Epigenetic Modification of CFTR in Head and Neck Cancer
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Culture
2.2. Human Tissue and Ethical Approval
2.3. 5-Aza-CdR Treatment, Reverse Transcriptase (RT)-PCR, and Real-Time PCR
2.4. Western Blot Analysis
2.5. Immunofluorescence
2.6. Whole Cell Patch Clamp Recordings
2.7. Methylation-Specific PCR
2.8. Bisulfite Sequencing
2.9. GEO Dataset Analysis and PPI network (STRING)
2.10. Small Interfering RNA Transfection
2.11. Proliferation Assay (CCK-8 Assay and EdU Click-iT™ Assay)
2.12. Apoptosis Assay (PE/annexin-V and Caspase 3/7 Assays)
2.13. Single Cell Motility Study (Time-Lapse Imaging)
2.14. Matrigel Invasion Assay
2.15. Statistical Analysis
3. Results
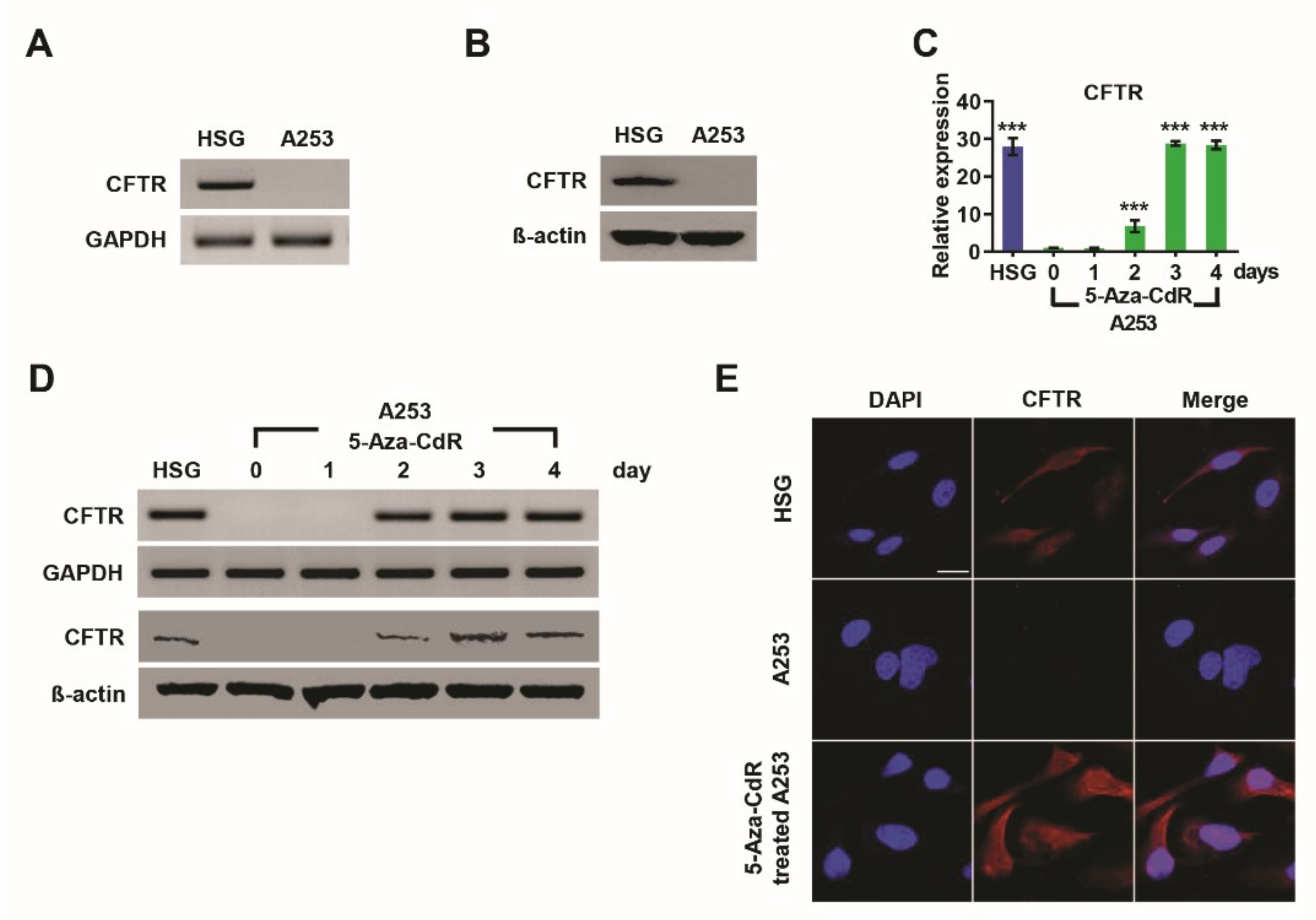
3.1. Silencing and Reactivation of CFTR in A253 Cells
3.2. Functional Analysis of CFTR in A253 Cells
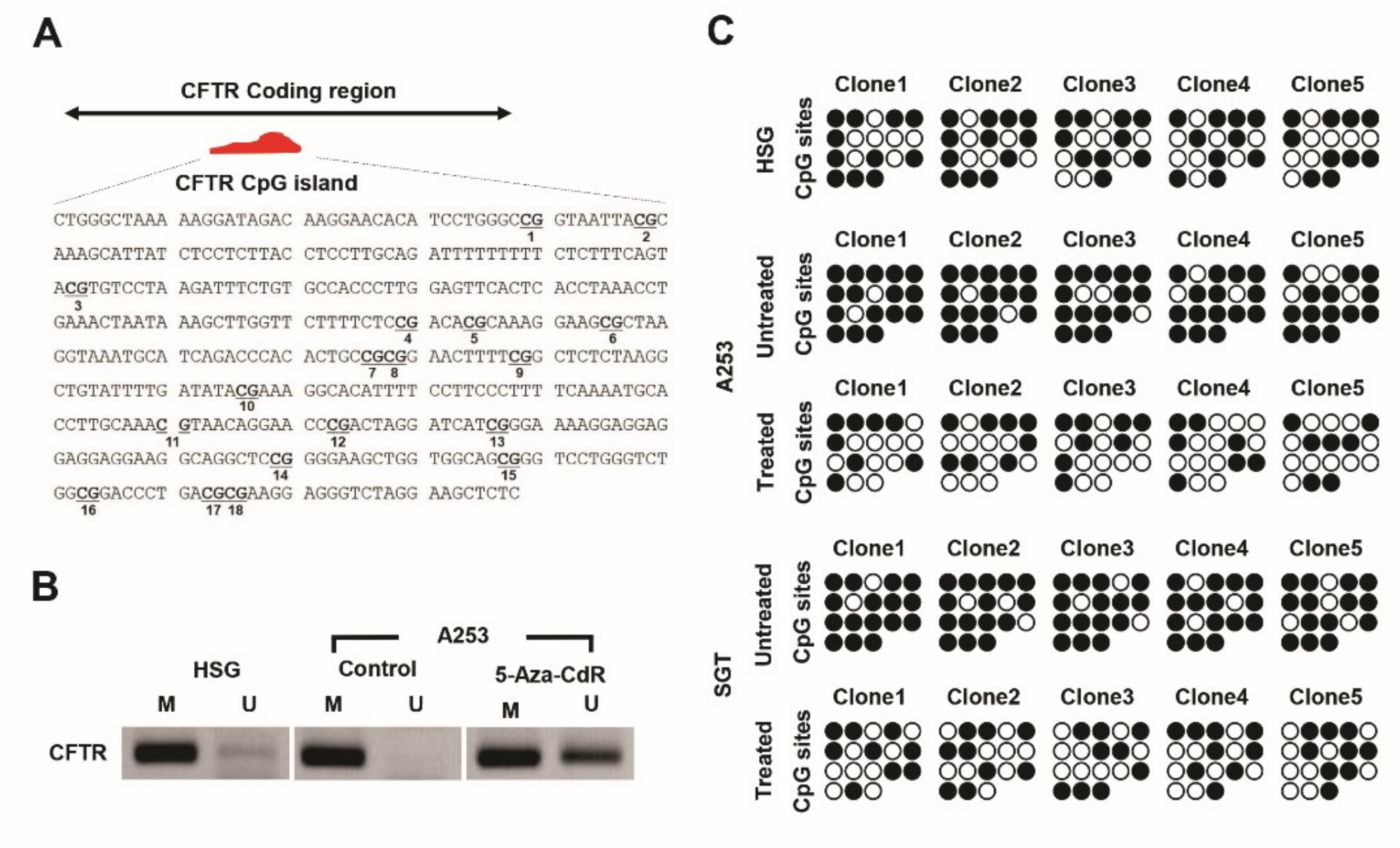
3.3. Methylation Pattern and Status of CFTR in HSG and A253 Cells
3.4. Epigenetic Analysis of CFTR CpG Islands in Human Tissue
3.5. Epigenetic Regulation of CFTR in Cancer-Related Genes in HNC Cells and DNA Methylation Analyses of Head and Neck Cancer Tissue
3.6. Epigenetic Induction of CFTR Regulates Antiproliferation and Apoptosis
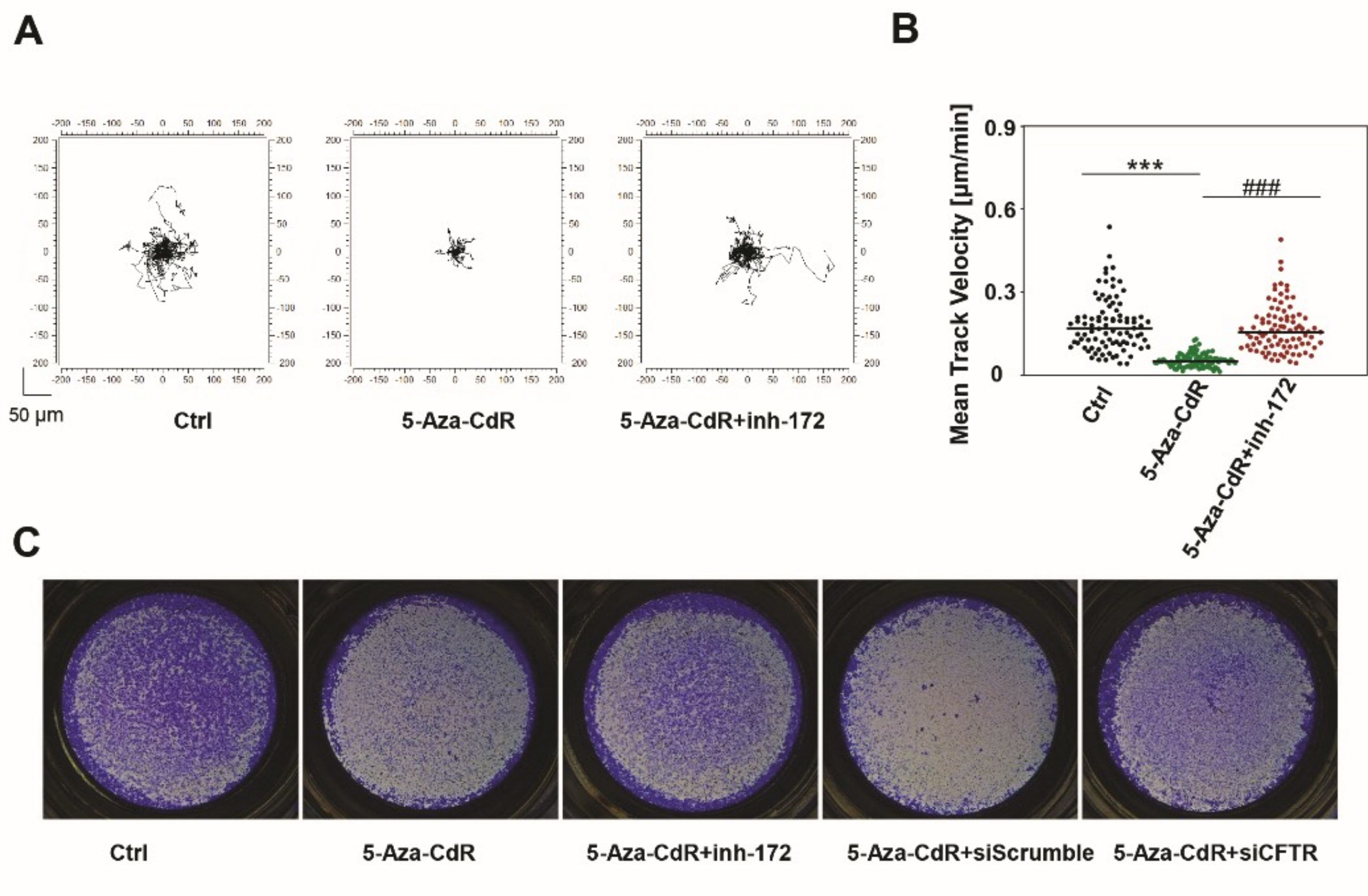
3.7. Epigenetic Induction of CFTR Negatively Regulates Cell Motility and Invasion.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA methylation in cancer: Too much, but also too little. Oncogene 2002, 21, 5400–5413. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Grant, P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. In Epigenetics: Development and Disease; Kundu, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 289–317. [Google Scholar] [CrossRef]
- Esteller, M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene 2002, 21, 5427–5440. [Google Scholar] [CrossRef]
- Robertson, K.D.; Uzvolgyi, E.; Liang, G.; Talmadge, C.; Sumegi, J.; Gonzales, F.A.; Jones, P.A. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: Coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999, 27, 2291–2298. [Google Scholar] [CrossRef]
