Abstract
Propionibacterium are anaerobic/aero-tolerant rod Gram-positive bacteria, and numerous studies are associated with primary and secondary endodontic infections. The data in the literature on the prevalence of Propionibacterium are conflicting, and there are studies that report conflicting data on the prevalence in primary and secondary endodontic infections. This review aims to clarify the prevalence of bacteria of the genus Propionibacterium in endodontic lesions. The present systematic review work was performed on the basis of the Prisma protocol. A search was carried out on the PubMed and Scopus databases with the use of keywords. The research produced 410 records, which, after the elimination of the overlaps and the application of the inclusion and exclusion criteria, led to a number of 36 included articles divided by the three outcomes. The first outcome concerns prevalence of bacteria of the genus Propionibacterium in primary and secondary endodontic lesions. The secondary outcome, differences in the prevalence of bacteria of the genus Propionibacterium between primary endodontic infections and secondary endodontic infections. The tertiary outcome, differences in the prevalence of Propionibacterium Acnes compared to Propionibacterium propionicum in endodontic infections. The results of the meta-analysis show that the genus Propionibacterium bacteria are more prevalent in secondary endodontic infections and that P. acnes has a higher prevalence than P. propionicum.
1. Introduction
The bacteria involved in primary endodontic lesions are mainly aerobic and facultative anaerobes. In the literature the bacterial associations most frequently associated with primary infections are: Fusobacterium nucleatum, Porphyromonas endodontalis, Peptostreptococcus micros, Campylobacter rectus [1]; Prevotella intermedia, P. micros, and P. anaerobius eubacteria [2]; and eubacteria Prevotella, and Peptostreptococcus [3]. For persistent intraradicular and extraradicular secondary infections, the scientific literature focuses on the role of the enterococci (enterococcus faecalisis) of streptococci (Gram-positive optional anaerobic bacteria) while calling into question the role of bacteria of the genus Actinomyces and of the genus Propionibacterium for persistent infections involving the extraradicular apical surface with the formation of bacterial biofilms. Propionibacterium are anaerobic/aerotolerant rod Gram-positive bacteria, and numerous studies are associated with primary and secondary endodontic infections; however, the prevalence data are actually conflicting. In fact, Sundqvist et al., 1989 [4] reports the presence of Propionibacterium on 1 in 72 in apical periodontitis, while Niazi et al., 2010 reports data with a prevalence of 18 out of 20 in refractory endodontic lesions and Rocas et al., 2011 [5] 26 on 43 necrotic teeth.
Previous systematic reviews have not investigated the role of Propionibacterium. The latest review conducted by Prada et al., 2019 on the role of bacteria in endodontic infections did not perform meta-analysis on the prevalence of Propionibacterium [6].
This review aims to clarify the prevalence of bacteria of the genus Propionibacterium in endodontic lesions. This knowledge is important given the presence of bacteria of the Order of Actinomycetes in persistent endodontic infections refractory to endodontic treatments [7].
2. Materials and Methods
The following systematic review was conducted based on the indications of the Prisma protocol.
The study was constructed on the population, intervention, control, and outcome (PICO) questions: population (patients with teeth with primary and secondary endodontic infections), intervention (bacteria of the genus Propionibacterium), control (patients with teeth that have no Propionibacterium infections), and outcome (prevalence of bacteria of the genus Propionibacterium in primary and secondary endodontic infections).
The formulation of the PICO question is as follows: What is the prevalence of bacteria of the genus Propionibacterium in primary and secondary endodontic infections (primary outcome)? Other questions were also raised: if there is a greater prevalence of Propionibacterium in secondary endodontic infections than primary infections (secondary questions) and which among the species of the genus Propionibacterium (P. acnes and P. propionicum) has the greatest prevalence in endodontic lesions (tertiary questions)?
After an initial selection phase of the records identified in the databases, the potentially eligible articles are qualitatively evaluated in order to investigate the role of bacteria in endodontic infections and in apical periodontitis, with particular attention to the role of Propionibacterium in endodontic infections.
2.1. Eligibility Criteria
The works taken into consideration are clinical studies concerning the role of bacteria in endodontic bacterial infections. In particular, all studies that have investigated the presence of microorganisms within the dental elements subject to endodontic treatment or retreatment conducted in recent years and published with abstracts in English have been considered potentially eligible.
It was decided to choose articles of the last 40 years, because more and more new bacterial species have been identified since 1980 [8], and moreover, the way of classifying and dividing them in the various families has changed. [9] Moreover, the identification methods of bacteria have improved with the introduction of PCR.
The potentially eligible articles were finally subjected to a full-text analysis to verify use for a qualitative analysis and quantitative analysis.
The inclusion and exclusion criteria applied in the full-text analysis are the following:
- Include all those studies that identified the presence of bacteria of the genus Propionibacterium in the dental elements subjected to endodontic treatment or retreatment or in the teeth subjected to apicectomy or extraction following endodontic failure.
- The exclusion criteria are: to exclude all those studies that do not report the prevalence data of the bacteria of the genus Propionibacterium in the primary and secondary lesions of the dental elements, to exclude all studies reporting a number of teeth examined below 20 for excessive risk of bias, and those not written in English and were published before 1980.
2.2. Research Methodology
Studies have been identified through bibliographic research on electronic databases.
The literature search was conducted on the search engines “PubMed” and “Scopus”. The search on the providers was conducted between 07.12.2019 and 22.12.2019, and the last search for a partial update of the literature was conducted on 26/12/2019.
The following search terms were used on PubMed and Scopus: “Propionibacterium” AND “endodontic” OR “apical parodontitis” (PubMed 67), “persistent intraradicular infection” OR “primary endodontic infection” (PubMed 36), “endodontic failure” OR “endodontic microbiologic” (PubMed 201), persistent intraradicular infection (Scopus 23), “persistent extraradicular infection” (Scopus 18), and “Propionibacterium” AND “endodontic” (Scopus 65) (Table 1).

