Smartphones and Apps to Control Glycosylated Hemoglobin (HbA1c) Level in Diabetes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
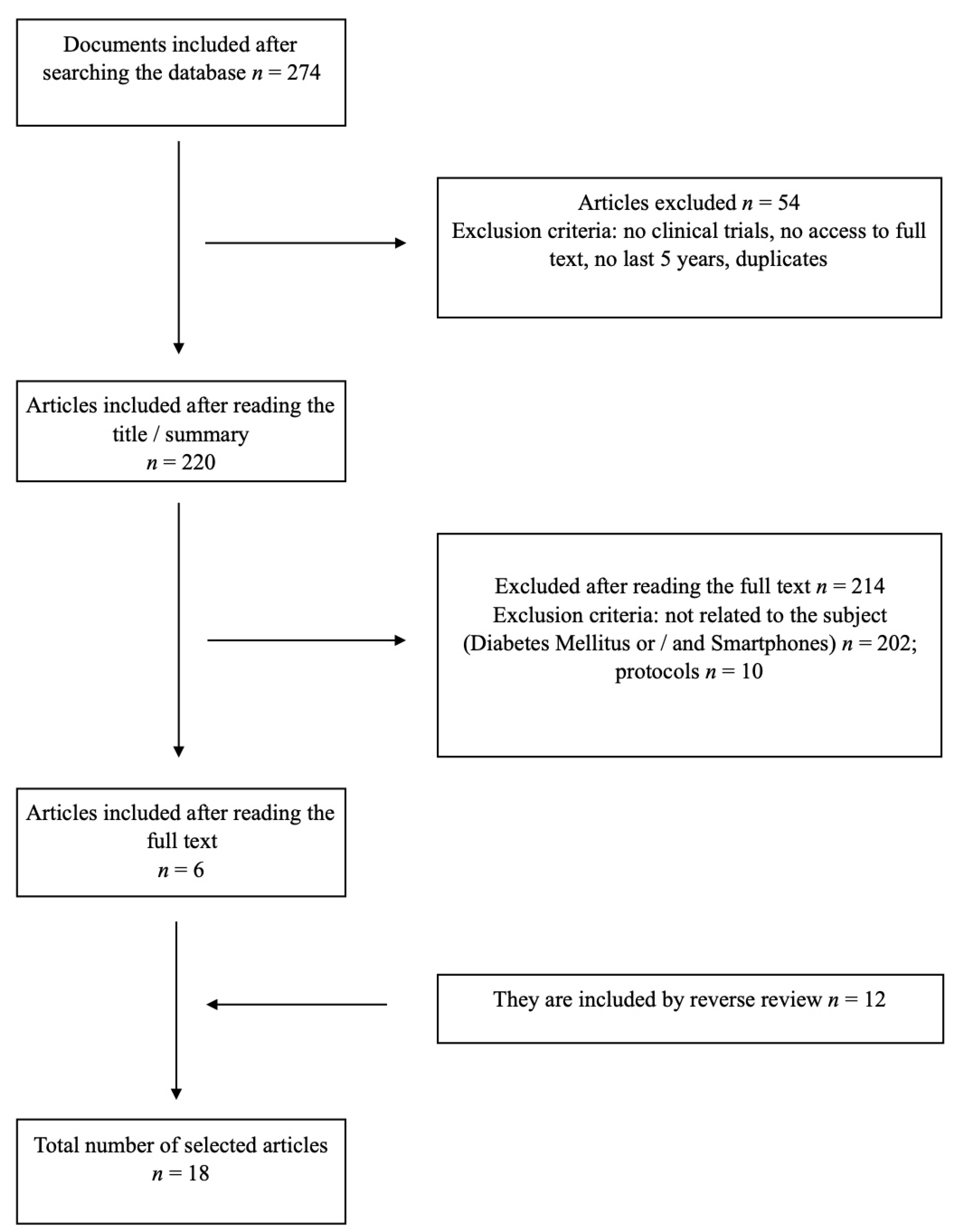
2. Material and Methods
2.1. Design
2.1.1. Search Strategy
2.1.2. Study Selection
2.1.3. Data Extraction
2.2. Critical Review and Level of Evidence
2.3. Data Analysis
3. Results
3.1. Apps and Mobile Phones for the Control of DM1
3.2. Apps and Mobile Phones for the Control of DM2
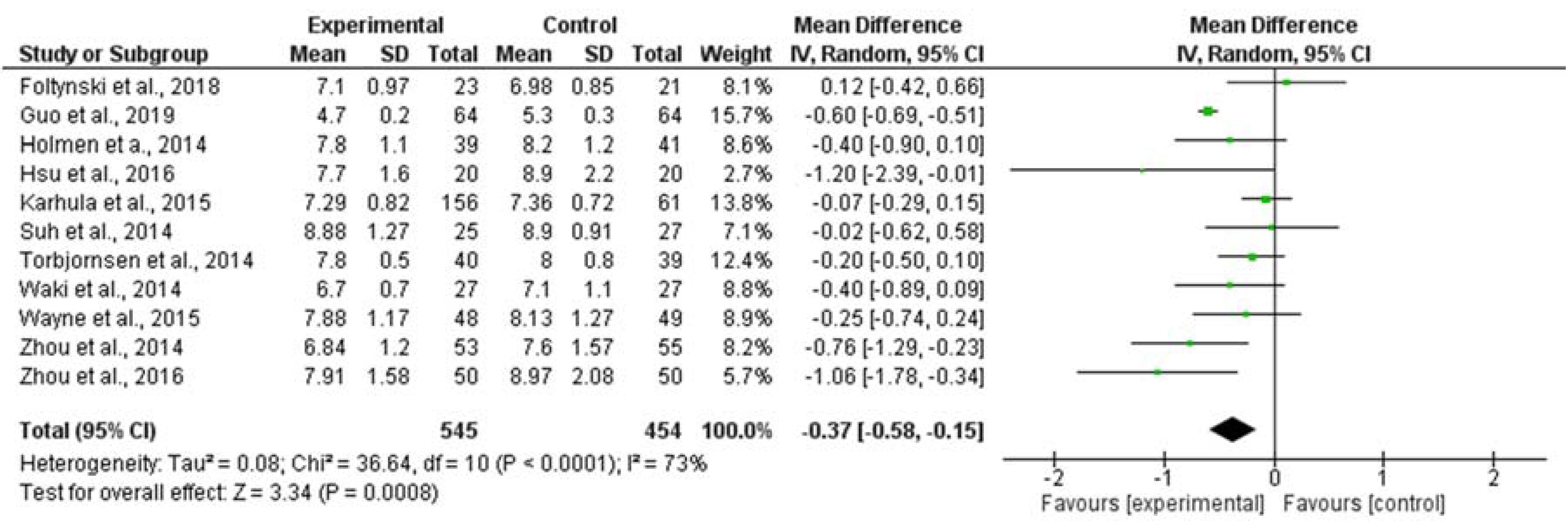
3.3. Meta-Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; IDF: Brussels, Belgium, 2017. [Google Scholar]
- Ogurtsova, K.; Fernandes, J.D.R.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 28, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes. 2016. Available online: https://www.who.int/diabetes/global-report/es/ (accessed on 20 November 2019).
- Roberts, S.; Barry, E.; Craig, D.; Airoldi, M.; Bevan, G.; Greenhalgh, T. Preventing type 2 diabetes: Systematic review of studies of cost-effectiveness of lifestyle programmes and metformin, with and without screening, for pre-diabetes. BMJ Open 2017, 7, e017184. [Google Scholar] [CrossRef]
- Boyle, L.; Grainger, R.; Hall, R.M.; Krebs, J.D. Use of and beliefs about mobile phone apps for diabetes self-management: Surveys of people in a hospital diabetes clinic and diabetes health professionals in New Zealand. JMIR mHealth uHealth 2017, 5, e85. [Google Scholar] [CrossRef]
- Ubaid Ur Rehman, M.; Aleem, M.; Islam, M.A.; Ahmed, S. Smart applications for diabetes management: A comprehensive survey and ranking. Health Inform. J. 2019. [Google Scholar] [CrossRef]
- Debong, F.; Mayer, H.; Kober, J. Real-World assessments of my Sugr Mobile Health App. Diabetes Technol. Ther. 2019, 21, S2–S35. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalg, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The Oxford 2011 Levels of Evidence. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence (accessed on 10 January 2020).