- Ramos, M.-P.; Wijetunga, N.A.; McLellan, A.S.; Suzuki, M.; Greally, J.M. DNA demethylation by 5-aza-2′-deoxycytidine is imprinted, targeted to euchromatin, and has limited transcriptional consequences. Epigenet. Chromatin 2015, 8, 11. [Google Scholar] [CrossRef]
- Schmelz, K.; Sattler, N.; Wagner, M.; Lübbert, M.; Dörken, B.; Tamm, I. Induction of gene expression by 5-Aza-2′-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNA-methylation-dependent and -independent mechanisms. Leukemia 2005, 19, 103–111. [Google Scholar] [CrossRef]
- Juergens, R.A.; Wrangle, J.; Vendetti, F.P.; Murphy, S.C.; Zhao, M.; Coleman, B.; Sebree, R.; Rodgers, K.; Hooker, C.M.; Franco, N.; et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011, 1, 598–607. [Google Scholar] [CrossRef]
- Connolly, R.M.; Li, H.; Jankowitz, R.C.; Zhang, Z.; Rudek, M.A.; Jeter, S.C.; Slater, S.A.; Powers, P.; Wolff, A.C.; Fetting, J.H.; et al. Combination Epigenetic Therapy in Advanced Breast Cancer with 5-Azacitidine and Entinostat: A Phase II National Cancer Institute/Stand Up to Cancer Study. Clin. Cancer Res. 2017, 23, 2691–2701. [Google Scholar] [CrossRef]
- Azad, N.; Zahnow, C.A.; Rudin, C.M.; Baylin, S.B. The future of epigenetic therapy in solid tumours—Lessons from the past. Nat. Rev. Clin. Oncol. 2013, 10, 256–266. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Lee, S.-W.; Shin, Y.-H.; Lee, J.-H.; Jahng, J.W.; Park, K. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget 2017, 8, 48972. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H. The establishment of salivary gland tumor cell line (SGT-1 Lee cell line) from human submandibular gland adenocarcinoma. J. Korean Assoc. Oral. Maxillofac. Surg. 1996, 20, 22–30. [Google Scholar]
- Shin, Y.H.; Jin, M.; Hwang, S.M.; Choi, S.K.; Namkoong, E.; Kim, M.; Park, M.Y.; Choi, S.Y.; Lee, J.H.; Park, K. Epigenetic modulation of the muscarinic type 3 receptor in salivary epithelial cells. Lab. Investig. 2015, 95, 237–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pei, S.-G.; Wang, J.-X.; Wang, X.-L.; Zhang, Q.-J.; Zhang, H. Correlation of survivin, p53 and Ki-67 in laryngeal cancer Hep-2 cell proliferation and invasion. Asian Pac. J. Trop. Med. 2015, 8, 636–642. [Google Scholar] [CrossRef]
