The Prognostic Impact of Comorbidities in Patients with De-Novo Diffuse Large B-Cell Lymphoma Treated with R-CHOP Immunochemotherapy in Curative Intent
Abstract
1. Introduction
2. Methods
2.1. Patients and Methods
2.2. Statistics
3. Results
3.1. Clinical Characteristics
3.2. Charlson Comorbidity Index
3.3. Hematopoietic Cell Transplantation-Specific Comorbidity Index
3.4. Association between Comorbidities and Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by who subtype in the united states, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Effect of age on the characteristics and clinical behavior of non-hodgkin’s lymphoma patients. The non-hodgkin’s lymphoma classification project. Ann. Oncol. 1997, 8, 973–978.
- Smith, A.; Crouch, S.; Howell, D.; Burton, C.; Patmore, R.; Roman, E. Impact of age and socioeconomic status on treatment and survival from aggressive lymphoma: A UK population-based study of diffuse large b-cell lymphoma. Cancer Epidemiol. 2015, 39, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Hamlin, P.; Soubeyran, P.; Stauder, R.; Wadhwa, P.; Aapro, M.; Lichtman, S. Diffuse large b-cell lymphoma in the elderly: Impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An international society of geriatric oncology (siog) expert position paper. J. Geriatr. Oncol. 2015, 6, 141–152. [Google Scholar] [CrossRef]
- A predictive model for aggressive non-hodgkin’s lymphoma. The international non-hodgkin’s lymphoma prognostic factors project. N. Engl. J. Med. 1993, 329, 987–994.
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. Chop chemotherapy plus rituximab compared with chop alone in elderly patients with diffuse large-b-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Schubert, J.; Ziepert, M.; Schmits, R.; Mohren, M.; Lengfelder, E.; Reiser, M.; Nickenig, C.; Clemens, M.; Peter, N.; et al. Six versus eight cycles of bi-weekly chop-14 with or without rituximab in elderly patients with aggressive cd20+ b-cell lymphomas: A randomised controlled trial (ricover-60). Lancet Oncol. 2008, 9, 105–116. [Google Scholar] [CrossRef]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-chop versus chop alone or with maintenance rituximab in older patients with diffuse large b-cell lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef]
- Feugier, P.; Van Hoof, A.; Sebban, C.; Solal-Celigny, P.; Bouabdallah, R.; Ferme, C.; Christian, B.; Lepage, E.; Tilly, H.; Morschhauser, F.; et al. Long-term results of the r-chop study in the treatment of elderly patients with diffuse large b-cell lymphoma: A study by the groupe d’etude des lymphomes de l’adulte. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 4117–4126. [Google Scholar] [CrossRef]
