Does Combining Biomarkers and Brain Images Provide Improved Prognostic Predictive Performance for Out-Of-Hospital Cardiac Arrest Survivors before Target Temperature Management?
Abstract
1. Introduction
2. Experimental Section
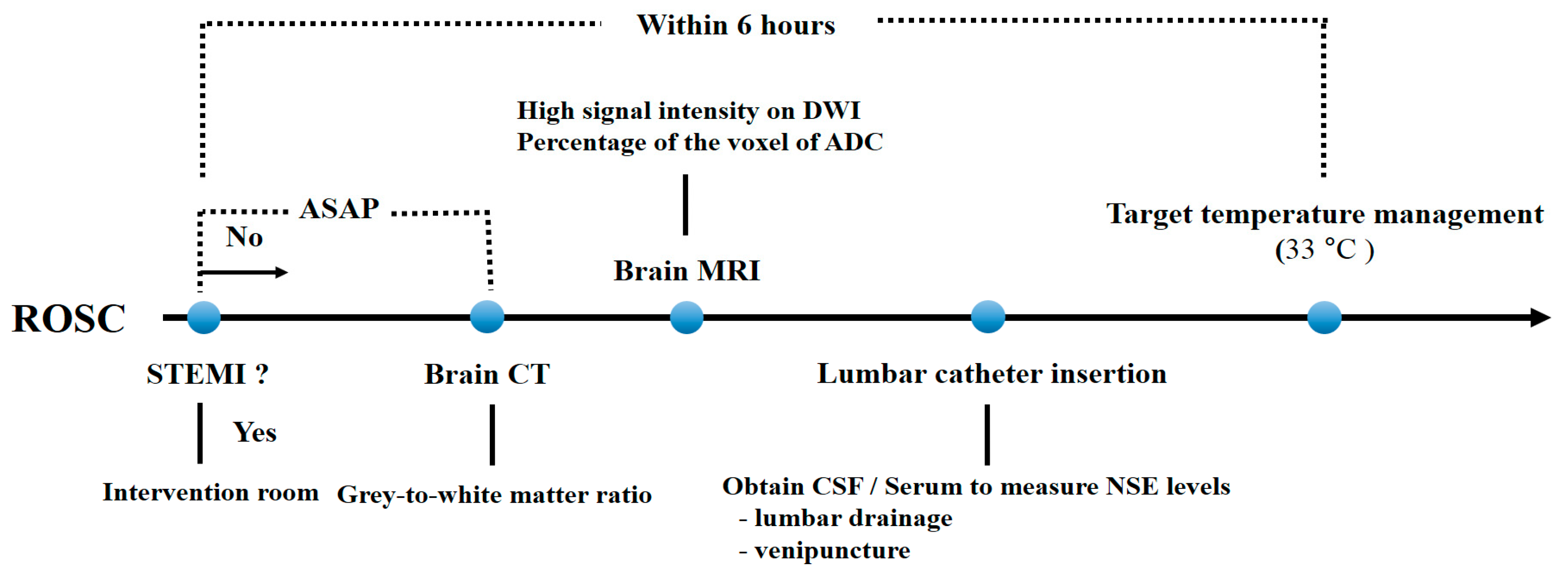
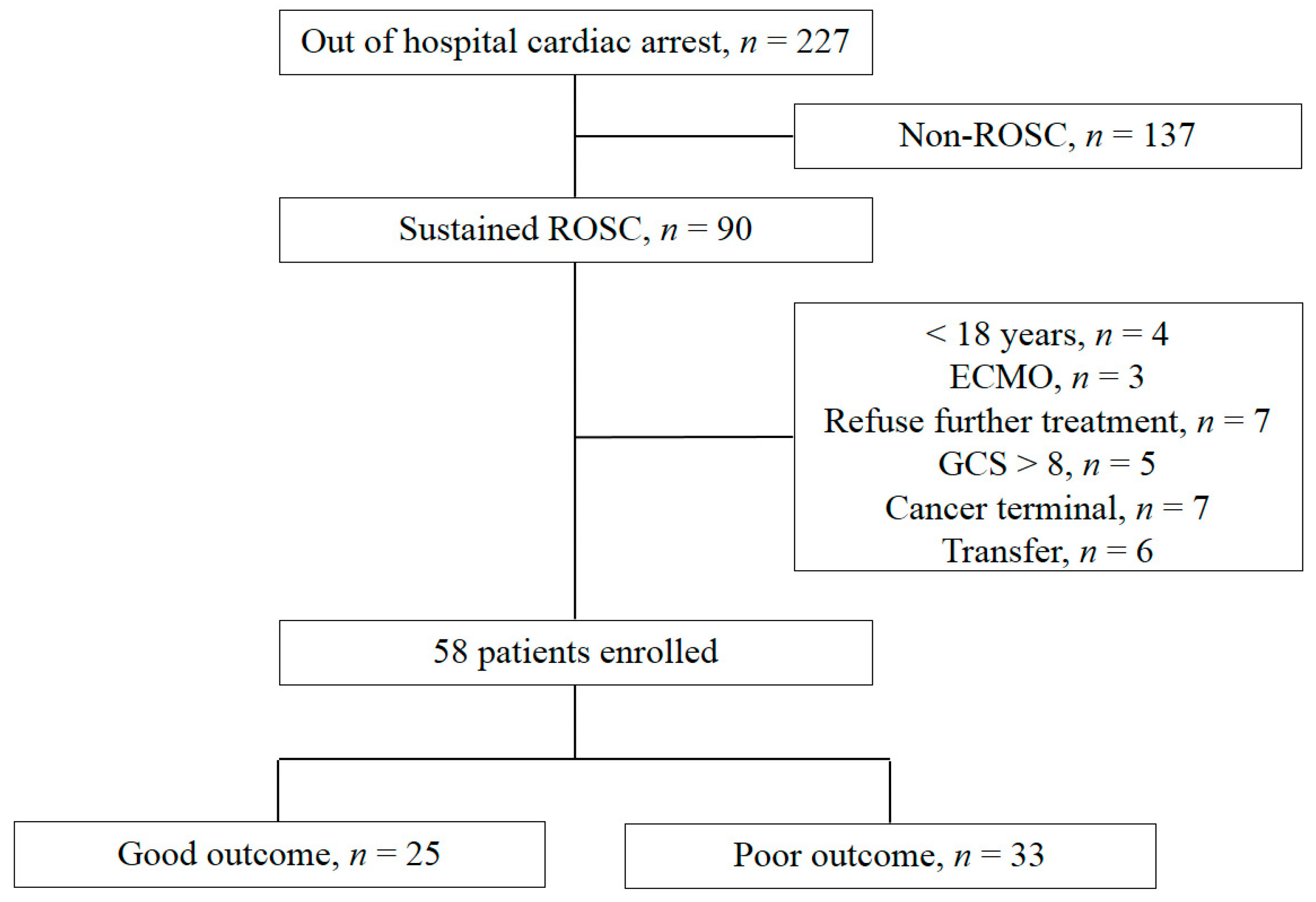
2.1. Study Design and Population
2.2. TTM Protocol
2.3. Measurement of NSE Levels in CSF and Serum
2.4. Grey-To-White Matter Ratio Measurement Using Brain CT
2.5. MRI (High Signal Intensity in DWI and Percentage of Voxels of ADC)
2.6. Outcome
2.7. Data Collection
2.8. Data Analysis
3. Results
3.1. Patient Demographics
3.2. Comparison of Neurologic Outcome Using Each Method
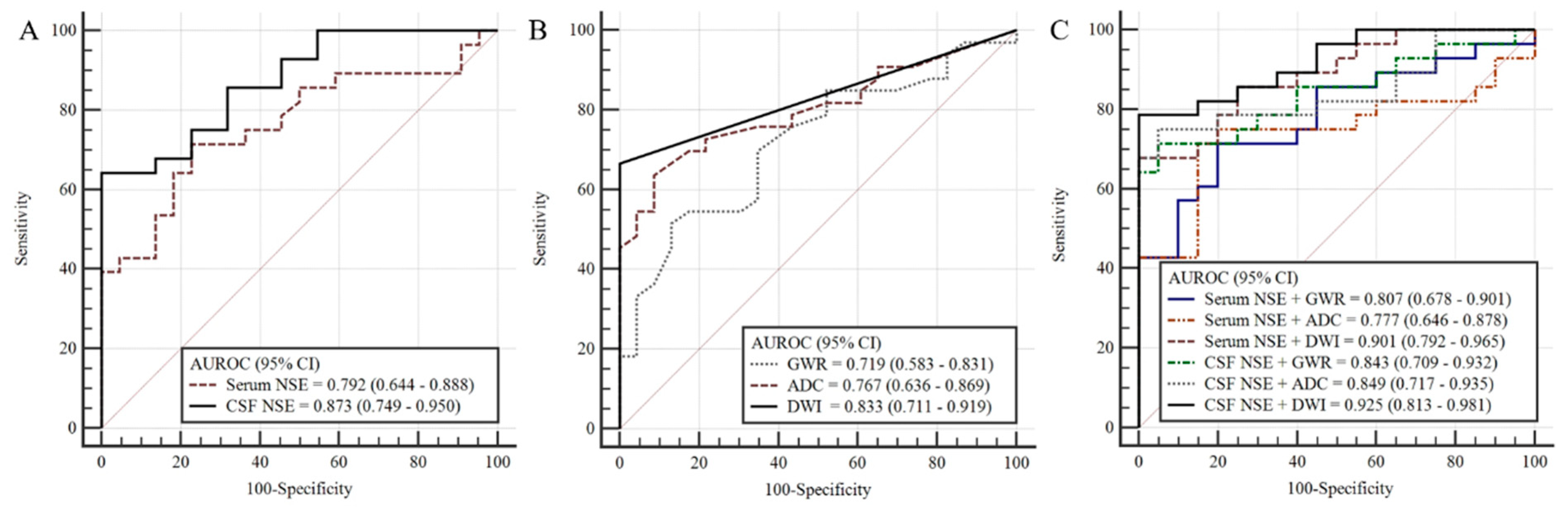
3.3. Prognostic Performance of Each Method
3.4. Prognostic Performance of Combining NSE Levels and Brain Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zandbergen, E.G.; de Haan, R.J.; Reitsma, J.B.; Hijdra, A. Survival and recovery of consciousness in anoxic-ischemic coma after cardiopulmonary resuscitation. Intensive Care Med. 2003, 29, 1911–1915. [Google Scholar] [CrossRef]
- Geocadin, R.G.; Callaway, C.W.; Fink, E.L.; Golan, E.; Greer, D.M.; Ko, N.U.; Lang, E.; Licht, D.J.; Marino, B.S.; McNair, N.D.; et al. Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e517–e542. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Torres, C.; Aufderheide, T.P.; Austin, M.A.; Callaway, C.W.; Golan, E.; Herren, H.; Jasti, J.; Kudenchuk, P.; Scales, D.C.; et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016, 102, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, E.A.; Mlynash, M.; Caulfield, A.F.; Eyngorn, I.; Wijman, C.A.C. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit. Care 2011, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Karapetkova, M.; Koenig, M.A.; Jia, X. Early prognostication markers in cardiac arrest patients treated with hypothermia. Eur. J. Neurol. 2016, 23, 476–488. [Google Scholar] [CrossRef]