- Borhani, N.; Manoochehri, M.; Gargari, S.S.; Novin, M.G.; Mansouri, A.; Omrani, M.D. Decreased expression of proapoptotic genes caspase-8-and BCL2-associated agonist of cell death (BAD) in ovarian cancer. Clin. Ovarian Other Gynecol. Cancer 2014, 7, 18–23. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, Y.; Xie, K.; Zhang, X.; Zhi, X.; Wang, W.; Li, Z.; Zhang, Q.; Wang, L.; Wang, J.; et al. The role of the AMOP domain in MUC4/Y-promoted tumour angiogenesis and metastasis in pancreatic cancer. J. Exp. Clin. Cancer Res. 2016, 35, 91. [Google Scholar] [CrossRef]
- Zeng, W.; Lee, M.G.; Yan, M.; Diaz, J.; Benjamin, I.; Marino, C.R.; Kopito, R.; Freedman, S.; Cotton, C.; Muallem, S. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am. J. Physiol.-Cell Physiol. 1997, 273, C442–C455. [Google Scholar]
- Anderson, M.P.; Rich, D.P.; Gregory, R.J.; Smith, A.E.; Welsh, M.J. Generation of cAMP-activated chloride currents by expression of CFTR. Science 1991, 251, 679–682. [Google Scholar] [CrossRef]
- Lobert, S.; Graichen, M.E.; Hamilton, R.D.; Pitman, K.T.; Garrett, M.R.; Hicks, C.; Koganti, T. Prognostic biomarkers for HNSCC using quantitative real-time PCR and microarray analysis: Beta-tubulin isotypes and the p53 interactome. Cytoskeleton (Hoboken) 2014, 71, 628–637. [Google Scholar] [CrossRef]
- Poage, G.M.; Butler, R.A.; Houseman, E.A.; McClean, M.D.; Nelson, H.H.; Christensen, B.C.; Marsit, C.J.; Kelsey, K.T. Identification of an epigenetic profile classifier that is associated with survival in head and neck cancer. Cancer Res. 2012, 72, 2728–2737. [Google Scholar] [CrossRef]
- Chang, F.; Kim, J.M.; Choi, Y.; Park, K. MTA promotes chemotaxis and chemokinesis of immune cells through distinct calcium-sensing receptor signaling pathways. Biomaterials 2018, 150, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Offenbacher, S. Epigenetics: Connecting Environment and Genotype to Phenotype and Disease. J. Dent. Res. 2009, 88, 400–408. [Google Scholar] [CrossRef]