- Tilly, H.; Gomes da Silva, M.; Vitolo, U.; Jack, A.; Meignan, M.; Lopez-Guillermo, A.; Walewski, J.; Andre, M.; Johnson, P.W.; Pfreundschuh, M.; et al. Diffuse large b-cell lymphoma (dlbcl): Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2015, 26 (Suppl. 5), v11–v125. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Trumper, L.; Kloess, M.; Schmits, R.; Feller, A.C.; Rube, C.; Rudolph, C.; Reiser, M.; Hossfeld, D.K.; Eimermacher, H.; et al. Two-weekly or 3-weekly chop chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: Results of the nhl-b2 trial of the dshnhl. Blood 2004, 104, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Hutchings, M.; Ladetto, M.; Goede, V.; Mey, U.; Soubeyran, P.; Spina, M.; Stauder, R.; Trneny, M.; Wedding, U.; et al. Esmo consensus conference on malignant lymphoma: General perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann. Oncol. 2017, 29, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The revised international prognostic index (r-ipi) is a better predictor of outcome than the standard ipi for patients with diffuse large b-cell lymphoma treated with r-chop. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sehn, L.H.; Rademaker, A.W.; Gordon, L.I.; Lacasce, A.S.; Crosby-Thompson, A.; Vanderplas, A.; Zelenetz, A.D.; Abel, G.A.; Rodriguez, M.A.; et al. An enhanced international prognostic index (nccn-ipi) for patients with diffuse large b-cell lymphoma treated in the rituximab era. Blood 2014, 123, 837–842. [Google Scholar] [CrossRef]
- Morrison, V.A.; Hamlin, P.; Soubeyran, P.; Stauder, R.; Wadhwa, P.; Aapro, M.; Lichtman, S.M. Approach to therapy of diffuse large b-cell lymphoma in the elderly: The international society of geriatric oncology (siog) expert position commentary. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2015, 26, 1058–1068. [Google Scholar] [CrossRef]
- Stauder, R.; Nosslinger, T.; Pfeilstocker, M.; Sperr, W.R.; Wimazal, F.; Krieger, O.; Valent, P. Impact of age and comorbidity in myelodysplastic syndromes. J. Natl. Compr. Cancer Netw. JNCCN 2008, 6, 927–934. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Asmis, T.R.; Ding, K.; Seymour, L.; Shepherd, F.A.; Leighl, N.B.; Winton, T.L.; Whitehead, M.; Spaans, J.N.; Graham, B.C.; Goss, G.D. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: A review of national cancer institute of Canada clinical trials group trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 54–59. [Google Scholar] [CrossRef]
- Wieringa, A.; Boslooper, K.; Hoogendoorn, M.; Joosten, P.; Beerden, T.; Storm, H.; Kibbelaar, R.E.; Veldhuis, G.J.; van Kamp, H.; van Rees, B.; et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large b-cell lymphoma treated with r-chop: A population-based cohort study. Br. J. Haematol. 2014, 165, 489–496. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Miura, K.; Hojo, A.; Hatta, Y.; Tanaka, T.; Kurita, D.; Iriyama, N.; Kobayashi, S.; Takeuchi, J. Charlson comorbidity index is an independent prognostic factor among elderly patients with diffuse large b-cell lymphoma. J. Cancer Res. Clin. Oncol. 2011, 137, 1079–1084. [Google Scholar] [CrossRef]