Table 1.
Complete overview of the search methodology. Records identified by databases: 410 and records selected for quantitative analysis: 36.
2.3. Screening Methodology
The keywords to be searched and their combinations were decided before the identification phase of the records in common agreement between the two reviewers (with the task of selecting potentially eligibile articles). The records obtained were subsequently examined by two independent reviewers (M.D. and C.Q.), and a third reviewer (G.T.) acted as a decision-maker in situations of doubt.
The screening included the analysis of the title and the abstract and, in doubtful cases, of text analysis to eliminate the records not related to the topics of the review. The articles obtained were subjected to full-text analysis by the two reviewers (80 articles), from which the ones eligible for the qualitative analysis and inclusion in the meta-analysis for the three outcomes were identified. The results sought by the two reviewers were:
- (1)
- Primary outcome, the prevalence of bacteria of the genus Propionibacterium in primary and secondary endodontic lesions;
- (2)
- Secondary outcome, differences in the prevalence of bacteria of the genus Propionibacterium between primary endodontic infections and secondary endodontic infections;
- (3)
- Tertiary outcome, differences in the prevalence of Propionibacterium Acnes compared to Propionibacterium propionicum in endodontic infections.
The K agreement between the two screening reviewers was 0.8584 (Table 2) [10]. The K agreement was based on the formulas of the Cochrane Handbook for Systematic Reviews [11].

Table 2.
K agreement calculation: Po = 0.925 (proportion of agreement) and Pe = 0.4701 (agreement expected), and so K agreement = 0.8584. No agreement, 0.0–0.20: slight agreement, 0.21–0.40: fair agreement, 0.41–0.60: moderate agreement, 0.61–0.80: substantial agreement, and 0.81–1.00: almost perfect agreement. The K agreement was calculated from the 36 articles to include 15 articles with the application of the inclusion and exclusion criteria.
The entire selection and screening procedures are described in a flow chart (Figure 1).
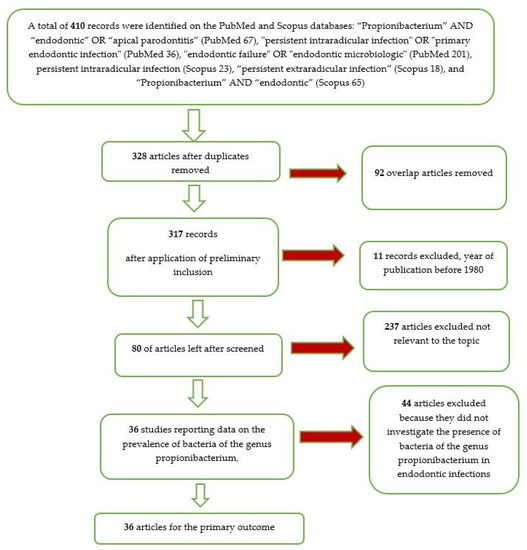
Figure 1.
Flow chart of the different phases of the systematic review.
The Newcastle–Ottawa Scale for case control studies was used to assess the risk of bias in the included studies in primary, secondary, and tertiary outcomes [12]. The cumulative meta-analysis for the first outcome was performed using the software Open Meta-Analyst version 10; the quantitative analysis for the secondary and tertiary outcomes was performed with the Rev Manager software 5.3 (Cochrane Collaboration, Copenhagen, Denmark). The data have been processed following the indications of the Cochrane Handbook for Systematic Reviews Chapters 7.4, 7.5, and 7.6 [11].
3. Results
From the searches in the PubMed and Scopus databases, 410 records were identified. With the use of the end-note software, the overlaps were removed, obtaining 328 records. After the elimination of the articles prior to 1980, we reach a number of records of 317. With the application of the eligibility criteria (all the studies that investigated the presence of bacteria in endodontic infection), we reach a number of 80 articles.
Applying the inclusion and exclusion criteria, we included 36 articles in the meta-analysis.
- Thirty-six articles for the primary outcome: all studies reporting data on the prevalence of bacteria of the genus Propionibacterium, further divided into two subgroups A and B. A—prevalence of the genus Propionibacterium in primary endodontic infections in untreated canals, pulp necrosis, and pulpits (21 articles) and B—prevalence of the genus Propionibacterium in secondary endodontic infections in treated channels, in cases of endodontic failure and in cases of endodontic retreatment (20 articles);
- Seven articles for the secondary outcome: all studies reporting data differences in the prevalence of bacteria of the genus Propionibacterium between primary endodontic infections and secondary endodontic infections; and
- Fourteen articles for the tertiary outcome: all studies reporting data differences in the prevalence of Propionibacterium Acnes compared to Propionibacterium propionicum in endodontic infections.