- Review Manager (RevMan) [Computer Program]; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014.
- Foltynski, P.; Ladyzynski, P.; Pankowska, E.; Mazurczak, K. Efficacy of automatic bolus calculator with automatic speech recognition in patients with type 1 diabetes: A randomized cross-over trial. J. Diabetes 2018, 10, 600–618. [Google Scholar] [CrossRef]
- Klee, P.; Bussien, C.; Castellsague, M.; Combescure, C.; Dirlewanger, M.; Girardin, C.; Mando, J.L.; Perrenoud, L.; Salomon, C.; Schneider, F.; et al. An intervention by patient-designed do-it-yourself mobile device app reduces HbA1c in children and adolescents with type 1 diabetes: A randomized double-crossover study. Diabetes Technol. Ther. 2018, 20, 797–805. [Google Scholar] [CrossRef]
- Suh, S.; Jean, C.; Koo, M.; Lee, S.Y.; Cho, M.J.; Sim, K.H.; Jin, S.M.; Bae, J.C.; Kim, J.H. A randomized controlled trial of an internet-based mentoring program for type 1 diabetes patients with inadequate glycemic control. Diabetes Metab. J. 2014, 38, 134–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drion, I.; Pameijer, L.R.; Van Dijk, P.R.; Groenier, K.H.; Kleefstra, N.; Bilo, H.J.G. The effects of a mobile phone application on quality of life in patients with type I diabetes mellitus: A randomized controlled trial. J. Diabetes Sci. Technol. 2015, 9, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Skrovseth, S.O.; Arsand, E.; Godtliebsen, F.; Joakimsen, R.M. Data-driven personalized feedback to patients with type 1 diabetes: A randomized trial. Diabetes Technol. Ther. 2015, 17, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Holmen, H.; Torbjornsen, A.; Wahl, A.K.; Jenum, A.K.; Smastuen, C.; Arsand, E.; Holmen, H.; Wahl, A.K.; Ribu, L. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: One year results from the Norwegian randomized controlled trial renewing health. JMIR mHealth uHealth 2014, 2, e57. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kwak, S.H.; Jung, H.S.; Kao, B.K.; Moon, M.K.; Lim, S.; Jang, H.C.; Park, K.S.; Cho, Y.M. The effect of a Smartphone-based patient centered diabetes care system in patients with type 2 diabetes: A randomized controlled trial for 24 weeks. Diabetes Care 2019, 42, 3–9. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Li, P.; Zhou, P.; Li, S.Y. Evaluation the effects of mobile health intervention on weight management, glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus. J. Endocrinol. Investig. 2019, 42, 709–714. [Google Scholar] [CrossRef]
- Karhula, T.; Vuorinen, A.; Raapysjarvi, K.; Pakanen, M.; Itkonen, P.; Tepponen, M.; Junno, U.M.; Jokinen, T.; van Gils, M.; Lähteenmäki, J.; et al. Telemonitoring and mobile phone-based health coaching among finnish diabetic and heart disease patients: Randomized controlled trial. J. Med. Internet Res. 2015, 17, e153. [Google Scholar] [CrossRef]
- Fang, R.; Denn, X. Electronic messaging intervention for management of cardiovascular risk factors in type 2 Diabetes Mellitus: A randomised controlled trial. J. Clin. Nurs. 2018, 27, 612–620. [Google Scholar] [CrossRef]
- Zhou, P.; Xu, L.; Liu, X.; Huang, J.; Xu, W.; Chen, W. Web-based telemedicine for management of type 2 diabetes through glucose uploads: A randomized controlled trial. Int. J. Clin. Exp. Pathol. 2014, 7, 8848–8854. [Google Scholar] [PubMed]
- Hsu, W.C.; Lau, K.H.K.; Huang, R.; Ghiloni, S.; Le, H.; Gilroy, S.; Abrahamson, M.; Moore, J. Utilization of a cloud-based diabetes management program for insuling initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol. Ther. 2016, 2, 59–66. [Google Scholar] [CrossRef]