- Lin, T.L.; Kuo, M.C.; Shih, L.Y.; Dunn, P.; Wang, P.N.; Wu, J.H.; Tang, T.C.; Chang, H.; Hung, Y.S. The impact of age, charlson comorbidity index, and performance status on treatment of elderly patients with diffuse large b cell lymphoma. Ann. Hematol. 2012, 91, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Hamilton, L.; Ogbonnaya, A.; Raju, A.; Hennenfent, K.; Galaznik, A. Treatment approaches for older and oldest patients with diffuse large b-cell lymphoma—Use of non-r-chop alternative therapies and impact of comorbidities on treatment choices and outcome: A humedica database retrospective cohort analysis, 2007–2015. J. Geriatr. Oncol. 2019, 11, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (hct)-specific comorbidity index: A new tool for risk assessment before allogeneic hct. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Kerbauy, D.M.; Chyou, F.; Gooley, T.; Sorror, M.L.; Scott, B.; Pagel, J.M.; Myerson, D.; Appelbaum, F.R.; Storb, R.; Deeg, H.J. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2005, 11, 713–720. [Google Scholar] [CrossRef]
- Sorror, M.L.; Sandmaier, B.M.; Storer, B.E.; Franke, G.N.; Laport, G.G.; Chauncey, T.R.; Agura, E.; Maziarz, R.T.; Langston, A.; Hari, P.; et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA 2011, 306, 1874–1883. [Google Scholar] [CrossRef]
- Raimondi, R.; Tosetto, A.; Oneto, R.; Cavazzina, R.; Rodeghiero, F.; Bacigalupo, A.; Fanin, R.; Rambaldi, A.; Bosi, A. Validation of the hematopoietic cell transplantation-specific comorbidity index: A prospective, multicenter gitmo study. Blood 2012, 120, 1327–1333. [Google Scholar] [CrossRef]
- Mian, M.; Wasle, I.; Gamerith, G.; Mondello, P.; Melchardt, T.; Jager, T.; Linkesch, W.; Fiegl, M. R-chop versus r-comp: Are they really equally effective? Clin. Oncol. (R. Coll. Radiol. (Gt. Br.)) 2014, 26, 648–652. [Google Scholar] [CrossRef]
- Mian, M.; Augustin, F.; Kocher, F.; Gunsilius, E.; Willenbacher, W.; Zabernigg, A.; Zangerl, G.; Oexle, H.; Schreieck, S.; Schnallinger, M.; et al. A success story: How a single targeted-therapy molecule impacted on treatment and outcome of diffuse large b-cell lymphoma. Anticancer Res. 2014, 34, 2559–2564. [Google Scholar]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised response criteria for malignant lymphoma. J. Chin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an international workshop to standardize response criteria for non-hodgkin’s lymphomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 1–14. [Google Scholar] [CrossRef]
- Wasterlid, T.; Mohammadi, M.; Smedby, K.E.; Glimelius, I.; Jerkeman, M.; Bottai, M.; Eloranta, S. Impact of comorbidity on disease characteristics, treatment intent and outcome in diffuse large b-cell lymphoma: A Swedish lymphoma register study. J. Intern. Med. 2019, 285, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Hermet, E.; Cabrespine, A.; Guieze, R.; Garnier, A.; Tempescul, A.; Lenain, P.; Bouabdallah, R.; Vilque, J.P.; Frayfer, J.; Bordessoule, D.; et al. Autologous hematopoietic stem cell transplantation in elderly patients (>/= 70 years) with non-hodgkin’s lymphoma: A French society of bone marrow transplantation and cellular therapy retrospective study. J. Geriatr. Oncol. 