- Jeon, C.H.; Park, J.S.; Lee, J.H.; Kim, H.; Kim, S.C.; Park, K.H.; Yi, K.S.; Kim, S.M.; Youn, C.S.; Kim, Y.M.; et al. Comparison of brain computed tomography and diffusion-weighted magnetic resonance imaging to predict early neurologic outcome before target temperature management comatose cardiac arrest survivors. Resuscitation 2017, 118, 21–26. [Google Scholar] [CrossRef]
- You, Y.; Park, J.; Min, J.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; Cho, S.; et al. Relationship between time related serum albumin concentration, optic nerve sheath diameter, cerebrospinal fluid pressure, and neurological prognosis in cardiac arrest survivors. Resuscitation 2018, 131, 42–47. [Google Scholar] [CrossRef]
- Moon, H.K.; Jang, J.; Park, K.N.; Kim, S.H.; Lee, B.K.; Oh, S.H.; Jeung, K.W.; Choi, S.P.; Cho, I.S.; Youn, C.S. Quantitative analysis of relative volume of low apparent diffusion coefficient value can predict neurologic outcome after cardiac arrest. Resuscitation 2018, 126, 36–42. [Google Scholar] [CrossRef]
- You, Y.; Park, J.S.; Min, J.; Yoo, I.; Ahn, H.J.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; Cho, S.; et al. The usefulness of neuron-specific enolase in cerebrospinal fluid to predict neurological prognosis in cardiac arrest survivors who underwent target temperature management: A prospective observational study. Resuscitation 2019, 145, 185–191. [Google Scholar] [CrossRef]
- Longstreth, W.T., Jr.; Nichol, G.; Van Ottingham, L.; Hallstrom, A.P. Two simple questions to assess neurologic outcomes at 3 months after out-of-hospital cardiac arrest: Experience from the public access defibrillation trial. Resuscitation 2010, 81, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Boone, R.H.; Tomlinson, G.; Detsky, A.S. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004, 291, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, C.; D’Arrigo, S.; Nolan, J.P. Prognostication after cardiac arrest. Crit. Care 2018, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Lee, D.H.; Oh, J.H.; Lee, S.H.; Choi, Y.H.; Kim, S.H.; Min, J.H.; Kim, S.J.; Park, Y.S. Korean Hypothermia Network Investigators. Grey-white matter ratio measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation 2019, 140, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.W.; Kim, H.; Min, J.H.; Kang, J.H.; Yi, K.S.; Park, K.H.; Lee, B.K. Efficacy