Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro
Abstract
1. Introduction
2. Methods and Materials
2.1. Porous Implants Production and Characterization
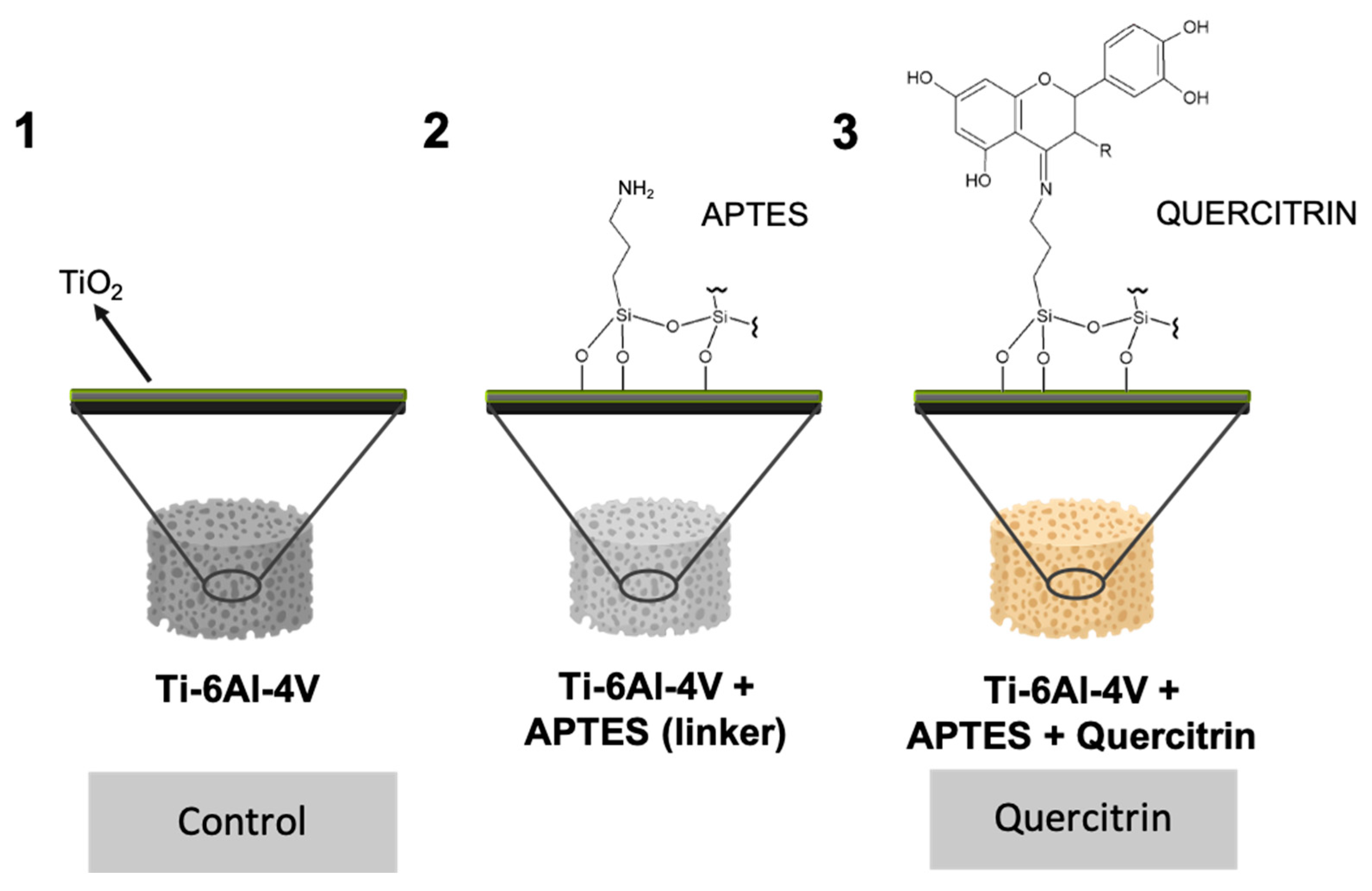
2.2. Implant Coating
2.3. Mechanical Characterization of Coated and Uncoated Implants
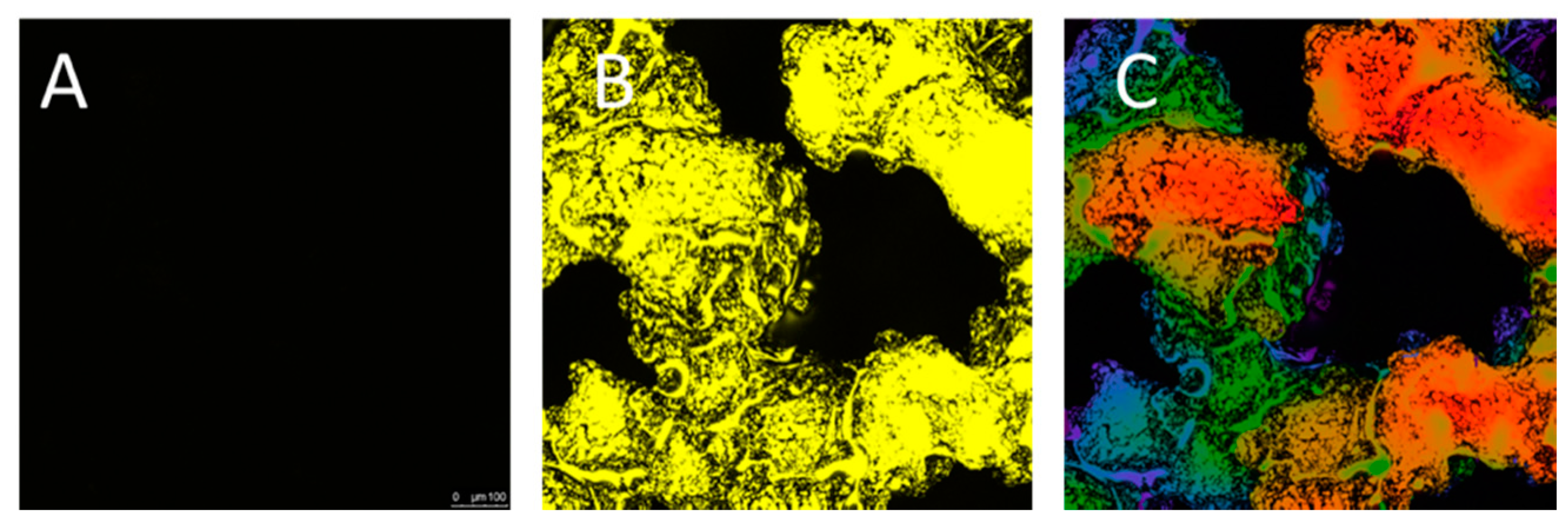
2.4. Visualization of Quercitrin Coating Onto the 3D Implants by Confocal Microscopy
2.5. Cell Culture
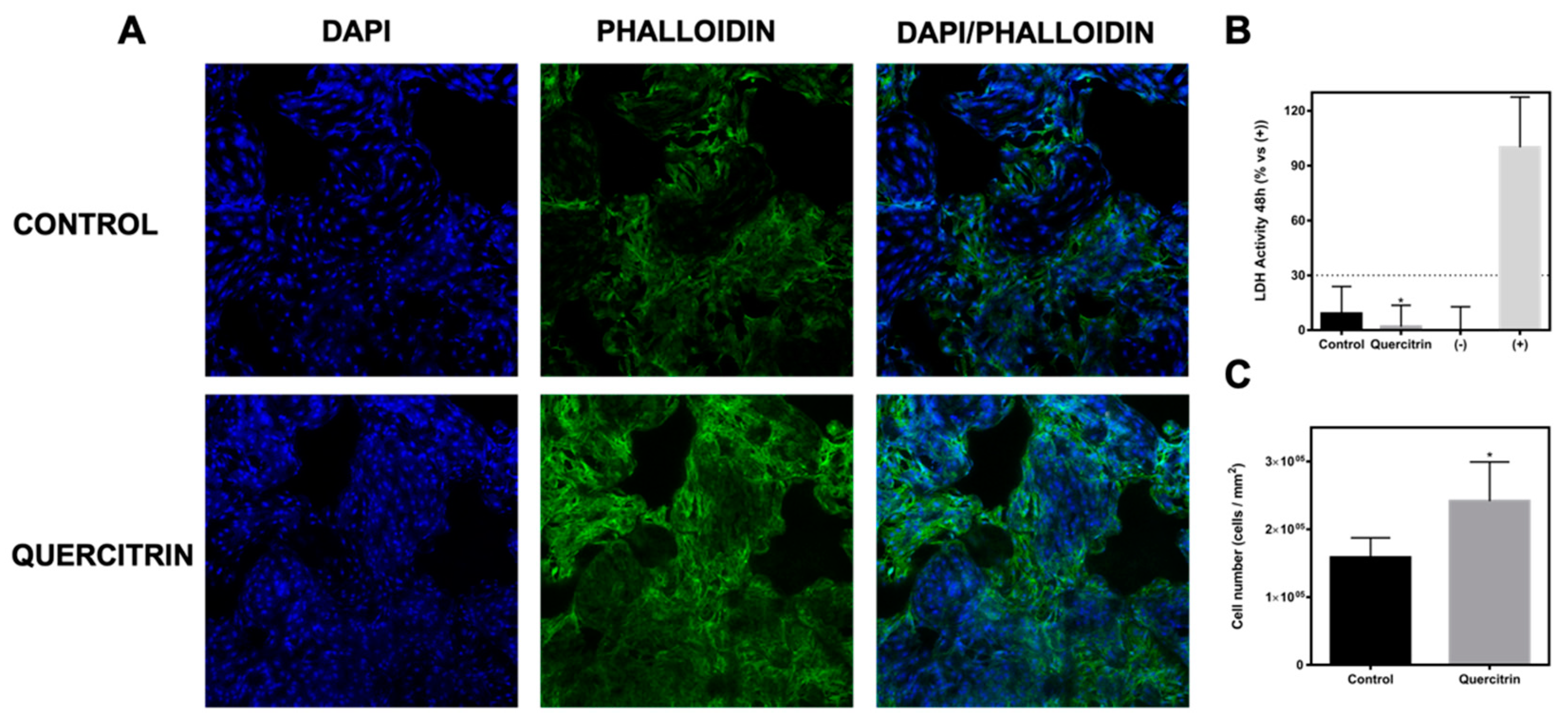
2.6. Determination of Cell Viability: LDH Activity
2.7. Immunofluorescence
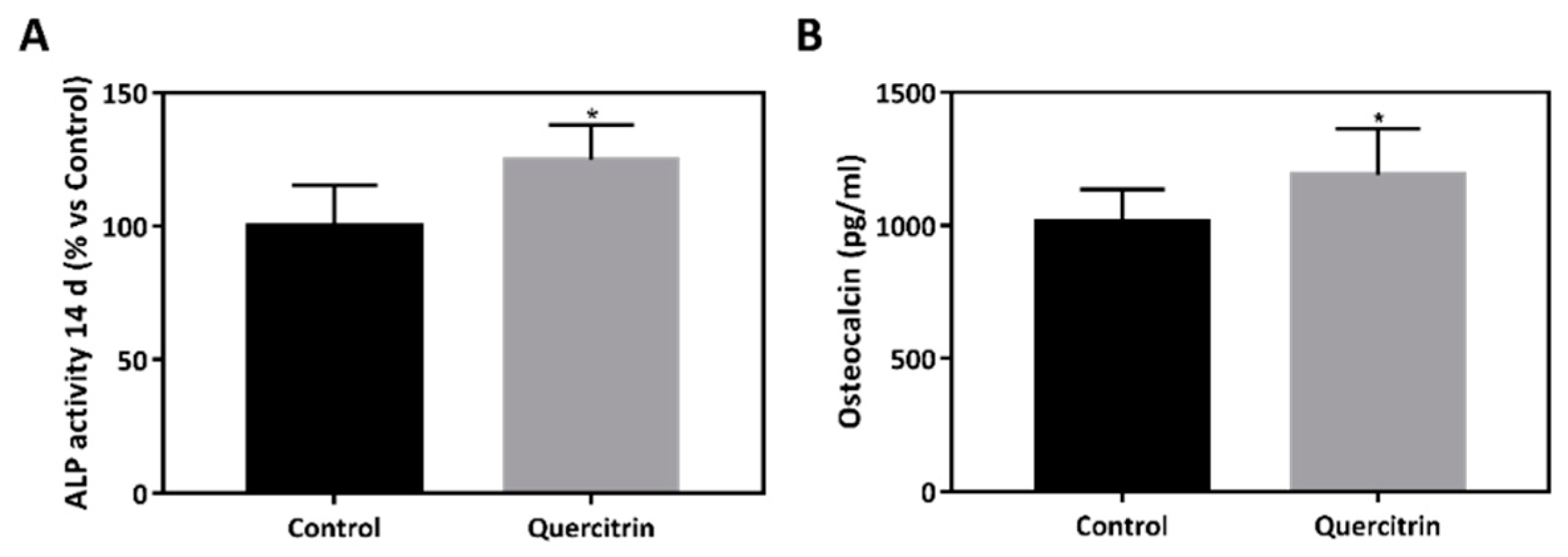
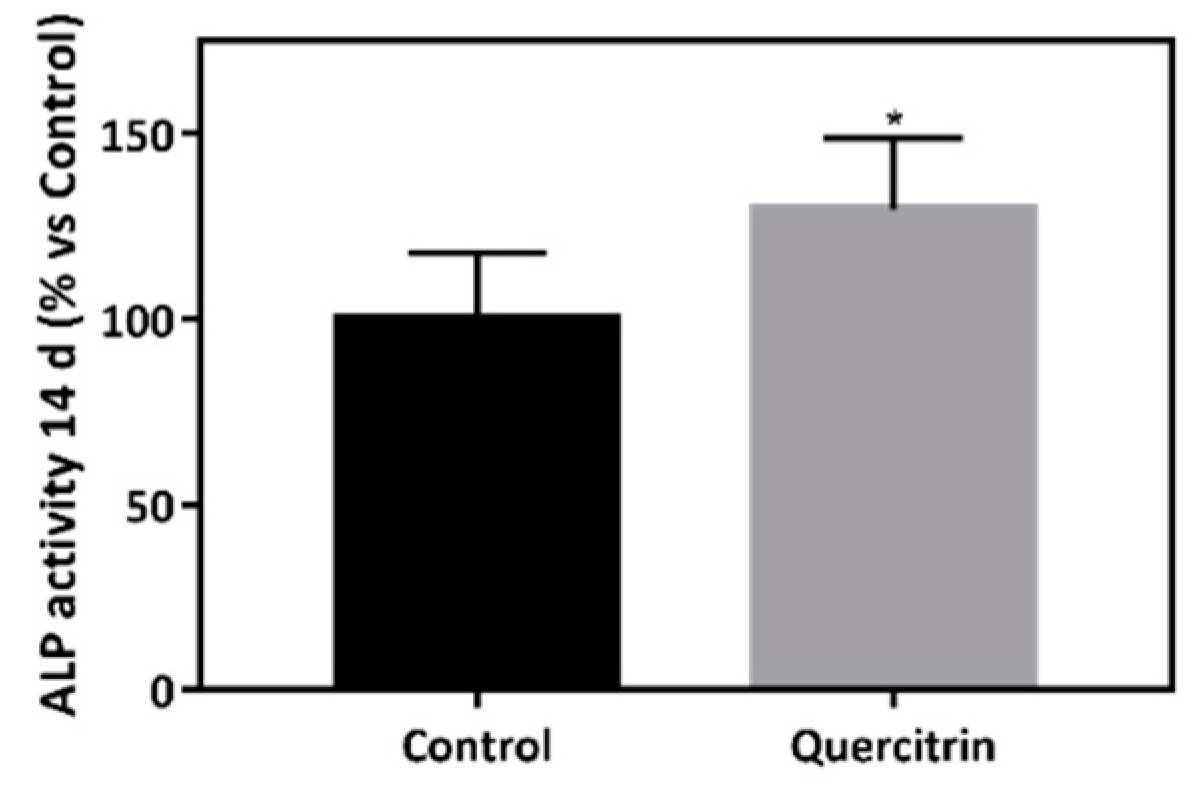
2.8. Alkaline Phosphatase (ALP) Activity
2.9. Osteocalcin Content
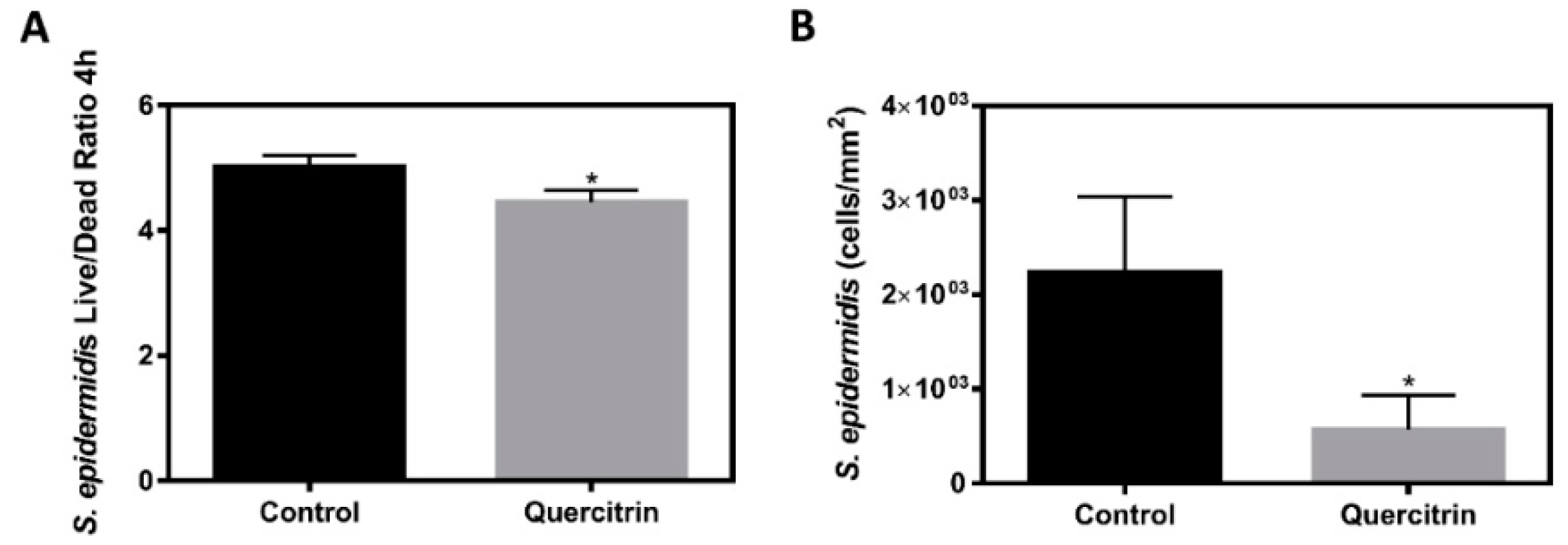
2.10. Staphylococcus epidermidis Live/Dead Ratio and Adhesion
2.11. Statistics
3. Results
3.1. Porous Ti-6Al-4V Implants Characterization
3.2. Biocompatibility and Bioactivity of the Implants
3.3. Bioactivity of the Implants under Bacterial Challenging Conditions
