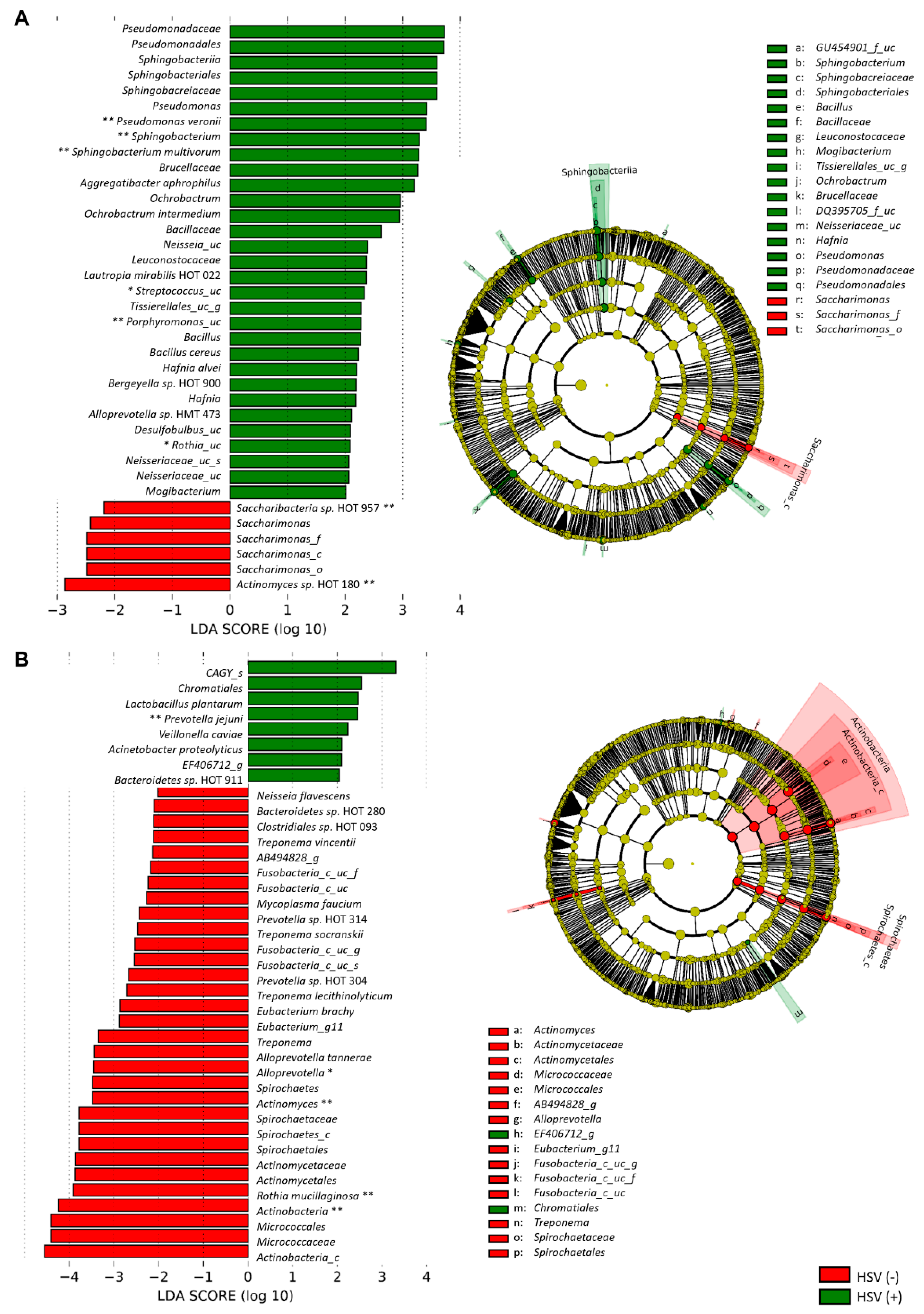
3.3. The Presence of HSV-1 after Chemotherapy Is Associated with the Dysbiosis of Buccal Bacteriota
3.4. The Severity of OM Is Correlated with Depletion of Commensals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scully, C.; Sonis, S.; Diz, P.D. Oral mucositis. Oral Dis. 2006, 12, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, H.-K.; Park, S.; Lee, A.; Lee, Y.-H.; Shin, D.-Y.; Koh, Y.; Choi, J.-Y.; Yoon, S.-S.; Choi, Y. Strong association between herpes simplex virus-1 and chemotherapy-induced oral mucositis in patients with hematologic malignancies. Korean J. Intern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wardley, A.M.; Jayson, G.C.; Swindell, R.; Morgenstern, G.R.; Chang, J.; Bloor, R.; Fraser, C.J.; Scarffe, J.H. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br. J. Haematol. 2000, 110, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.B.; Sonis, S.T.; Monopoli, M.M.; Sonis, A.L. A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 1993, 72, 1612–1617. [Google Scholar] [CrossRef]
- McGuire, D.B.; Fulton, J.S.; Park, J.; Brown, C.G.; Correa, M.E.; Eilers, J.; Elad, S.; Gibson, F.; Oberle-Edwards, L.K.; Bowen, J.; et al. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral, O. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 3165–3177. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Hou, H.A.; Chow, J.M.; Chen, Y.C.; Hsueh, P.R.; Tien, H.F. The impact of oral herpes simplex virus infection and candidiasis on chemotherapy-induced oral mucositis among patients with hematological malignancies. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 753–759. [Google Scholar] [CrossRef]
- De Mendonça, R.M.H.; de Araújo, M.; Levy, C.E.; Morari, J.; Silva, R.A.; Yunes, J.A.; Brandalise, S.R. Prospective evaluation of HSV, Candida spp., and oral bacteria on the severity of oral mucositis in pediatric acute lymphoblastic leukemia. Support. Care Cancer 2012, 20, 1101–1107. [Google Scholar] [CrossRef]
- Laheij, A.M.; de Soet, J.J.; von dem Borne, P.A.; Kuijper, E.J.; Kraneveld, E.A.; van Loveren, C.; Raber-Durlacher, J.E. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support. Care Cancer 2012, 20, 3231–3240. [Google Scholar] [CrossRef]
- Napenas, J.J.; Brennan, M.T.; Coleman, S.; Kent, M.L.; Noll, J.; Frenette, G.; Nussbaum, M.L.; Mougeot, J.L.; Paster, B.J.; Lockhart, P.B.; et al. Molecular methodology to assess the impact of cancer chemotherapy on the oral bacterial flora: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 554–560. [Google Scholar] [CrossRef]