3.1. Study Characteristics and Data Extraction
The included studies for the quantitative analysis were:First Outcome: Pourhajibagher et al., 2018 [13]; Grgurevic et al., 2017 [14]; Lysakowska et al., 2016 [15]; Tennert et al., 2014 [16]; Halbauer et al., 2013 [17]; Signoretti et al., 2013 [18]; Anderson et al., 2013 [19]; Rocas et al., 2012 [20]; Rocas et al., 2011 [5]; Chugal et al., 2011 [21]; Ledezma-Rasillo et al., 2010 [22]; Mindere et al., 2010 [23]; Niazi et al., 2010 [24]; Fujii et al., 2009 [25]; Vianna et al., 2007 [26]; Chu et al., 2005 [27]; Chavez de Paz et al., 2005 [28]; Gomes et al., 2004 [29]; Chavez de Paz et al. [30]; Siqueira et al., 2004 [31]; Hommez et al., 2004 [32]; Pinheiro et al., 2003 [33]; Siqueira et al., 2003 [34]; Sunde et al., 2002 [35]; Peters et al., 2002 [36]; Rolph et al., 2001 [37]; Sundqvist et al., 1998 [38]; Molander et al., 1998 [39]; Vigil et al., 1997 [40]; Sjogren et al., 1997 [41]; Gomes et al., 1996 [42]; Brauner et al., 1995 [43]; Debelian et al., 1995 [44]; Sundqvist et al., 1992 [45]; Fukushima et al., 1990 [46]; and Sundqvist et al., 1998 [4].Second Outcome: Lysakowska et al., 2016 [15]; Signoretti et al., 2013 [18]; Chugal et al., 2011 [21]; Gomes et al., 2004 [29]; Hommez et al., 2004 [32]; Rolph et al., 2001 [37]; and Siqueira et al., 2003 [34].Third Outcome: Lysakowska et al., 2016 [15]; Tennert et al., 2014 [16]; Halbauer et al., 2013 [17]; Ledezma-Rasillo et al., 2010 [22]; Niazi et al., 2010 [24]; Vianna et al., 2007 [26]; Chu et al., 2005 [27]; Chavez de Paz et al., 2005 [28]; Chavez de Paz et al., 2004 [30]; Pinheiro et al., 2003 [33]; Siqueira et al., 2003 [34]; Sunde et al., 2002 [35]; Peters et al., 2002 [36]; Sundqvist et al., 1998 [38]; Sjogren et al., 1997 [41]; and Debelian et al., 1995 [44].
The extraction of the data and the methods in which they have been reported follow the indications of the Cochrane Handbook for Systematic Reviews of Interventions Chapter 7 (Selection of Studies and Data Collection), specifically from pages 152 to 182.
The extracted data included the magazine (author, data, and journal); the bacterium species of the genus Propionibacterium investigated (genus, species, and number of dental elements with the presence of the bacterium); the number of samples examined; types of samples (necrotic or vital tooth, endodontic canal, tooth in pulpitis or apical periodontitis, tooth previously treated endodontically, and tooth with failure subject to extraction or endodontic surgery); the number of samples per pathology with the presence of Propionibacterium; and the bacterium identification method (PCR or culture). If the data on the prevalence in the single studies were reported only for the individual species of Propionibacterium and the overall data was not present or it was not possible to obtain it, the data pertaining to the species was considered for the purpose of the meta-analysis that, in the single study, presented the higher prevalence. If the data was reported as a percentage, the number was calculated through the use of proportions.

Table 3.
Primary outcome (the data regarding the prevalence of bacteria of the genus Propionibacterium in the various studies are reported).

Table 4.
Secondary outcome (difference in the prevalence of bacteria of the genus Propionibacterium between primary endodontic infections and secondary endodontic infections).

Table 5.
Tertiary outcome difference in the prevalence of Propionibacterium Acnes compared to Propionibacterium propionicum in endodontic infections.
3.2. Risk of Bias
The risk of bias was assessed through the Newcastle–Ottawa case control scale, modified for the cumulative meta-analysis. The results are reported in detail in Table 6. For each category, a value of one to three was assigned (one = low and three = high).

Table 6.
Assessment of risk of bias within the studies (Newcastle–Ottawa scale) with scores 7 to 12 = low quality, 13 to 20 = intermediate quality, and 21 to 24 = high quality.
Studies presenting a high risk of bias were not included in the meta-analyzes. Articles with high bias risk were excluded from the scale and eliminated during the inclusion phase. Other articles were excluded, because, for the outcomes investigated, they presented the same data and samples. Some studies, although presenting a number of samples greater than or equal to 20, do not report precise data on the exact number of Propionibacterium in relation to the dental elements subject to endodontic lesions (for example, Francisco et al., 2018 [47,48]). Other studies, such as that conducted by Mussano et al., 2018 [48], even if starting from a number of patients equal to 121 with acute apical periodontitis, only 10 biopsy specimens were examined. The bias risk assessment of the 36 articles included was conducted by the first reviewer (M.D.).
For the first outcome, the risk of bias between studies was very high and was evidenced by the high heterogeneity between studies (I2 92.78%). For the secondary outcome, the risk of bias between studies was low, as represented by the funnel plot (Figure 2A). For the tertiary outcome, the risk was average, with a heterogeneity between studies of I2 65%, and was assessed with the funnel plot (Figure 2B).
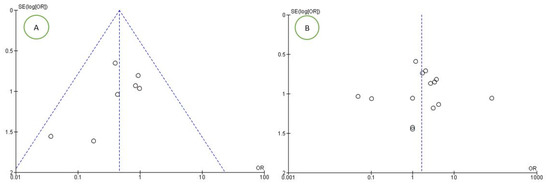
Figure 2.
Funnel plots of the evaluation of heterogeneity for the (A) second and (B) third outcomes.
3.3. Meta-Analysis
The statistical analysis of the data was performed using the Rev Manager 5.3 software (Copenhagen, 153 Denmark, The Nordic Cochrane Centre, The Nordic Cochrane Collaboration, 2014), and cumulative meta-analysis for the first outcome was performed using the software Open Meta-Analyst version 10. The results were represented by forest plots for each of the outcomes.
For the first outcome, prevalence of bacteria of the genus Propionibacterium in primary and secondary endodontic lesions, the heterogeneity was very high, with I2 equal to 92.78%. For this reason, a random effects model was used. The cumulative meta-analysis presents an Overvall (I2= 92.78%; p-value < 0.001) of 0.202 (0.169 and 0.279) with a ratio between events and samples examined equal to 322\1658 (Figure 3).
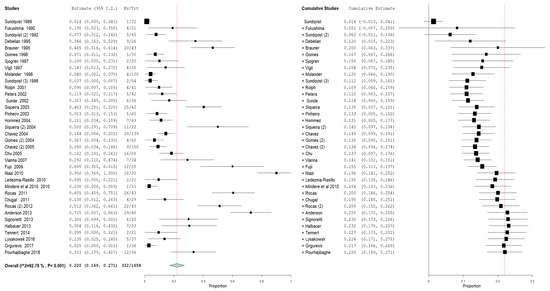
Figure 3.