- Sun, Y.; Birnbaumer, L.; Singh, B.B. TRPC1 regulates calcium-activated chloride channels in salivary gland cells. J. Cell. Physiol. 2015, 230, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Singh, B.B.; Ambudkar, I.S. ATP-dependent activation of K(Ca) and ROMK-type K(ATP) channels in human submandibular gland ductal cells. J. Biol. Chem. 1999, 274, 25121–25129. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lou, H.H.; Boyer, J.L.; Limberis, M.P.; Vandenberghe, L.H.; Hackett, N.R.; Leopold, P.L.; Wilson, J.M.; Crystal, R.G. Functional cystic fibrosis transmembrane conductance regulator expression in cystic fibrosis airway epithelial cells by AAV6. 2-mediated segmental trans-splicing. Hum. Gene Ther. 2009, 20, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Kim, M.; Kim, N.; Choi, S.K.; Namkoong, E.; Choi, S.Y.; Lee, J.H.; Cha, S.; Park, K. Epigenetic alteration of the purinergic type 7 receptor in salivary epithelial cells. Biochem. Biophys. Res. Commun. 2015, 466, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Mo, J.; Liu, D.; Cao, D.; Wang, H.; He, Y.; Wang, H. Global DNA 5-Hydroxymethylcytosine and 5-Formylcytosine Contents Are Decreased in the Early Stage of Hepatocellular Carcinoma. Hepatology 2019, 69, 196–208. [Google Scholar] [CrossRef]
- Nikitakis, N.G.; Pentenero, M.; Georgaki, M.; Poh, C.F.; Peterson, D.E.; Edwards, P.; Lingen, M.; Sauk, J.J. Molecular markers associated with development and progression of potentially premalignant oral epithelial lesions: Current knowledge and future implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 650–669. [Google Scholar] [CrossRef]
- Marx, J.L. Cell growth control takes balance: The uncontrolled division of cancer cells may result either from excessive growth stimulation or deficient growth inhibition. Science 1988, 239, 975–977. [Google Scholar] [CrossRef]
- Wells, A.; Grahovac, J.; Wheeler, S.; Ma, B.; Lauffenburger, D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol. Sci. 2013, 34, 283–289. [Google Scholar] [CrossRef]