- Waki, K.; Fujita, H.; Uchimura, Y.; Omae, K.; Aramaki, E.; Kato, S.; Lee, H.; Kobayashi, H.; Kadowaki, T.; Ohe, K. DialBetics: A novel smartphone-based self-management support system for type 2 diabetes patients. J. Diabetes Sci. Technol. 2014, 8, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Peters, A.L.; Burnet, E.; Lam, C.N.; Menchine, M. Trial to examine text message based mHealth in emergency department patients with diabetes (text-med): A randomized controlled trial. Ann. Emerg. Med. 2014, 6, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Torbjornsen, A.; Jenum, A.K.; Smastuen, M.C.; Arsand, E.; Holmen, H.; Wahl, A.K.; Ribu, L. A low intensity mobile health intervention with a without health counselling for persons with type 2 diabetes, part 1: Baseline and short term results from a randomized controlled trial in the Norwegian part of renewing health. JMIR mHealth uHealth 2014, 2, e52. [Google Scholar] [CrossRef] [PubMed]
- Wayne, N.; Perez, D.F.; Kaplan, D.M.; Ritvo, P. Health coaching reduces HbA1c in type 2 diabetic patients from a lower-socioeconomic status community: A randomized controlled trial. J. Med. Internet Res. 2015, 17, e224. [Google Scholar] [CrossRef]
- Anzaldo-Campos, M.C.; Contreras, S.; Vargas-Ojeda, A.; Menchaca-Díaz, R.; Fortmann, A.; Philis-Tsimikas, A. Dulce Wireless Tijuana: A randomized control trial evaluating the impact of project dulce and short-term mobile technology on glycemic control in a family medicine clinic in Northern Mexico. Diabetes Technol. Ther. 2016, 18, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, M.; Yuan, J.; Sun, Y. Welltang—A smart phone-based diabetes management application improves blood glucose control in Chinese people with diabetes. Diabetes Res. Clin. Pract. 2016, 116, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Grohman-Izay, B.; Forisch, M. Use of digital tracking devices in the management of the diabetes mellitus: A systematic review and meta-analysis. Diabetes 2015, 64, 137. [Google Scholar]
- Skrøvseth, S.O.; Årsand, E.; Godtliebsen, F.; Joakimsen, R.M. Model-driven diabetes care: Study protocol for a randomized controlled trial. Trials 2013, 14, 139. [Google Scholar] [CrossRef]
- Feuerstein, C.; Bzdick, S.; Padmanabhuni, A.; Bains, P.; Roe, C.; Weinstock, R.S. Use of a smartphone application to reduce hypoglycemia in type 1 diabetes: A pilot study. J. Diabetes Sci. Technol. 2018, 12, 1192–1199. [Google Scholar] [CrossRef]
- Charpentier, G.; Benahmou, P.Y.; Dardadi, D.; Clergeot, A.; Franc, S.; Schaepelunck, P.; Catargi, B.; Melki, V.; Chaillous, L.; Farret, A.; et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients. Diabetes Care 2011, 34, 533–539. [Google Scholar] [CrossRef]
- Vallejo, M.R.; Carreira, M.; Anarte, M.T.; Linares, F.; Olveira, G.; González, S. Bolus calculator reduces hypoglycemia in the short term and fear of hypoglycemia in the long term in subjects with type 1 diabetes (CBMDI Study). Diabetes Technol. Ther. 2017, 19, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Taushmann, M.; Allen, J.M.; Wilinska, M.E.; Thabit, H.; Acerini, C.L.; Dunger, D.B.; Hovorka, R. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: A 3-week, free-living, randomized crossover trial. Diabetes Care 2016, 39, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.G.; Youssef, J.E.; Reddy, R.; Resalat, R.; Branigan, D.; Condon, J.; Preiser, N.; Ramsey, K.; Jones, M.; Edwards, C.; et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes. Metab. 2016, 18, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Höchsmann, C.; Walz, S.P.; Schäfer, J.; Holopainen, J.; Hanssen, H.; Schmidt-Trucksäss, A. Mobile exergaming for health-effects of a serious game application for smartphones on physical activity and exercise adherence in type 2 Diabetes Mellitus—Study protocol for a randomized controlled trial. Trials 2017, 18, 1–17. [Google Scholar] [CrossRef]