2015, 6, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Goldstein, J.S.; Ayer, T.; Rai, A.; Flowers, C.R. A population-based multistate model for diffuse large b-cell lymphoma-specific mortality in older patients. Cancer 2019, 125, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Jia, X.; Hill, B.; Dean, R.; Pohlman, B.; Smith, M.R.; Jagadeesh, D. Impact of comorbidities on outcomes of elderly patients with diffuse large b-cell lymphoma. Am. J. Hematol. 2017, 92, 989–996. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Prins, M.C.; Stauder, R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk. Res. 2014, 38, 275–283. [Google Scholar] [CrossRef]
- Antic, D.; Jelicic, J.; Trajkovic, G.; Balint, M.T.; Bila, J.; Markovic, O.; Petkovic, I.; Nikolic, V.; Andjelic, B.; Djurasinovic, V.; et al. Is it possible to improve prognostic value of nccn-ipi in patients with diffuse large b cell lymphoma? The prognostic significance of comorbidities. Ann. Hematol. 2018, 97, 267–276. [Google Scholar] [CrossRef]
- Kwak, L.W.; Halpern, J.; Olshen, R.A.; Horning, S.J. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1990, 8, 963–977. [Google Scholar] [CrossRef]
- Pettengell, R.; Schwenkglenks, M.; Bosly, A. Association of reduced relative dose intensity and survival in lymphoma patients receiving chop-21 chemotherapy. Ann. Hematol. 2008, 87, 429–430. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.L.; Feng, R.; Li, J.T.; Tian, Y.; Wang, T. Validation and refinement of the age, comorbidities, and albumin index in elderly patients with diffuse large b-cell lymphoma: An effective tool for comprehensive geriatric assessment. Oncologist 2018, 23, 722–729. [Google Scholar] [CrossRef]
- Eyre, T.A.; Martinez-Calle, N.; Hildyard, C.; Eyre, D.W.; Plaschkes, H.; Griffith, J.; Wolf, J.; Fields, P.; Gunawan, A.; Oliver, R.; et al. Impact of intended and relative dose intensity of r-chop in a large, consecutive cohort of elderly diffuse large b-cell lymphoma patients treated with curative intent: No difference in cumulative incidence of relapse comparing patients by age. J. Intern. Med. 2019, 285, 681–692. [Google Scholar] [CrossRef]
- Goede, V.; Stauder, R. Multidisciplinary care in the hematology clinic: Implementation of geriatric oncology. J. Geriatr. Oncol. 2019, 10, 497–503. [Google Scholar] [CrossRef]
- Peyrade, F.; Bologna, S.; Delwail, V.; Emile, J.F.; Pascal, L.; Ferme, C.; Schiano, J.M.; Coiffier, B.; Corront, B.; Farhat, H.; et al. Combination of ofatumumab and reduced-dose chop for diffuse large b-cell lymphomas in patients aged 80 years or older: An open-label, multicentre, single-arm, phase 2 trial from the lysa group. Lancet Haematol. 2017, 4, e46–e55. [Google Scholar] [CrossRef]
- Peyrade, F.; Jardin, F.; Thieblemont, C.; Thyss, A.; Emile, J.F.; Castaigne, S.; Coiffier, B.; Haioun, C.; Bologna, S.; Fitoussi, O.; et al. Attenuated immunochemotherapy regimen (r-minichop) in elderly patients older than 80 years with diffuse large b-cell lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011, 12, 460–468. [Google Scholar] [CrossRef]