of diffusion-weighted magnetic resonance imaging performed before therapeutic hypothermia in predicting clinical outcome in comatose cardiopulmonary arrest survivors. Resuscitation 2015, 88, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.P.; Park, K.N.; Park, H.K.; Kim, J.Y.; Youn, C.S.; Ahn, K.J.; Yim, H.W. Diffusion-weighted magnetic resonance imaging for predicting the clinical outcome of comatose survivors after cardiac arrest: A cohort study. Crit. Care 2010, 14, R17. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; In, Y.N.; You, Y.H.; Min, J.H.; Ahn, H.J.; Yoo, I.S.; Kim, S.W.; Lee, J.W.; Ryu, S.; Jeong, W.J.; et al. Ultra-early neurologic outcome prediction of out-of-hospital cardiac arrest survivors using combined diffusion-weighted imaging findings and quantitative analysis of apparent diffusion coefficient. Resuscitation 2020, 148, 39–48. [Google Scholar] [CrossRef]
- Oren, N.C.; Chang, E.; Yang, C.W.; Lee, S.K. Brain Diffusion Imaging Findings May Predict Clinical Outcome after Cardiac Arrest. J. Neuroimaging 2019, 29, 540–547. [Google Scholar] [CrossRef]
- Moseley, M.E.; Cohen, Y.; Mintorovitch, J.; Chileuitt, L.; Shimizu, H.; Kucharczyk, J.; Wendland, M.F.; Weinstein, P.R. Early detection of regional cerebral ischemia in cats: Comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn. Reson. Med. 1990, 14, 330–346. [Google Scholar] [CrossRef]
- McGarry, B.L.; Jokivarsi, K.T.; Knight, M.J.; Grohn, O.H.J.; Kauppinen, R.A. Magnetic Resonance Imaging Protocol for Stroke Onset Time Estimation in Permanent Cerebral Ischemia. J. Vis. Exp. 2017, e55277. [Google Scholar] [CrossRef]
- Wijman, C.A.; Mlynash, M.; Caulfield, A.F.; Hsia, A.W.; Eyngorn, I.; Bammer, R.; Fischbein, N.; Albers, G.W.; Moseley, M. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann. Neurol. 2009, 65, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Baddeley, C.; O’Sullivan, M.J.; Bells, S.; Pasternak, O.; Jones, D.K. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 2012, 59, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Berlot, R.; Metzler-Baddeley, C.; Jones, D.K.; O’Sullivan, M.J. CSF contamination contributes to apparent microstructural alterations in mild cognitive impairment. Neuroimage 2014, 92, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Vondrakova, D.; Kruger, A.; Janotka, M.; Malek, F.; Dudkova, V.; Neuzil, P.; Ostadal, P. Association of neuron-specific enolase values with outcomes in cardiac arrest survivors is dependent on the time of sample collection. Crit. Care. 2017, 21, 172. [Google Scholar] [CrossRef]
- Echeverria-Palacio, C.M.; Agut, T.; Arnaez, J.; Valls, A.; Reyne, M.; Garcia-Alix, A. Neuron-Specific Enolase in Cerebrospinal Fluid Predicts Brain Injury After Sudden Unexpected Postnatal Collapse. Pediatr. Neurol. 2019, 101, 71–77. [Google Scholar] [CrossRef]
- Lee, B.K.; Jeung, K.W.; Lee, H.Y.; Jung, Y.H.; Lee, D.H. Combining brain computed tomography and serum neuron specific enolase improves the prognostic performance compared to either alone in comatose cardiac arrest survivors treated with therapeutic hypothermia. Resuscitation 2013, 84, 1387–1392. [Google Scholar] [CrossRef]
| Characteristics | Cohort (n = 58) | Good Outcome (n = 25) | Poor Outcome (n = 33) | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 53.5 (37.6–69.0) | 50.5 (43.0–58.1) | 55.3 (48.8–61.7) | 0.347 |
| Sex, male, n (%) | 40 (69.0) | 20 (80.0) | 20 (75.8) | 0.155 |
| Charlson Comorbidity Index score, median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–1.50) | 0.975 |
| Arrest characteristics | ||||
| Witness arrest, n (%) | 36 (62.1) | 21 (84.0) | 15 (45.5) | 0.003 |
| Bystander CPR, n (%) | 41 (70.7) | 21 (84.0) | 20 (62.5) | 0.085 |
| Shockable rhythm, n (%) | 19 (33.3) | 16 (64.0) | 3 (9.4) | 0.000 |
| Cardiac aetiology, n (%) | 17 (30.4) | 13 (52.0) | 4 (12.9) | 0.002 |
| No flow time, min (IQR) | 3.5 (0.0–16.0) | 0.0 (0.0–5.0) | 12.0 (1.0–42.0) | 0.002 |
| Low flow time, min (IQR) | 20.0 (9.0–33.0) | 9.0 (5.5–16.5) | 30.0 (19.5–42.5) | <0.001 |
| ROSC to CT time, min (IQR) | 79.0 (43.0–129.0) | 77.0 (40.5–106.5) | 95.0 (43.0–152.0) | 0.271 |
| ROSC to MRI time, min (IQR) | 180.5 (128.0–240.8) | 154.0 (113.5–286.5) | 194.0 (129.5–288.5) | 0.713 |