- Valentiner, L.S.; Ried-Larsen, M.; Karstoft, K.; Brinkløv, C.F.; Brøns, C.; Nielsen, R.O.; Christensen, R.; Nielsen, J.S.; Vaag, A.A.; Pedersen, B.K.; et al. Long-term effect of smartphone-delivered interval walking training on physical activity in patients with type 2 diabetes: Protocol for a parallel group single-blinded randomised controlled trial. BMJ Open 2017, 7, e014036. [Google Scholar] [CrossRef]
- Valentiner, L.S.; Thorsen, I.K.; Kongstad, M.B.; Brinkløv, C.F.; Larsen, R.T.; Karstoft, K.; Nielsen, J.S.; Pedersen, B.K.; Langberg, H.; Ried-Larsen, M. Effect of ecological momentary assessment, goal-setting and personalized phone-calls on adherence to interval walking training using the InterWalk application among patients with type 2 diabetes—A pilot randomized controlled trial. PLoS ONE 2019, 14, e0208181. [Google Scholar] [CrossRef]
- Waki, K.; Aizawa, K.; Kato, S.; Fujita, H.; Lee, H.; Kobayashi, H.; Ogawa, M.; Mouri, K.; Kadowaki, T.; Ohe, K. DialBetics with a multimedia food recording tool, FoodLog: Smartphone-based self-management for type 2 diabetes. J. Diabetes Sci. Technol. 2015, 9, 534–540. [Google Scholar] [CrossRef]
- Kim, G.; Cheol, J.; Kee, B.; Yeon, K.; Kyung, D.; Lee, M.K.; Kim, J.H.; Jin, S.M. An information and communication technology-based centralized clinical trial to determine the efficacy and safety of insulin dose adjustment education based on a smartphone personal health record application: A randomized controlled trial. BMC Med. Inform. Decis. Mak. 2017, 17, 109. [Google Scholar] [CrossRef]
- Alonso, R.; García, L.; Patino, M.C.; Sánchez, N.; Gómez, M.A.; Recio, J.I. Effectiveness of a multifactorial intervention in increasing adherence to the Mediterranean diet among patients with diabetes mellitus type 2: A controlled and randomized study (EMID Study). Nutrients 2019, 11, 162. [Google Scholar] [CrossRef]
- Goh, G.; Chuan, N.; Malhotra, R.; Padmanabhan, U.; Barbier, S.; Allen, J.C., Jr.; Østbye, T. Short-term trajectories of use of a caloric-monitoring mobile phone app among patients with type 2 diabetes mellitus in a primary care setting. J. Med. Internet. Res. 2015, 17, e33. [Google Scholar] [CrossRef]
- Luo, L.; Pang, B.; Chen, J.; Li, Y.; Xie, X. Assessing the Impact of Lifestyle Interventions on Diabetes Prevention in China: A Modeling Approach. Int. J. Environ. Res. Public Health 2019, 16, 1677. [Google Scholar] [CrossRef] [PubMed]
- Ayman, A.A.H.; Asirvatham, A.R.; Mohamed, A.A.D. Differences of freestyle libre flash glucose monitoring system and finger pricks on clinical charactersitcis and glucose monitoring satisfaction in type 1 diabetes using insulin pump. Clin. Med. Insight Endocrinol. Diabetes 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Heinemann, L.; Freckmann, G.; Ehrman, D.; Faber-Heinemann, G.; Guerra, S.; Waldenmaier, D.; Hermanns, N. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired treated with multiple daily insulin injections (hypoed): A multicenter randomized controlled trial. Lancet 2018, 391, 1367–1777. [Google Scholar] [CrossRef]
- Hermanss, N.; Ehrmann, D.; Schipfer, M.; Krogger, J.; Haak, T.; Kulzer, B. The impact of a structured education and treatment programme (FLASH) for people with diabetes using a flash sensor-based glucose monitoring system. Results of a randomized controlled trial. Diabetes Res. Clin. Pract. 2019, 150, 111–121. [Google Scholar] [CrossRef]
- Al Hayek, A.A.; Robert, A.A.; Al Dawish, M.; Ahmed, R.A.; Al Sabaan, F.S. The evolving role of short term professional continuous glucose monitoring on glycemic control and hypoglycemia among Saudi patients with type 1 diabetes: A prospective study. Diabetes Ther. 2015, 6, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Higging, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
| Author, Country (Year) | Design | Sample | Intervention | Main Results | Level of Evidence/Degree of Education |
|---|---|---|---|---|---|