Forest plot of the random effects model of the meta-analysis cumulative of the primary outcome.
Furthermore, an analysis of the subgroups was performed: subgroups A—prevalence of the genus Propionibacterium in primary endodontic infections in untreated canals, pulp necrosis, and pulpits (Figure 4). For subgroup A, the heterogeneity is very high, with I2 equal to 86.62%. For this reason, a random effects model was used. The cumulative meta-analysis presents an Overvall (I2 = 86.62%; p-value < 0.001) of 0.159 (0.109 and 0.210) with a ratio between events and samples examined equal to 143\869. B—prevalence of the genus Propionibacterium in secondary endodontic infections in treated channels in cases of endodontic failure and in cases of endodontic retreatment (Figure 5). For subgroup B, the heterogeneity is very high, with I2 equal to 94.52%. For this reason, a random effects model was used. The cumulative meta-analysis presents an Overvall (I2 = 94.52%; p-value < 0.001) of 0.258 (0.172 and 0.344) with a ratio between events and samples examined equal to 143\651.
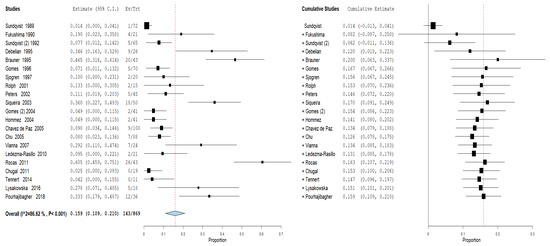
Figure 4.
Forest plot of the random effects model of the meta-analysis cumulative of the primary outcome subgroup A.
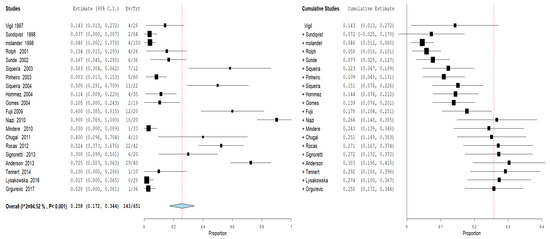
Figure 5.
Forest plot of the random effects model of the meta-analysis cumulative of the primary outcome subgroup B.
For the secondary outcome, differences in the prevalence of bacteria of the genus Propionibacterium between primary endodontic infections and secondary endodontic infections, the comparison showed absence of heterogeneity among the studies, with an I2 equal to 0%. For this reason, for the second outcome, a fixed effects model was applied. For the second outcome, the forest plot is in favor of the subject group primary endodontic infection in a statistically significant way.
All studies show data in favor of a higher prevalence of Propionibacterium in secondary endodontic infections. The forest plot shows the rhombus that does not intersect the noneffect line, moved towards primary endodontic infections having fewer events (presence of Propionibacterium) in relation to the population (teeth with infection) compared to the group of secondary endodontic infections (Figure 6).
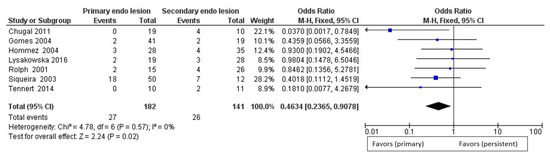
Figure 6.
Forest plot of the fixed effects model of the meta-analysis of the secondary outcome.
For the tertiary outcome: difference in the prevalence of Propionibacterium Acnes compared to Propionibacterium propionicum in endodontic infections. The comparison showed high heterogeneity between the studies, with an I2 of 68%; a random effects model was applied. Through an exploration of the sources of heterogeneity, we noted that, from excluding the studies of Chavez de Paz et al., 2004 [30] and Chavez de Paz et al., 2005 [28], heterogeneity decreases from 68% to 32% (as shown in Figure 7).
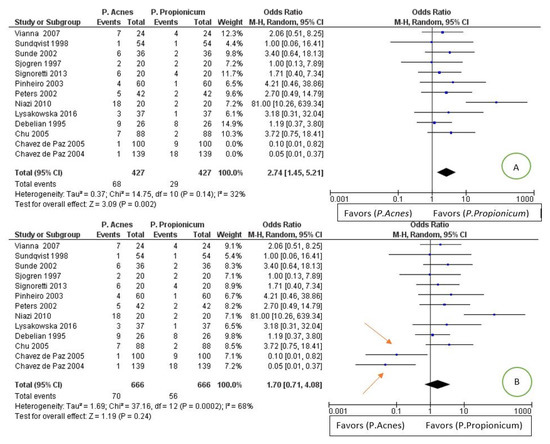
Figure 7.
Image A: forest plot of the random effects model of the meta-analysis of the tertiary outcome; the arrows indicate the main sources of heterogeneity. Image B: forest plot with the exclusion of heterogeneity sources.
The forest plot is in favor of the Propionibacterium propionicum group, having a smaller number of events in relation to the population in proportions less than the group of the Propionibacterium acnes.
4. Discussion
The endodontic treatment is a reasonably predictable procedure with success rates between 86% and 98%. The success or failure of this treatment is also assessed by clinical signs and symptoms, as well as radiological results of the treated tooth [49].
A number of studies have focused on the detection and identification of microorganisms in the root canal of root-filled teeth [33,50,51], and the persistence of microorganisms in the apical part of the root canal was recognized as the main cause of failure of endodontic treatment, even after performing lege artis of endodontic procedures [52]. This can occur due to the inability of endodontic instruments and irrigants to reach all parts of the canal system and effectively remove microorganisms [53,54]. The root canal microflora between primary endodontic cases and retreatment cases differs [38].
The main problem is that, in most cases, the apico-coronal seal is inadequate; therefore, tissue fluids rich in glycoproteins go into the root canal, providing a substrate for the remaining microorganisms, which can proliferate and reach a sufficient number to generate or perpetuate a periradicular lesion [55]. On the other hand, there are situations in which the sealed root canals may be contaminated by the oral cavity: infiltration through temporary or permanent restoration materials, fracture or loss of restoration, fracture of the tooth structure, recurring caries that expose the root-filling material, or delay in the application of the final restoration material. In these circumstances, if a root filling does not prevent saliva from entering, microorganisms can invade and recolonize the canal system [56].