- Liotta, L.A.; Stracke, M.L.; Aznavoorian, S.A.; Beckner, M.E.; Schiffmann, E. Tumor cell motility. Semin. Cancer Biol. 1991, 2, 111–114. [Google Scholar] [PubMed]
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Guan, X.; Yang, Z.; Li, C. Emerging role of cystic fibrosis transmembrane conductance regulator-an epithelial chloride channel in gastrointestinal cancers. World J. Gastrointest. Oncol. 2016, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. CMLS 2017, 74, 93–115. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jiang, X.; Chan, H.C. Cystic fibrosis transmembrane conductance regulator—Emerging regulator of cancer. Cell. Mol. Life Sci. 2018, 75, 1737–1756. [Google Scholar] [CrossRef]
- Fardi, M.; Solali, S.; Farshdousti Hagh, M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis. 2018, 5, 304–311. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef]
- Momparler, R.L.; Cote, S.; Eliopoulos, N. Pharmacological approach for optimization of the dose schedule of 5-Aza-2′-deoxycytidine (Decitabine) for the therapy of leukemia. Leukemia 1997, 11, 175–180. [Google Scholar] [CrossRef]
- Mendizabal, I.; Yi, S.V. Diversity of Human CpG Islands. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 265–280. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Momparler, R.L. Cancer epigenetics. Oncogene 2003, 22, 6479–6483. [Google Scholar] [CrossRef]
- Park, J.W.; Han, J.W. Targeting epigenetics for cancer therapy. Arch. Pharmacal Res. 2019, 42, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Laird, P.W. Cancer-epigenetics comes of age. Nat. Genet. 1999, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Almeida, L.O. Epigenetic Modifications and Head and Neck Cancer: Implications for Tumor Progression and Resistance to Therapy. Int. J. Mol. Sci. 2017, 18, 1506. [Google Scholar] [CrossRef]
- Esteller, M.; Fraga, M.F.; Guo, M.; Garcia-Foncillas, J.; Hedenfalk, I.; Godwin, A.K.; Trojan, J.; Vaurs-Barrière, C.; Bignon, Y.-J.; Ramus, S. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum. Mol. Genet. 2001, 10, 3001–3007. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Zhang, J.T.; Jiang, X.H.; Xie, C.; Cheng, H.; Da Dong, J.; Wang, Y.; Fok, K.L.; Zhang, X.H.; Sun, T.T.; Tsang, L.L. Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 2961–2969. [Google Scholar] [CrossRef]