- Ye, Y.; Carlsson, G.; Agholme, M.B.; Wilson, J.A.; Roos, A.; Henriques-Normark, B.; Engstrand, L.; Modeer, T.; Putsep, K. Oral bacterial community dynamics in paediatric patients with malignancies in relation to chemotherapy-related oral mucositis: A prospective study. Clin. Microbiol. Infect. 2013, 19, E559–E567. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Colevas, A.D.; Setser, A.; Rusch, V.; Jaques, D.; Budach, V.; Langer, C.; Murphy, B.; Cumberlin, R.; Coleman, C.N. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 2003, 13, 176–181. [Google Scholar] [CrossRef]
- Sonis, S.T.; Eilers, J.P.; Epstein, J.B.; LeVeque, F.G.; Liggett, W.H., Jr.; Mulagha, M.T.; Peterson, D.E.; Rose, A.H.; Schubert, M.M.; Spijkervet, F.K.; et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer 1999, 85, 2103–2113. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.A.; Boeckh, M.J.; Center for International Blood and Marrow Research; et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, Y.; Yoon, H.J.; Baek, K.J.; Alam, J.; Park, H.K.; Choi, Y. The presence of bacteria within tissue provides insights into the pathogenesis of oral lichen planus. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Vasconcelos, R.M.; Sanfilippo, N.; Paster, B.J.; Kerr, A.R.; Li, Y.; Ramalho, L.; Queiroz, E.L.; Smith, B.; Sonis, S.T.; Corby, P.M. Host-Microbiome Cross-talk in Oral Mucositis. J. Dent. Res. 2016, 95, 725–733. [Google Scholar] [CrossRef]
- Miranda-Silva, W.; Knebel, F.H.; Tozetto-Mendozo, T.R.; Palmieri, M.; da Fonseca, F.P.; Camargo, A.A.; Braz-Silva, P.H.; Fregnani, E.R. Herpesviruses in the oral cavity of patients subjected to allogeneic hematopoietic stem cell transplantation and its relationship with oral mucositis. Clin. Oral. Investig. 2020. [Google Scholar] [CrossRef]
- Mohamadi, J.; Motaghi, M.; Panahi, J.; Havasian, M.R.; Delpisheh, A.; Azizian, M.; Pakzad, I. Anti-fungal resistance in candida isolated from oral and diaper rash candidiasis in neonates. Bioinformation 2014, 410, 667–670. [Google Scholar] [CrossRef]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. (Weinheim) 2018, 351, e1800141. [Google Scholar] [CrossRef] [PubMed]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Laheij, A.M.G.A.; Raber-Durlacher, J.E.; Koppelmans, R.G.A.; Huysmans, M.D.N.J.M.; Potting, C.; van Leeuwen, S.J.M.; Hazenberg, M.D.; Brennan, M.T.; von Bültzingslöwen, I.; Johansson, J.E.; et al. Microbial changes in relation to oral mucositis in autologous hematopoietic stem cell transplantation recipients. Sci. Rep. 2019, 9, 16929. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Kim, Y.T. Prevalence of Antibody to Herpes Simplex Virus. Korean J. Dermatol. 1993, 31, 38–46. [Google Scholar]
- Khanna, K.M.; Lepisto, A.J.; Decman, V.; Hendricks, R.L. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 2004, 16, 463–469. [Google Scholar] [CrossRef]
- Righini-Grunder, F.; Hurni, M.; Warschkow, R.; Rischewski, J. Frequency of Oral Mucositis and Local Virus Reactivation in Herpes Simplex Virus Seropositive Children with Myelosuppressive Therapy. Klin. Padiatr. 2015, 227, 335–338. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Guimaraes, A.L.; Victoria, J.M.; Gomes, C.C.; Gomez, R.S. Herpes simplex virus type 1 shedding in the oral cavity of seropositive patients. Oral Dis. 2005, 11, 13–16. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Diefenbach, R.J.; Miranda-Saksena, M.; Bosnjak, L.; Kim, M.; Jones, C.; Douglas, M.W. The cycle of human herpes simplex virus infection: Virus transport and immune control. J. Infect. Dis. 2006, 194 (Suppl S1), S11–S18. [Google Scholar] [CrossRef]
- Miklos, Z.; Danis, V.A.; Adams, S.; Lloyd, A.R.; Adrian, D.L.; Cunningham, A.L. In vivo production of cytokines and β (CC) chemokines in human recurrent herpes simplex lesions—Do herpes simplex virus-infected keratinocytes contribute to their production? J. Infect. Dis. 1998, 177, 827–838. [Google Scholar] [CrossRef]
- Aruni, A.W.; Dou, Y.; Mishra, A.; Fletcher, H.M. The Biofilm Community-Rebels with a Cause. Curr. Oral. Health Rep. 2015, 2, 48–56. [Google Scholar] [CrossRef]
- Horton, L.E.; Haste, N.M.; Taplitz, R.A. Rethinking Antimicrobial Prophylaxis in the Transplant Patient in the World of Emerging Resistant Organisms-Where Are We Today? Curr. Hematol. Malig. Rep. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]



| Control (n = 15) | Patients Undergoing Autologous HSCT | p Values | ||||
|---|---|---|---|---|---|---|
| Total (n = 46) | OM-Free (n = 26) | OM-Ex (n = 20) | p1 | p2 | ||
| Age | 38.1±11.6 | 52.7 ± 9.8 | 52.0 ± 10.0 | 53.5 ± 9.8 | < 0.001 a | 0.626 a |
| Gender (% male) | 40 | 54.3 | 42.3 | 70 | 0.338 b | 0.062 b |
| Type of disease | ||||||