Bacterial survival is closely related to their ability to adapt to hostile environments, and biofilm formation is considered an effective survival strategy and a common cause of persistent infection [57].
To survive in a sealed channel, microorganisms must withstand intracanal disinfection measures and must adapt to an environment with poor nutrient availability. Therefore, only the species that have these abilities may be involved in endodontic failure. Furthermore, bacteria located in areas such as apical deltas, lateral canals, irregularities, and dentinal tubules can often escape endodontic disinfection procedures, and it is likely that the supply of bacterial nutrients remains unchanged after treatment [58].
The microorganisms that reach the environment beyond the foramen of the root canal are recognized by the immune system, initiating a local inflammatory response—a series of events with the aim of eliminating the infection and providing conditions for restoring the balance of the guest [59].
However, many microorganisms could survive due to their ability to bypass, respond to, or resist host defense mechanisms, colonizing the external root surface and forming a biofilm [60]. Microbial species associated with bacterial complexes organized in a biofilm possess characteristics that differ from their planktonic forms, such as greater diversity and metabolic efficiency, resistance to phagocytic cells, antimicrobial agents and environmental stresses, and increased pathogenicity [61,62].
Bacterial colonization of root canal spaces has been demonstrated as the main etiological factor of apical periodontitis [63,64]. In most cases, it is impossible to distinguish between periapical granulomas and radicular cysts without resorting to a biopsy [65]. Radicular cysts are believed to form from the proliferation of Malassez epithelial cell remains in inflamed periradicular tissues [66]; their reported incidences among periapical lesions varies from 6% to 55% [67].
In two paradigmatic studies of Ricucci and Siqueira [64,68], bacterial biofilm varied, and no single model for endodontic infections was identified. Bacteria could change the severity and the prognosis of apical periodontitis, and yet, surprisingly, little information is available in the scientific literature comparing the microbiota within periapical granulomas and radicular cysts. The application of high throughput amplicon target sequencing (HTS) to study microbial ecology has been seen over the past two years to estimate microbial diversity in different ecosystems using the 16S rRNA gene as a target [69].
Microbial identification by molecular methods has been widely used in microbiological research applied to dentistry [70,71,72,73,74,75]. Culture-independent molecular biology methods have advantages over bacterial identification procedures based on phenotypic features, such as greater sensitivity and specificity and ability to identify noncultivable bacteria [70,73,76], which generates more reliable results regarding microbial content.
Endodontic surgical treatment is recommended for teeth with long-lasting apical lesions that persist even after careful conventional endodontic retreatment [77,78]. The main objective of apical surgery is to remove the etiological agent, which is normally associated with extraradicular biofilm on long-lasting apical lesions [79,80], periapical actinomycosis [81], foreign body reactions triggered by extruded endodontic materials [82], accumulation of endogenous cholesterol crystals in apical tissues [83], or unresolved cystic lesions [67].
The bacteria that are most frequently found in the root canals of teeth with post-treatment endodontic disease when using culture techniques are predominantly Gram-positive, including cocci (e.g., Enterococcus spp. and Streptococcus spp.) and rods (e.g., Actinomyces and Propionibacterium) [33,38,39,84].
Although several bacterial species from the oral cavity have been found in the infected root canals, there is a limited set of species that are most frequently present in some types of endodontic infections and, therefore, are recognized as the main group of endodontic [85]. Among this set, there are some species of the genus Propionibacterium that have often been isolated from both intraradicular and extraradicular infections [38,86,87].
The species of Propionibacterium detected in the endodontic infections described in the literature are P. acnes, P. propionicum (the P. propionicum and P. propionicus are the same bacterium with different names), P. acidipropionici, P. granulosum, P. avidium, and P. acidifaciens. From the qualitative analysis of the articles, it appears that the Propionibacterium most associated with the presence of endodontic infections is P. acnes, followed by P. propionicum and P. granulosam.
The results of our meta-analysis for the first outcome shows how the presence of Propionibacterium in endodontic infections has a prevalence of 0.202 (0.169 and 0.279) (relationship between teeth with the presence of Propionibacterium and teeth with infections) that from the analysis of the subgroups increased from subgroup A (primary endodontic infections) 0.159 to B (secondary endodontic infections) 0.258. It could be assumed that the bacteria of the genus Propionibacterium are more present in secondary infections, but further data and analyses are necessary.
In fact, for the second outcome, the data provide us with significant information, thanks to the direct comparison (within the studies) between primary and secondary lesions. Propionibacterium is more present in endodontic secondary lesions significantly, confirming in part the data of the analysis of subgroups A and B. The studies by Lysakowska et al., 2016 [15]; Signoretti et al., 2013 [18]; Gomes et al., 2004 [29]; Hommez et al., 2004 [32]; Rolph et al., 2001 [37]; and Siqueira et al., 2003 [34] all report data with a lower presence of Propionibacterium in primary endodontic infections but with confidence intervals that intersect the noneffect line, with the exception of Chugal et al., 2011 [21]. The absence of heterogeneity between the studies and the set of studies instead shows a statistically significant result in favor of a lower presence of Propionibacterium in primary endodontic infections.
The meta-analysis of the tertiary outcome reports data that are in favor of a higher prevalence of P. acnes than P. propionicum in a statistically not significant way but with a high heterogeneity of I2 68%. Through a search for the sources of heterogeneity, and excluding the articles of Chavez de Paz et al., 2004 [30] and Chavez de Paz et al., 2005 [28], the heterogeneity is lowered to 32%, and the data become significant and are in favor for fewer lesions/infections in which the P. propionicum is present.