| Anzaldo-Campos et al., Mexico (2016) | Randomized Clinical Trial | Diabetes mellitus 2 patients. Control group (n = 100) Intervention group (n = 102). | Project Dulce technology-enhaced intervention (10 months). Care management by multidisciplinary team, and peer-led group education component. Glucose levels were send by the cell phone and patients received text messages, short educational videos, brochures, and took interactive surveys. | The HbA1c levels statiscally decreased 3.02% after 10 months while in the control group the decrease was 1.30%. The difference between both groups was statistically significant. | 1a/A |
| Arora et al., USA (2014) | Randomized Clinical Trial | Diabetes mellitus 1 and 2 patients. Control group (n = 64) Intervention group (n = 64). | TExT-MED (6 months). Daily text messages delivered to patients’ mobile phones. The messages enhanced patient motivation, self-efficacy, and ability to perform diabetes self-care behaviors, including information about diabetes, medication reminders, healthy-living challenges, and questions. Two messages were send twice a day (9 AM and 5 PM). | HbA1c decreased by 1.05% in the intervention group and 0.6% in the control group after 6 months. The mean difference was not statistically significant between groups. | 1a/A |
| Drion et al., Netherlands (2015). | Randomized Clinical Trial | Diabetes mellitus 1 patients. Control group (n = 32) Intervention group (n = 31). | Diabetes Under Control app (3 months). The app let patients introduce diabetes-related self-care data (blood glucose, carbohydrate intake, medication, physical exercise, and notes). | No significant differences were found between groups in the HbA1c levels after the intervention. | 1a/A |
| Fang, China (2018) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 62) Intervention group (n = 67). | The intervention group received microletters and short messages with diabetes related information while the control group received short calls. | After 1 year the intervention group level of HbA1c decreased from 6.96 (± 1.30) in pre-intervention to 6.55 ± 1.06 in post-intervention (p < 0.05). In the control group the HbA1c level increased (p > 0.05). The difference in HbA1c levels between groups in pre- and post-intervention were statistically significant. | 1a/A |
| Foltynski, Polonia (2018) | Randomized clinical trial (crossed) | Diabetes mellitus 1 patients. Control group (n = 27) Intervention group (n = 27). | During Period 1 (4 d) a group received support from the VoiceDiab system in insulin bolus calculations (Treatment A) or performed manual bolus calculations (Treatment B). At 14 d, the patients move to Period 2 (4 d) and received the opposite treatment plan to that received in Period 1. | No differences in the HbA1c levels were found between groups. | 1a/A |
| Guo et al., China (2019) | Randomized clinical trial | Pregnant women with gestiational diabetes mellitus. Control group (n = 60) Intervention group, mHealth group (n = 64). | The intervention group had the application “Dnurse App” to monitor fasting and post-prandial glucose. It also included an online instruction done by a nurse every day for 2 h to answer questions about diet, exercise, blood glucose, and other relevant topics. They were notified if they uploaded an abnormal blood glucose result. | The HbA1c mean was lower in the intervention group (4.07) than in the control group (5.3) with p < 0.001. | 1a/A |
| Holmen et al., Norway (2014) | Three arm randomized controlled clinical trial | Diabetes mellitus 2 patients. Control group (n = 50) Intervention group 1: Few Touch App group, (n = 51).Intervention group 2: Few Touch with Health Counseling group (n = 50). | The Few Touch App provided the user a diabetes diary app designed to increase self-managament through awareness, overview of relevant factors, and motivational feedback. It also included registration of food and physical-activity habits. The other group added Health Counseling to the app for the first 4 months. | After one year, the level of HbA1c decreases in all groups. However, the change in the level of HbA1c did not differ significantly between groups. | 1a/A |