| ROSC to LP time, min (IQR) | 256.5 (223.8–364.8.0) | 239.0 (193.0–430.0) | 272.0 (229.0–334.0) | 0.303 |
| Characteristics | Good Neurologic Outcome (n = 25) | Poor Neurologic Outcome (n = 33) | p-Value |
|---|---|---|---|
| Serum NSE, median (IQR), 57 * | 26.1 (19.4–33.3), 25 * | 48.1 (30.0–90.2), 32 * | <0.001 |
| CSF NSE, median (IQR), 51 * | 19.1 (11.8–33.2), 23 * | 94.7 (19.2–183.8), 28 * | <0.001 |
| GWR, median (IQR), 58 * | 1.24 (1.19–1.29), 25 * | 1.16 (1.11–1.24), 33 * | 0.005 |
| HSI on DWI, number (%), 57 * | 0 (0.0%), 24 * | 22 (66.7%), 33 * | <0.001 |
| PV 400 ** on ADC, median (IQR), 57 * | 2.28 (0.32–2.93), 24 * | 3.90 (2.24–29.24), 33 * | <0.001 |
| Characteristics | AUC (95% CI) | p-Value | Cut-Off | Sensitivity/Specificity (%) | PPV | NPV | TP | TN | FP | FN |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum NSE, 57 * | 0.792 (0.664–0.888) | <0.001 | 54.8 | 46.9/100 | 100.0 | 59.5 | 15 | 26 | 0 | 16 |
| CSF NSE, 51 * | 0.873 (0.749–0.950) | <0.001 | 53.7 | 64.3/100 | 100.0 | 68.7 | 18 | 23 | 0 | 10 |
| DWI (HSI), 57 * | 0.833 (0.711–0.919) | <0.001 | HSI positive | 66.7/100 | 100.0 | 68.6 | 22 | 24 | 0 | 11 |
| ADC (PV 400 **), 57 * | 0.767 (0.636–0.869) | <0.001 | 4.3 | 45.5/100 | 100.0 | 57.1 | 15 | 24 | 0 | 18 |
| GWR, 58 * | 0.719 (0.583–0.831) | 0.002 | 1.07 | 18.2/100 | 100.0 | 46.0 | 6 | 26 | 0 | 26 |
| DWI + Serum NSE, 56 * | 0.901 (0.792–0.965) | <0.001 | 71.9/100 | 100.0 | 72.7 | 23 | 24 | 0 | 9 | |
| DWI + CSF NSE, 49 * | 0.925 (0.813–0.981) | <0.001 | 77.8/100 | 100.0 | 72.7 | 22 | 21 | 0 | 6 | |
| ADC + Serum NSE, 56 * | 0.777 (0.646–0.878) | <0.001 | 78.6/100 | 100.0 | 77.8 | 16 | 24 | 0 | 16 | |
| ADC + CSF NSE, 49 * | 0.849 (0.717–0.935) | <0.001 | 67.9/100 | 100.0 | 70.0 | 18 | 21 | 0 | 10 | |
| GWR + Serum NSE, 56 * | 0.807 (0.678–0.901) | <0.001 | 50.0/100 | 100.0 | 59.0 | 16 | 24 | 0 | 16 | |
| GWR + CSF NSE, 49 * | 0.855 (0.724–0.940) | <0.001 | 64.3/100 | 100.0 | 48.8 | 18 | 21 | 0 | 10 |
| Characteristics | AUC (95% CI) | p-Value | Sensitivity (%) | Specificity (%) | PPV | NPV |
|---|---|---|---|---|---|---|
| CN/CC | 0.705 (0.568–0.819) | 0.003 | 33.3 | 100.0 | 100.0 | 51.1 |