All the studies except for the Chavez de Paz et al., 2004 [30] and Chavez de Paz et al., 2005 [28] and Niazi et al., 2010 [57] report confidence intervals that intersect the line of noneffect. In fovor studies for a greater prevalence of P. propionicum are Chavez de Paz et al., 2004 [63] and Chavez de Paz et al., 2005 [61], while the studies of Sundqvist et al., 1998 [38] and Sjogren et al., 1997 report identical results for the two groups. The remaining studies are in favor of a greater presence of P. acnes in endodontic infections.
5. Conclusions
In conclusion, we can say that Propionibacterium is definitely present in endodontic infections with a higher prevalence for secondary endodontic lesions and that the Propionibacterium that has the highest prevalence is P. acnes.
Author Contributions
Conceptualization, M.D., M.A., V.C., R.A., D.S., L.L.R., E.L., G.M., and G.T.; methodology, M.D.; software, M.D.; data analysis, M.D and D.S.; visualization, M.D.; supervision and project administration, L.L.M.; writing, M.D. and C.Q.; and reviewing and editing, M.D. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors acknowledge Lorenzo Lo Muzio, the Director of the Department of Clinical and Experimental Medicine of the University of Foggia, (Foggia, Italy) whose help in writing this article has been fundamental.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ruviere, D.B.; Leonardo, M.R.; da Silva, L.A.; Ito, I.Y.; Nelson-Filho, P. Assessment of the microbiota in root canals of human primary teeth by checkerboard DNA-DNA hybridization. J. Dent. Child. 2007, 74, 118–123. [Google Scholar]
- Guven, Y.; Ustun, N.; Aksakal, S.D.; Topcuoglu, N.; Aktoren, O.; Kulekci, G. Assessment of the endodontic microbiota of abscessed primary teeth using microarray technology. Indian J. Dent. Res. 2018, 29, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Luo, T.; Lee, K.H.; Guerreiro, D.; Botero, T.M.; McDonald, N.J.; Rickard, A.H. Deciphering Endodontic Microbial Communities by Next-generation Sequencing. J. Endod. 2018, 44, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G.; Johansson, E.; Sjogren, U. Prevalence of black-pigmented bacteroides species in root canal infections. J. Endod. 1989, 15, 13–19. [Google Scholar] [CrossRef]
- Rocas, I.N.; Siqueira, J.F., Jr.; Debelian, G.J. Analysis of symptomatic and asymptomatic primary root canal infections in adult Norwegian patients. J. Endod. 2011, 37, 1206–1212. [Google Scholar] [CrossRef]
- Prada, I.; Mico-Munoz, P.; Giner-Lluesma, T.; Mico-Martinez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Arena, C.; Caponio, V.C.A.; Caloro, G.A.; Zhurakivska, K.; Adipietro, I.; Troiano, G.; Lo Muzio, L. Inspection of the Microbiota in Endodontic Lesions. Dent. J. 2019, 7. [Google Scholar] [CrossRef]
- Approved lists of bacterial names. Med. J. Aust. 1980, 2, 3–4. [CrossRef]
- Cummins, C.S.; Johnson, J.L. Corynebacterium parvum: A synonym for Propionibacterium acnes? J. Gen. Microbiol. 1974, 80, 433–442. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Collaboration. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Chichester, UK; Hoboken, NJ, USA, 2008; p. 649. [Google Scholar]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Bahador, A. An in vivo evaluation of microbial diversity before and after the photo-activated disinfection in primary endodontic infections: Traditional phenotypic and molecular approaches. Photodiagn. Photodyn. Ther. 2018, 22, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Grgurevic, J.; Ivanisevic Malcic, A.; Tambic Andrasevic, A.; Prpic Mehicic, G.; Kuzmac, S.; Jukic, S. Frequency of bacetrial content finding in persistant periapical lesions. Acta Stomatol. Croat. 2017, 51, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lysakowska, M.E.; Ciebiada-Adamiec, A.; Sienkiewicz, M.; Sokolowski, J.; Banaszek, K. The cultivable microbiota of primary and secondary infected root canals, their susceptibility to antibiotics and association with the signs and symptoms of infection. Int. Endod. J. 2016, 49, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Fuhrmann, M.; Wittmer, A.; Karygianni, L.; Altenburger, M.J.; Pelz, K.; Hellwig, E.; Al-Ahmad, A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014, 40, 670–677. [Google Scholar] [CrossRef]
- Halbauer, K.; Prskalo, K.; Jankovic, B.; Tarle, Z.; Panduric, V.; Kalenic, S. Efficacy of ozone on microorganisms in the tooth root canal. Coll. Antropol. 2013, 37, 101–107. [Google Scholar]
- Signoretti, F.G.; Gomes, B.P.; Montagner, F.; Jacinto, R.C. Investigation of cultivable bacteria isolated from longstanding retreatment-resistant lesions of teeth with apical periodontitis. J. Endod. 2013, 39, 1240–1244. [Google Scholar] [CrossRef]
- Anderson, A.C.; Al-Ahmad, A.; Elamin, F.; Jonas, D.; Mirghani, Y.; Schilhabel, M.; Karygianni, L.; Hellwig, E.; Rehman, A. Comparison of the bacterial composition and structure in symptomatic and asymptomatic endodontic infections associated with root-filled teeth using pyrosequencing. PLoS ONE 2013, 8, e84960. [Google Scholar] [CrossRef]
- Rocas, I.N.; Siqueira, J.F., Jr. Characterization of microbiota of root canal-treated teeth with posttreatment disease. J. Clin. Microbiol. 2012, 50, 1721–1724. [Google Scholar] [CrossRef]
- Chugal, N.; Wang, J.K.; Wang, R.; He, X.; Kang, M.; Li, J.; Zhou, X.; Shi, W.; Lux, R. Molecular characterization of the microbial flora residing at the apical portion of infected root canals of human teeth. J. Endod. 2011, 37, 1359–1364. [Google Scholar] [CrossRef]