| Hsu et al., USA (2016) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 20) Intervention group (n = 20). | A cloud-based diabetes management program was used for 14 w by people with diabetes through an app. It included self-tracking informing about medication, medication adherence, and blood glucose and it emphasized other factors like diet and exercise. The app also included weekly charts with healthcare professionals (virtual visits with audio, video, and shared screen control). | Both groups decreased their levels of HbA1c after the intervention. The decrease in the intervention groups was higher than in the control group (3.2 vs. 2.0) with p < 0.05. | 1a/A |
| Karhula et al., Finland (2015) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 70) Intervention group (n = 180). | Mobile personal health record app. The patients sent health information through the app once a week. | After 1 year, no significant statistical differences were found in HbA1c levels between groups or in pre- andpost-intervention levels in the intervention group. | 1a/A |
| Kim et al., Korea (2019) | Randomized clinical trial | Diabetes mellitus 2 patients. mDiabetes group (n = 90) pLog-book group (n = 82). | The mDiabetes was used for 24 w (application that used an algorithm to send inmediate feedback messages according to the glycemic control). | The reduction of HbA1c was higher in the experimental group -0.40 compared to the control group -0.06. The mean difference between groups was 0.35% (p < 0.05). | 1a/A |
| Klee et al., Switzerland (2018) | Randomized clinical trial | Diabetes mellitus 1 patients (children and adolescents). Experimental group used Webdia app (n = 20) Control group (n = 13). | Webdia app to help in calculating the insulin dose and information about meals. Patients used the app for 3 months. | After 3 months, when taking into account patients with HbA1c higher than 8% the reduction in the intervention group was 0.33 while the control group had increased their HbA1c level (0.21) with p < 0.05. | 1a/A |
| Skrovseth et al., Norway (2015) | Randomized clinical trial | Diabetes mellitus 1 patients. Control group (n = 15) Intervention group (n = 15). | Diabetes Diary App (18 w). The app included a daily and weekly blood glucose graphic, blood glucose trends, situations matching when injecting insulin, physical activity, and carbohydrate registration. | Both groups decreased their levels of HbA1c, but the differences in the mean values of HbA1c were not statistically significant. | 1a/A |
| Suh, Korea (2014) | Randomized clinical trial | Diabetes mellitus 1 patients. Control group (n = 27) Intervention group (n = 25). | The intervention group received individual feedback on their results. They sent the results of their glucometer to a website. Then they received support by a mentor on insulin dosing, physical activity, and food intake within 48 h of transmission. Text messages were sent to notify the assigned mentors when their trainees uploaded their data. The control group did not receive any comments but were able to review and interpret their own website data as often as necessary. | After 12 w the control group HbAc1 level decreased from 9.52 ± 1.01 to 8.9 ± 0.91. After 12 w the intervention group HbAc1 decreased from 9.39 ± 1.21 to 8.88 ± 1.27. Without statistical significance the subjects who improved their HbA1c levels more than 1% were 68.8% of patients in the intervention group vs. 41.7% in the control group. | 1a/A |