| P/CC | 0.652 (0.513–0.775) | 0.048 | 6.06 | 100.0 | 100.0 | 42.6 |
| T/CC | 0.692 (0.554–0.808) | 0.007 | 18.18 | 100.0 | 100.0 | 46.0 |
| CN/PIC | 0.607 (0.468–0.735) | 0.161 | 12.12 | 100.0 | 100.0 | 44.2 |
| P/PIC | 0.559 (0.420–0.691) | 0.464 | 3.0 | 100.0 | 100.0 | 41.8 |
| T/PIC | 0.588 (0.448–0.718) | 0.254 | 6.06 | 100.0 | 100.0 | 42.6 |
| Average GWR | 0.687 (0.549–0.804) | 0.01 | 18.18 | 100.0 | 100.0 | 46.0 |
| Average (CC) * | 0.719 (0.583–0.831) | 0.002 | 18.18 | 100.0 | 100.0 | 46.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.H.; Lee, I.H.; Park, J.S.; Yoo, I.S.; Kim, S.W.; Lee, J.W.; Ryu, S.; You, Y.; Min, J.H.; Cho, Y.C.; et al. Does Combining Biomarkers and Brain Images Provide Improved Prognostic Predictive Performance for Out-Of-Hospital Cardiac Arrest Survivors before Target Temperature Management? J. Clin. Med. 2020, 9, 744. https://doi.org/10.3390/jcm9030744
Son SH, Lee IH, Park JS, Yoo IS, Kim SW, Lee JW, Ryu S, You Y, Min JH, Cho YC, et al. Does Combining Biomarkers and Brain Images Provide Improved Prognostic Predictive Performance for Out-Of-Hospital Cardiac Arrest Survivors before Target Temperature Management? Journal of Clinical Medicine. 2020; 9(3):744. https://doi.org/10.3390/jcm9030744
Chicago/Turabian StyleSon, Seung Ha, In Ho Lee, Jung Soo Park, In Sool Yoo, Seung Whan Kim, Jin Woong Lee, Seung Ryu, Yeonho You, Jin Hong Min, Yong Chul Cho, and et al. 2020. "Does Combining Biomarkers and Brain Images Provide Improved Prognostic Predictive Performance for Out-Of-Hospital Cardiac Arrest Survivors before Target Temperature Management?" Journal of Clinical Medicine 9, no. 3: 744. https://doi.org/10.3390/jcm9030744
APA StyleSon, S. H., Lee, I. H., Park, J. S., Yoo, I. S., Kim, S. W., Lee, J. W., Ryu, S., You, Y., Min, J. H., Cho, Y. C., Jeong, W. J., Oh, S. K., Cho, S. U., Ahn, H. J., Kang, C., Lee, D. H., Lee, B. K., & Youn, C. S. (2020). Does Combining Biomarkers and Brain Images Provide Improved Prognostic Predictive Performance for Out-Of-Hospital Cardiac Arrest Survivors before Target Temperature Management? Journal of Clinical Medicine, 9(3), 744. https://doi.org/10.3390/jcm9030744