- Ledezma-Rasillo, G.; Flores-Reyes, H.; Gonzalez-Amaro, A.M.; Garrocho-Rangel, A.; Ruiz-Rodriguez Mdel, S.; Pozos-Guillen, A.J. Identification of cultivable microorganisms from primary teeth with necrotic pulps. J. Clin. Pediatr. Dent. 2010, 34, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Mindere, A.; Kundzina, R.; Nikolajeva, V.; Eze, D.; Petrina, Z. Microflora of root filled teeth with apical periodontitis in Latvian patients. Stomatologija 2010, 12, 116–121. [Google Scholar] [PubMed]
- Niazi, S.A.; Clarke, D.; Do, T.; Gilbert, S.C.; Mannocci, F.; Beighton, D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J. Clin. Microbiol. 2010, 48, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Saito, Y.; Tokura, Y.; Nakagawa, K.I.; Okuda, K.; Ishihara, K. Characterization of bacterial flora in persistent apical periodontitis lesions. Oral Microbiol. Immunol. 2009, 24, 502–505. [Google Scholar] [CrossRef]
- Vianna, M.E.; Horz, H.P.; Conrads, G.; Zaia, A.A.; Souza-Filho, F.J.; Gomes, B.P. Effect of root canal procedures on endotoxins and endodontic pathogens. Oral Microbiol. Immunol. 2007, 22, 411–418. [Google Scholar] [CrossRef]
- Chu, F.C.; Tsang, C.S.; Chow, T.W.; Samaranayake, L.P. Identification of cultivable microorganisms from primary endodontic infections with exposed and unexposed pulp space. J. Endod. 2005, 31, 424–429. [Google Scholar] [CrossRef]
- Chavez de Paz, L.; Svensater, G.; Dahlen, G.; Bergenholtz, G. Streptococci from root canals in teeth with apical periodontitis receiving endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 100, 232–241. [Google Scholar] [CrossRef]
- Gomes, B.P.; Pinheiro, E.T.; Gade-Neto, C.R.; Sousa, E.L.; Ferraz, C.C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef]
- Chavez de Paz, L.E.; Molander, A.; Dahlen, G. Gram-positive rods prevailing in teeth with apical periodontitis undergoing root canal treatment. Int. Endod. J. 2004, 37, 579–587. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 85–94. [Google Scholar] [CrossRef]
- Hommez, G.M.; Verhelst, R.; Claeys, G.; Vaneechoutte, M.; De Moor, R.J. Investigation of the effect of the coronal restoration quality on the composition of the root canal microflora in teeth with apical periodontitis by means of T-RFLP analysis. Int. Endod. J. 2004, 37, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Sousa, E.L.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N. Polymerase chain reaction detection of Propionibacterium propionicus and Actinomyces radicidentis in primary and persistent endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 215–222. [Google Scholar] [CrossRef]
- Sunde, P.T.; Olsen, I.; Debelian, G.J.; Tronstad, L. Microbiota of periapical lesions refractory to endodontic therapy. J. Endod. 2002, 28, 304–310. [Google Scholar] [CrossRef]
- Peters, L.B.; van Winkelhoff, A.J.; Buijs, J.F.; Wesselink, P.R. Effects of instrumentation, irrigation and dressing with calcium hydroxide on infection in pulpless teeth with periapical bone lesions. Int. Endod. J. 2002, 35, 13–21. [Google Scholar] [CrossRef]
- Rolph, H.J.; Lennon, A.; Riggio, M.P.; Saunders, W.P.; MacKenzie, D.; Coldero, L.; Bagg, J. Molecular identification of microorganisms from endodontic infections. J. Clin. Microbiol. 2001, 39, 3282–3289. [Google Scholar] [CrossRef]
- Sundqvist, G.; Figdor, D.; Persson, S.; Sjogren, U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 86–93. [Google Scholar] [CrossRef]
- Molander, A.; Reit, C.; Dahlen, G.; Kvist, T. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 1998, 31, 1–7. [Google Scholar] [CrossRef]
- Vigil, G.V.; Wayman, B.E.; Dazey, S.E.; Fowler, C.B.; Bradley, D.V., Jr. Identification and antibiotic sensitivity of bacteria isolated from periapical lesions. J. Endod. 1997, 23, 110–114. [Google Scholar] [CrossRef]
- Sjogren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef]
- Gomes, B.P.; Lilley, J.D.; Drucker, D.B. Clinical significance of dental root canal microflora. J. Dent. 1996, 24, 47–55. [Google Scholar] [CrossRef]
- Brauner, A.W.; Conrads, G. Studies into the microbial spectrum of apical periodontitis. Int. Endod. J. 1995, 28, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Debelian, G.J.; Olsen, I.; Tronstad, L. Bacteremia in conjunction with endodontic therapy. Endod. Dent. Traumatol. 1995, 11, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G. Associations between microbial species in dental root canal infections. Oral Microbiol. Immunol. 1992, 7, 257–262. [Google Scholar] [CrossRef]
- Fukushima, H.; Yamamoto, K.; Hirohata, K.; Sagawa, H.; Leung, K.P.; Walker, C.B. Localization and identification of root canal bacteria in clinically asymptomatic periapical pathosis. J. Endod. 1990, 16, 534–538. [Google Scholar] [CrossRef]
- Francisco, P.A.; Delboni, M.G.; Lima, A.R.; Xiao, Y.; Siqueira, W.L.; Gomes, B. Proteomic profile of root canal contents in teeth with post-treatment endodontic disease. Int. Endod. J. 2019, 52, 451–460. [Google Scholar] [CrossRef]
- Mussano, F.; Ferrocino, I.; Gavrilova, N.; Genova, T.; Dell’Acqua, A.; Cocolin, L.; Carossa, S. Apical periodontitis: Preliminary assessment of microbiota by 16S rRNA high throughput amplicon target sequencing. BMC Oral Health 2018, 18, 55. [Google Scholar] [CrossRef]
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr. Taxonomic changes of bacteria associated with endodontic infections. J. Endod. 2003, 29, 619–623. [Google Scholar] [CrossRef]
- Troiano, G.; Perrone, D.; Dioguardi, M.; Buonavoglia, A.; Ardito, F.; Lo Muzio, L. In vitro evaluation of the cytotoxic activity of three epoxy resin-based endodontic sealers. Dent. Mater. J. 2018, 37, 374–378. [Google Scholar] [CrossRef]
- Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Gioia, G.D.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Dioguardi, M.; Cocco, A.; Zhurakivska, K.; Ciavarella, D.; Muzio, L.L. Increase in [corrected] the glyde path diameter improves the centering ability of F6 Skytaper. Eur. J. Dent. 2018, 12, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Lima, K.C.; Magalhaes, F.A.; Lopes, H.P.; de Uzeda, M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J. Endod. 1999, 25, 332–335. [Google Scholar] [CrossRef]
- Chavez de Paz, L.E. Redefining the persistent infection in root canals: Possible role of biofilm communities. J. Endod. 2007, 33, 652–662. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Lopes, H.P. Bacteria on the apical root surfaces of untreated teeth with periradicular lesions: A scanning electron microscopy study. Int. Endod. J. 2001, 34, 216–220. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Hornef, M.W.; Wick, M.J.; Rhen, M.; Normark, S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 2002, 3, 1033–1040. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. Journal of clinical periodontology 2005, 32 (Suppl. S6), 7–15. [Google Scholar] [CrossRef]
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The Effects of Surgical Exposures of Dental Pulps in Germ-Free and Conventional Laboratory Rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Ricucci, D.; Pascon, E.A.; Ford, T.R.; Langeland, K. Epithelium and bacteria in periapical lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.W.; Zhu, Y.Q.; Wang, X.Y. Six cases report of differential diagnosis of periapical diseases. Int. J. Oral Sci. 2011, 3, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shear, M. Cysts of the jaws: Recent advances. J. Oral Pathol. 1985, 14, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran Nair, P.N.; Pajarola, G.; Schroeder, H.E. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 81, 93–102. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N.; Alves, F.R.; Silva, M.G. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 721–726. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Zotta, T.; Ercolini, D. A comparison of bioinformatic approaches for 16S rRNA gene profiling of food bacterial microbiota. Int. J. Food Microbiol. 2018, 265, 9–17. [Google Scholar] [CrossRef]
- Munson, M.A.; Pitt-Ford, T.; Chong, B.; Weightman, A.; Wade, W.G. Molecular and cultural analysis of the microflora associated with endodontic infections. J. Dent. Res. 2002, 81, 761–766. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Molander, A.; Flannagan, S.E.; Nagel, A.C.; Appelbe, O.K.; Clewell, D.B.; Dahlen, G. Virulence, phenotype and genotype characteristics of endodontic Enterococcus spp. Oral Microbiol. Immunol. 2005, 20, 10–19. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J. Clin. Microbiol. 2005, 43, 3314–3319. [Google Scholar] [CrossRef]
- Rocas, I.N.; Siqueira, J.F., Jr. Detection of novel oral species and phylotypes in symptomatic endodontic infections including abscesses. FEMS Microbiol. Lett. 2005, 250, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Rocas, I.N.; Siqueira, J.F., Jr.; Benno, Y. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol. Immunol. 2006, 21, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.S.; Ferraz, C.C.R.; Zaia, A.A.; Almeida, J.F.A.; Gomes, B. Quantitative and qualitative analysis of microorganisms in root-filled teeth with persistent infection: Monitoring of the endodontic retreatment. Eur. J. Dent. 2013, 7, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N.; Cunha, C.D.; Rosado, A.S. Novel bacterial phylotypes in endodontic infections. J. Dent. Res. 2005, 84, 565–569. [Google Scholar] [CrossRef]
- Gutmann, J.L. Principles of endodontic surgery for the general practitioner. Dent. Clin. N. Am. 1984, 28, 895–908. [Google Scholar]
- Signoretti, F.G.; Endo, M.S.; Gomes, B.P.; Montagner, F.; Tosello, F.B.; Jacinto, R.C. Persistent extraradicular infection in root-filled asymptomatic human tooth: Scanning electron microscopic analysis and microbial investigation after apical microsurgery. J. Endod. 2011, 37, 1696–1700. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Noiri, Y.; Ehara, A.; Kawahara, T.; Takemura, N.; Ebisu, S. Participation of bacterial biofilms in refractory and chronic periapical periodontitis. J. Endod. 2002, 28, 679–683. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Apical actinomycosis as a continuum of intraradicular and extraradicular infection: Case report and critical review on its involvement with treatment failure. J. Endod. 2008, 34, 1124–1129. [Google Scholar] [CrossRef]
- Nair, P.N.; Sjogren, U.; Krey, G.; Sundqvist, G. Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. J. Endod. 1990, 16, 589–595. [Google Scholar] [CrossRef]
- Nair, P.N. Cholesterol as an aetiological agent in endodontic failures—A review. Aust. Endod. J. 1999, 25, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. A brief on bacterial biofilms. Nat. Genet. 2001, 29, 360. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N.; Souto, R.; de Uzeda, M.; Colombo, A.P. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J. Endod. 2002, 28, 168–172. [Google Scholar] [CrossRef]
- Happonen, R.P.; Soderling, E.; Viander, M.; Linko-Kettunen, L.; Pelliniemi, L.J. Immunocytochemical demonstration of Actinomyces species and Arachnia propionica in periapical infections. J. Oral Pathol. 1985, 14, 405–413. [Google Scholar] [CrossRef]
- Sjogren, U.; Happonen, R.P.; Kahnberg, K.E.; Sundqvist, G. Survival of Arachnia propionica in periapical tissue. Int. Endod. J. 1988, 21, 277–282. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).