3.4. Effect of the Implants on S. epidermidis Survival and Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deing, A.; Luthringer, B.; Laipple, D.; Ebel, T.; Willumeit, R. A Porous TiAl6V4 Implant Material for Medical Application. Int. J. Biomater. 2014, 2014, 904230. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Griesser, H.J.; Peleg, A.Y.; Traven, A. Anti-infective Surface Coatings: Design and Therapeutic Promise against Device-Associated Infections. PLOS Pathog. 2016, 12, e1005598. [Google Scholar] [CrossRef] [PubMed]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. BioMetals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Ma, Y.; Hu, Y.; Wu, Y.; Lan, F.; Weir, M.D.; Li, M.; Ren, B.; Oates, T.W.; et al. Two-staged time-dependent materials for the prevention of implant-related infections. Acta Biomater. 2019. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Guan, M.; Chen, Y.; Wei, Y.; Song, H.; Gao, C.; Cheng, H.; Li, Y.; Huo, K.; Fu, J.; Xiong, W. Long-lasting bactericidal activity through selective physical puncture and controlled ions release of polydopamine and silver nanoparticles-loaded TiO2 nanorods in vitro and in vivo. Int. J. Nanomed. 2019, 14, 2903–2914. [Google Scholar] [CrossRef]
- Peñarrieta-Juanito, G.M.; Costa, M.; Cruz, M.; Miranda, G.; Henriques, B.; Marques, J.; Magini, R.; Mata, A.; Caramês, J.; Silva, F.; et al. Bioactivity of novel functionally structured titanium-ceramic composites in contact with human osteoblasts. J. Biomed. Mater. Res.—Part A 2018, 106, 1923–1931. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A bioactive titanium foam scaffold for bone repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef]
- Satué, M.; Arriero, M.D.M.; Monjo, M.; Ramis, J.M. Quercitrin and Taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 2013, 86, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of Quercitrin as a Potential Therapeutic Agent for Periodontal Applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Córdoba, A.; Monjo, M.; Hierro-Oliva, M.; González-Martín, M.L.; Ramis, J.M. Bioinspired Quercitrin Nanocoatings: A Fluorescence-Based Method for Their Surface Quantification, and Their Effect on Stem Cell Adhesion and Differentiation to the Osteoblastic Lineage. ACS Appl. Mater. Interfaces 2015, 7, 16857–16864. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pacha-Olivenza, M.A.; Fernández-Calderón, M.C.; Córdoba, A.