- Xie, C.; Jiang, X.; Zhang, J.; Sun, T.; Dong, J.; Sanders, A.J.; Diao, R.; Wang, Y.; Fok, K.; Tsang, L. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene 2013, 32, 2282. [Google Scholar] [CrossRef]
- Noe, J.; Petrusca, D.; Rush, N.; Deng, P.; VanDemark, M.; Berdyshev, E.; Gu, Y.; Smith, P.; Schweitzer, K.; Pilewsky, J.; et al. CFTR regulation of intracellular pH and ceramides is required for lung endothelial cell apoptosis. Am. J. Respir. Cell Mol. Biol. 2009, 41, 314–323. [Google Scholar] [CrossRef]
- Rubera, I.; Duranton, C.; Melis, N.; Cougnon, M.; Mograbi, B.; Tauc, M. Role of CFTR in oxidative stress and suicidal death of renal cells during cisplatin-induced nephrotoxicity. Cell Death Dis. 2013, 4, e817. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, C.; Peng, X.; Zhang, H.; Huang, H.; Liu, H. CFTR inhibits the invasion and growth of esophageal cancer cells by inhibiting the expression of NF-κB. Cell Biol. Int. 2018, 42, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.C.; Wang, Y.; Da Silva, N.; Kim, B.; Diao, R.Y.; Hill, E.; Brown, D.; Chan, H.C.; Breton, S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J. Cell Sci. 2014, 127, 4396–4408. [Google Scholar] [CrossRef] [PubMed]
- Castellani, S.; Guerra, L.; Favia, M.; Di Gioia, S.; Casavola, V.; Conese, M. NHERF1 and CFTR restore tight junction organisation and function in cystic fibrosis airway epithelial cells: Role of ezrin and the RhoA/ROCK pathway. Lab. Investig. 2012, 92, 1527. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.T.; Wang, Y.; Cheng, H.; Xiao, H.Z.; Xiang, J.J.; Zhang, J.T.; Yu, S.B.; Martin, T.A.; Ye, L.; Tsang, L.L.; et al. Disrupted interaction between CFTR and AF-6/afadin aggravates malignant phenotypes of colon cancer. Biochim. Biophys. Acta 2014, 1843, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Ke, H.-L.; Huang, S.-P.; Wu, W.-J.; Chen, Y.-K.; Chang, L.-L. Increase sensitivity in detecting superficial, low grade bladder cancer by combination analysis of hypermethylation of E-cadherin, p16, p14, RASSF1A genes in urine. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 597–602. [Google Scholar] [CrossRef]