| Multiple myeloma (%) | 60.9 | 65.4 | 55 | 0.714 b | ||
| Lymphoma (%) | 32.6 | 26.9 | 40 | |||
| Others (%) | 6.5 | 7.7 | 5 | |||
| HSV-1 (%) | 0 | 23.9 | 19.2 | 30 | 0.038 b | 0.401 b |
| Candidaspp. (%) | 37 | 34.6 | 40 | 0.711 b | ||
| ANC < 500/μL(%) | 2.2 | 0 | 5 | 0.254 b | ||
| ALC < 1000/μL(%) | 69.6 | 65.4 | 75 | 0.487 b | ||
| Prophylactic antibiotic use (% yes) | 100 | 100 | 100 | 0.249 b | ||
| Prophylactic antifungal use (% yes) | 100 | 100 | 100 | |||
| Prophylactic acyclovir use (% yes) | 4.3 | 15.4 | 0 | 0.498 b | ||
| Variables | OM | OM (yes vs. no) | OM Severity (OMAS) | |||||
|---|---|---|---|---|---|---|---|---|
| No (n = 54) a | Yes (n = 25) a | Odds Ratio | 95% CI | pb | β | pb | ||
| HSV-1 | Negative | 63% | 25% | 3.668 | 1.512–8.895 | 0.004 | 0.321 ± 0.223 | 0.151 |
| Positive | 37% | 75% | ||||||
| Candida spp. | Negative | 64.8% | 66.7% | 1.008 | 0.362–2.807 | 0.988 | 0.064 ± 0.215 | 0.765 |
| Positive | 35.2% | 33.3% | ||||||
| Bacterial diversity c | 1.89 | 1.95 | 0.604 | 0.275–1.328 | 0.210 | −0.533 ± 0.220 | 0.015 | |
| (0.77–3.88) | (0.36–3.68) | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Hong, J.; Shin, D.-Y.; Koh, Y.; Yoon, S.-S.; Kim, P.-J.; Kim, H.-G.; Kim, I.; Park, H.-K.; Choi, Y. Association of HSV-1 and Reduced Oral Bacteriota Diversity with Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. J. Clin. Med. 2020, 9, 1090. https://doi.org/10.3390/jcm9041090
Lee A, Hong J, Shin D-Y, Koh Y, Yoon S-S, Kim P-J, Kim H-G, Kim I, Park H-K, Choi Y. Association of HSV-1 and Reduced Oral Bacteriota Diversity with Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. Journal of Clinical Medicine. 2020; 9(4):1090. https://doi.org/10.3390/jcm9041090
Chicago/Turabian StyleLee, Ahreum, Junshik Hong, Dong-Yeop Shin, Youngil Koh, Sung-Soo Yoon, Pil-Jong Kim, Hong-Gee Kim, Inho Kim, Hee-Kyung Park, and Youngnim Choi. 2020. "Association of HSV-1 and Reduced Oral Bacteriota Diversity with Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation" Journal of Clinical Medicine 9, no. 4: 1090. https://doi.org/10.3390/jcm9041090
APA StyleLee, A., Hong, J., Shin, D.-Y., Koh, Y., Yoon, S.-S., Kim, P.-J., Kim, H.-G., Kim, I., Park, H.-K., & Choi, Y. (2020). Association of HSV-1 and Reduced Oral Bacteriota Diversity with Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. Journal of Clinical Medicine, 9(4), 1090. https://doi.org/10.3390/jcm9041090