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Quercitrin-nanocoated titanium surfaces favour gingival cells against oral bacteria. Sci. Rep. 2016, 6, 22444. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Rubert, M.; Ramis, J.M.; Haugen, H.J.; Tiainen, H.; Lyngstadaas, S.P.; Monjo, M. TiO2 Scaffolds Sustain Differentiation of MC3T3-E1 Cells. J. Biomater. Tissue Eng. 2012, 2, 336–344. [Google Scholar] [CrossRef]
- Satué, M.; Petzold, C.; Córdoba, A.; Ramis, J.M.; Monjo, M. UV photoactivation of 7-dehydrocholesterol on titanium implants enhances osteoblast differentiation and decreases Rankl gene expression. Acta Biomater. 2013, 9, 5759–5770. [Google Scholar] [CrossRef]
- Guida, L.; Annunziata, M.; Carinci, F.; Di Feo, A.; Passaro, I.; Oliva, A. In vitro biologic response of human bone marrow stromal cells to enamel matrix derivative. J. Periodontol. 2007, 78, 2190–2196. [Google Scholar] [CrossRef]
- Geißler, S.; Barrantes, A.; Tengvall, P.; Messersmith, P.B.; Tiainen, H. Deposition Kinetics of Bioinspired Phenolic Coatings on Titanium Surfaces. Langmuir 2016, 32, 8050–8060. [Google Scholar] [CrossRef]