- Than, B.L.; Linnekamp, J.F.; Starr, T.K.; Largaespada, D.A.; Rod, A.; Zhang, Y.; Bruner, V.; Abrahante, J.; Schumann, A.; Luczak, T.; et al. CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 2016, 35, 4179–4187. [Google Scholar] [CrossRef]
- Son, J.W.; Kim, Y.J.; Cho, H.M.; Lee, S.Y.; Lee, S.M.; Kang, J.K.; Lee, J.U.; Lee, Y.M.; Kwon, S.J.; Choi, E. Promoter hypermethylation of the CFTR gene and clinical/pathological features associated with non-small cell lung cancer. Respirology 2011, 16, 1203–1209. [Google Scholar] [CrossRef]
- Chen, L.-H.; Hsu, W.-L.; Tseng, Y.-J.; Liu, D.-W.; Weng, C.-F. Involvement of DNMT 3B promotes epithelial-mesenchymal transition and gene expression profile of invasive head and neck squamous cell carcinomas cell lines. BMC Cancer 2016, 16, 431. [Google Scholar]
- Li, C.; Naren, A.P. CFTR chloride channel in the apical compartments: Spatiotemporal coupling to its interacting partners. Integr. Biol. 2010, 2, 161–177. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.; Kim, M.; Won, J.; Kim, J.; Oh, S.B.; Lee, J.-H.; Park, K. Epigenetic Modification of CFTR in Head and Neck Cancer. J. Clin. Med. 2020, 9, 734. https://doi.org/10.3390/jcm9030734
Shin Y, Kim M, Won J, Kim J, Oh SB, Lee J-H, Park K. Epigenetic Modification of CFTR in Head and Neck Cancer. Journal of Clinical Medicine. 2020; 9(3):734. https://doi.org/10.3390/jcm9030734
Chicago/Turabian StyleShin, Yonghwan, Minkyoung Kim, Jonghwa Won, Junchul Kim, Seog Bae Oh, Jong-Ho Lee, and Kyungpyo Park. 2020. "Epigenetic Modification of CFTR in Head and Neck Cancer" Journal of Clinical Medicine 9, no. 3: 734. https://doi.org/10.3390/jcm9030734
APA StyleShin, Y., Kim, M., Won, J., Kim, J., Oh, S. B., Lee, J.-H., & Park, K. (2020). Epigenetic Modification of CFTR in Head and Neck Cancer. Journal of Clinical Medicine, 9(3), 734. https://doi.org/10.3390/jcm9030734