- Soubeyran, P.; Terret, C.; Bellera, C.; Bonnetain, F.; Jean, O.S.; Galvin, A.; Chakiba, C.; Zwolakowski, M.D.; Mathoulin-Pelissier, S.; Rainfray, M. Role of geriatric intervention in the treatment of older patients with cancer: Rationale and design of a phase iii multicenter trial. BMC Cancer 2016, 16, 932. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: Asco guideline for geriatric oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]

| Valid Cases n | Clinical Characteristics n (%) | |
|---|---|---|
| Baseline characteristics | ||
| Total number | 181 | 100 |
| Median age (range), years | 60 (18–90) | |
| Women | 181 | 85 (47.0) |
| Age ≥ 60 years | 91 (50.3) | |
| B-symptoms | 156 | 65 (41.7) |
| Ann Arbor Stage | 181 | |
| I | 29 (16.0) | |
| II | 51 (28.2) | |
| III | 40 (22.1) | |
| IV | 61 (33.7) | |
| >1 Extranodal site | 172 | 52 (30.2) |
| WHO Performance Status ≥2 | 155 | 37 (23.9) |
| LDH > UNL | 160 | 91 (56.9) |
| IPI ≥ 3 | 148 | 63 (42.6) |
| Lymphadenopathy >5cm and/or Maximum spleen diameter ≥20cm | 166 | 77 (46.4) |
| Treatment | ||
| 1 cycle R-CHOP | 178 | 2 (1.1) |
| 2 cycles R-CHOP | 2 (1.1) | |
| 3 cycles R-CHOP | 3 (1.7) | |
| 4 cycles R-CHOP | 6 (3.4) | |
| 5 cycles R-CHOP | 7 (3.9) | |
| ≥6 cycles R-CHOP | 158 (88.8) | |
| Response | 181 | |
| CR | 135 (74.6) | |
| PR | 16 (8.8) | |
| SD | 1 (0.6) | |
| PD | 20 (11.0) | |
| Interruption | 3 (1.7) | |
| Death | 5 (2.8) | |
| Unknown | 1 (0.6) | |
| Relapses | 174 | 53 (30.5) |
| Charlson Comorbidity Index | Scoring Points | Comorbidities According to the CCI n (%) |
|---|---|---|
| Second solid tumor (non-metastatic) | 2 | 7 (3.9) |
| Diabetes (without complication) | 1 | 7 (3.9) |
| Chronic pulmonary disease | 1 | 7 (3.9) |
| Connective tissue disease | 1 | 6 (3.3) |
| Congestive heart failure | 1 | 4 (2.2) |
| Peripheral vascular disease | 1 | 4 (2.2) |
| Cerebrovascular disease (except hemiplegia) | 1 | 4 (2.2) |
| Ulcer disease | 1 | 4 (2.2) |
| Diabetes with end organ damage | 2 | 3 (1.7) |
| Myocardial infarction | 1 | 2 (1.1) |
| Moderate or severe liver disease | 3 | 2 (1.1) |
| Mild liver disease | 1 | 1 (0.6) |
| Hemiplegia | 2 | 1 (0.6) |
| Moderate or severe renal disease | 2 | - |
| Dementia | 1 | - |
| Leukemia | 2 | - |
| Lymphoma | 2 | - |
| Acquired immunodeficiency syndrome | 6 | - |
| Second solid tumor (metastatic) | 6 | - |
| Charlson Comorbidity Scoring points | ||
| CCI 0 | 139 (76.8) | |
| CCI 1 | 24 (13.3) | |
| CCI 2 | 13 (7.2) | |
| CCI 3 | 3 (1.7) | |
| CCI 4 | 2 (1.1) |
| HCT Comorbidity Index | Scoring Points | Comorbidities According to the HCT-CI n (%) |
|---|---|---|
| Previous solid tumor | 3 | 17 (9.4) |
| Cardiovascular comorbidity | 1 | 13 (7.2) |
| Renal comorbidity | 2 | 12 (6.6) |
| Diabetes | 1 | 10 (5.5) |
| Arrhythmia | 1 | 10 (5.5) |
| Rheumatologic comorbidity | 2 | 6 (3.3) |
| Heart valve disease | 3 | 5 (2.8) |
| Psychiatric disturbance | 1 | 5 (2.8) |
| Cerebrovascular disease | 1 | 4 (2.2) |
| Pulmonary comorbidity moderate | 2 | 4 (2.2) |
| Peptic ulcer | 2 | 4 (2.2) |
| Obesity | 1 | 3 (1.7) |
| Severe liver disease | 3 | 2 (1.1) |
| Pulmonary comorbidity severe | 3 | 1 (0.6) |
| Mild liver disease | 1 | 1 (0.6) |
| Inflammatory bowel disease | 1 | - |