- Kurth, D.G.; Bein, T. Thin Films of (3-Aminopropyl)triethoxysilane on Aluminum Oxide and Gold Substrates. Langmuir 1995, 11, 3061–3067. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Manzanaro-Moreno, N.; Colom, C.; Rønold, H.J.; Lyngstadaas, S.P.; Monjo, M.; Ramis, J.M. Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3319. [Google Scholar] [CrossRef]
- Yücel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.-H.; Cyganek, L.; Utikal, J.; et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 2935. [Google Scholar] [CrossRef]
- Badar, M.; Rahim, M.I.; Kieke, M.; Ebel, T.; Rohde, M.; Hauser, H.; Behrens, P.; Mueller, P.P. Controlled drug release from antibiotic-loaded layered double hydroxide coatings on porous titanium implants in a mouse model. J. Biomed. Mater. Res.—Part A 2015, 103, 2141–2149. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Gorgin Karaji, Z.; Lietaert, K.; Fluit, A.C.; Boel, C.H.E.; Vogely, H.C.; Vermonden, T.; Hennink, W.E.; Weinans, H.; Zadpoor, A.A.; et al. Simultaneous Delivery of Multiple Antibacterial Agents from Additively Manufactured Porous Biomaterials to Fully Eradicate Planktonic and Adherent Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 25691–25699. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llopis-Grimalt, M.A.; Arbós, A.; Gil-Mir, M.; Mosur, A.; Kulkarni, P.; Salito, A.; Ramis, J.M.; Monjo, M. Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro. J. Clin. Med. 2020, 9, 855. https://doi.org/10.3390/jcm9030855
Llopis-Grimalt MA, Arbós A, Gil-Mir M, Mosur A, Kulkarni P, Salito A, Ramis JM, Monjo M. Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro. Journal of Clinical Medicine. 2020; 9(3):855. https://doi.org/10.3390/jcm9030855
Chicago/Turabian StyleLlopis-Grimalt, Maria Antonia, Aina Arbós, Maria Gil-Mir, Aleksandra Mosur, Prathamesh Kulkarni, Armando Salito, Joana M. Ramis, and Marta Monjo. 2020. "Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro" Journal of Clinical Medicine 9, no. 3: 855. https://doi.org/10.3390/jcm9030855
APA StyleLlopis-Grimalt, M. A., Arbós, A., Gil-Mir, M., Mosur, A., Kulkarni, P., Salito, A., Ramis, J. M., & Monjo, M. (2020). Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro. Journal of Clinical Medicine, 9(3), 855. https://doi.org/10.3390/jcm9030855