| Infection | 1 | - |
| HCT-CI Scoring points ≥ 2 | ||
| HCT-CI 0 | 116 (64.1) | |
| HCT-CI 1 | 14 (7.7) | |
| HCT-CI 2 | 23 (12.7) | |
| HCT-CI 3 | 16 (8.8) | |
| HCT-CI 4 | 6 (3.3) | |
| HCT-CI 5 | 1 (0.6) | |
| HCT-CI 6 | - | |
| HCT-CI 7 | 4 (2.2) | |
| HCT-CI 8 | 1 (0.6) |
| Overall Survival | ||||
|---|---|---|---|---|
| Valid Cases n | 3-Year OS (%) | 5-Year OS (%) | p Value | |
| Whole cohort | 181 | 76.6 | 74.0 | - |
| Baseline characteristics | ||||
| Gender | ||||
| Male | 96 | 81.3 | 78.1 | 0.112 |
| Female | 85 | 72.8 | 69.0 | |
| Age * | ||||
| Age <60 years | 90 | 84.4 | 82.3 | <0.001 |
| Age ≥60 years | 91 | 69.0 | 65.8 | |
| Ann Arbor Stage* | ||||
| I-II | 80 | 90.9 | 90.9 | <0.001 |
| III-IV | 101 | 64.7 | 59.6 | |
| LDH * | ||||
| Normal | 69 | 90.9 | 88.9 | <0.001 |
| >UNL | 91 | 67.6 | 64.4 | |
| WHO Performance Status * | ||||
| 0–1 | 118 | 82.6 | 80.0 | <0.001 |
| ≥2 | 37 | 57.8 | 57.8 | |
| Extranodal sites * | ||||
| 0–1 | 120 | 82.2 | 79.8 | 0.040 |
| ≥2 | 52 | 63.3 | 59.6 | |
| IPI | ||||
| <3 | 85 | 91.2 | 89.4 | <0.001 |
| ≥3 | 63 | 56.4 | 53.7 | |
| Treatment | ||||
| ≥ 4 cycles R-CHOP | 7 | 33.3 | 33.3 | <0.001 |
| <4 cycles R-CHOP | 171 | 78.1 | 75.4 | |
| Response | ||||
| CR or PR | 151 | 88.1 | 85.1 | <0.001 |
| Other | 30 | 8.2 | 8.2 | |
| CCI * | ||||
| 0–1 | 163 | 81.3 | 78.3 | <0.001 |
| ≥2 | 18 | 38.9 | 38.9 | |
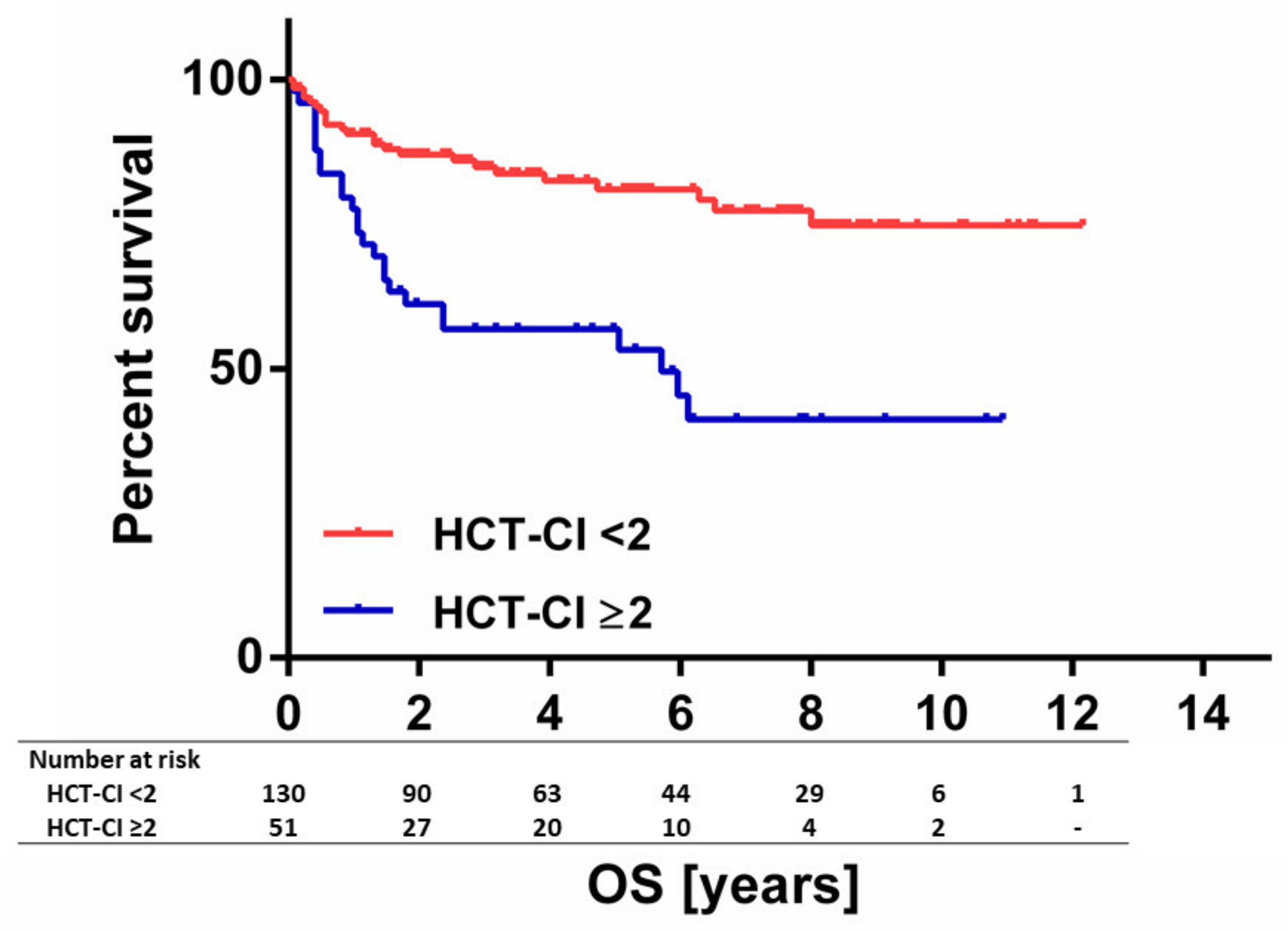
| HCT-CI * | ||||
| 0–1 | 130 | 84.9 | 81.0 | <0.001 |
| ≥2 | 51 | 56.8 | 56.8 | |
| HR (95% CI) | p Value | |
|---|---|---|
| Ann Arbor Stage ≥ III | 4.3 (1.8–10.6) | 0.001 |
| Performance Status ≥ 2 | 2.2 (1.1–4.2) | 0.023 |
| HCT-CI ≥ 2 | 2.6 (1.4–5.0) | 0.004 |
| elevated LDH | 2.1 (0.8–5.2) | 0.129 |
| ≥2 extranodal sites | 1.1 (0.6–2.3) | 0.722 |
| age ≥ 60years | 2.0 (0.9–4.4) | 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocher, F.; Mian, M.; Seeber, A.; Fiegl, M.; Stauder, R. The Prognostic Impact of Comorbidities in Patients with De-Novo Diffuse Large B-Cell Lymphoma Treated with R-CHOP Immunochemotherapy in Curative Intent. J. Clin. Med. 2020, 9, 1005. https://doi.org/10.3390/jcm9041005
Kocher F, Mian M, Seeber A, Fiegl M, Stauder R. The Prognostic Impact of Comorbidities in Patients with De-Novo Diffuse Large B-Cell Lymphoma Treated with R-CHOP Immunochemotherapy in Curative Intent. Journal of Clinical Medicine. 2020; 9(4):1005. https://doi.org/10.3390/jcm9041005
Chicago/Turabian StyleKocher, Florian, Michael Mian, Andreas Seeber, Michael Fiegl, and Reinhard Stauder. 2020. "The Prognostic Impact of Comorbidities in Patients with De-Novo Diffuse Large B-Cell Lymphoma Treated with R-CHOP Immunochemotherapy in Curative Intent" Journal of Clinical Medicine 9, no. 4: 1005. https://doi.org/10.3390/jcm9041005
APA StyleKocher, F., Mian, M., Seeber, A., Fiegl, M., & Stauder, R. (2020). The Prognostic Impact of Comorbidities in Patients with De-Novo Diffuse Large B-Cell Lymphoma Treated with R-CHOP Immunochemotherapy in Curative Intent. Journal of Clinical Medicine, 9(4), 1005. https://doi.org/10.3390/jcm9041005