| Torbjornsen et al., Norway (2014) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 44) Intervention group (n = 42). | Few Touch Application (4 months). It is a diary for type 2 diabetes included in the smartphone. The app registered blood glucose, food habits, physical activity, personal goal-setting system and general diabetes information. | The mean level of HbA1c decreased in all the intervention group and control group (0.23 vs. 0.39), but the mean difference was not statistically significant. | 1a/A |
| Waki, Japan (2014) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 27) Intervention group (n = 27). | DialBetics consisted of 4 modules:(1) data transmission module; (2) evaluation module; (3) communication module; (4) dietary evaluation module. A 3-month study was designed to assess safety and usability. | After 3 months, the DialBetics Group HbA1c decreased 0.4%, while in the Non-DiaBeltics Group it increased 0.1%. A statistically signifcant difference in HbA1c was found between groups after the intervention. | 1a/A |
| Wayne et al., Canada (2015) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 49) Intervention group (n = 48). | For 6 months patients recorded their information about blood glucose, physical activity, food intake, and mood in the app. Patients could comunnicate with a health coach through their phone (messaging, calls, and person meetings). The health coach could see the patients information. | After 6 months, both groups decreased their levels of HbA1c, but no significant differences were found between intervention and control group reductions (0.84% vs. 0.81%) | 1a/A |
| Zhou, China (2014) | Randomized clinical trial | Diabetes mellitus 2 patients. Control group (n = 55) Intervention group (n = 53). | Telemedicine group and a traditional group of face-to-face visits as a control | After 3 months, the telemedicine intervention HbA1c levels decreased in the control group from 8.22 ± 1.58 to 7.6 ± 1.57 and in the intervention group from 8.44 ± 1.58 to 6.84 ± 1.2. There was no significant difference between groups. | 1a/A |
| Zhou et al., China (2016) | Randomized clinical trial | Diabetes mellitus 1 and 2 patients. Control group (n = 50) Intervention group (n = 50). | Welltang app for 3 months. This was a diabetes management application and could be used by patients and clinicians. It had three main aims: knowledge, self-management, and communication between patients and clinicians. | After 3 months follow-up, the HbA1c mean was 7.91(±1.58) for the intervention group and 8.97(± 2.08) for the control group. The differences between groups were significant (p < 0.01). | 1a/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos-Cabrera, M.B.; Velando-Soriano, A.; Pradas-Hernández, L.; Suleiman-Martos, N.; Cañadas-De la Fuente, G.A.; Albendín-García, L.; Gómez-Urquiza, J.L. Smartphones and Apps to Control Glycosylated Hemoglobin (HbA1c) Level in Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 693. https://doi.org/10.3390/jcm9030693
Martos-Cabrera MB, Velando-Soriano A, Pradas-Hernández L, Suleiman-Martos N, Cañadas-De la Fuente GA, Albendín-García L, Gómez-Urquiza JL. Smartphones and Apps to Control Glycosylated Hemoglobin (HbA1c) Level in Diabetes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(3):693. https://doi.org/10.3390/jcm9030693
Chicago/Turabian StyleMartos-Cabrera, María Begoña, Almudena Velando-Soriano, Laura Pradas-Hernández, Nora Suleiman-Martos, Guillermo A. Cañadas-De la Fuente, Luis Albendín-García, and José L. Gómez-Urquiza. 2020. "Smartphones and Apps to Control Glycosylated Hemoglobin (HbA1c) Level in Diabetes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 3: 693. https://doi.org/10.3390/jcm9030693
APA StyleMartos-Cabrera, M. B., Velando-Soriano, A., Pradas-Hernández, L., Suleiman-Martos, N., Cañadas-De la Fuente, G. A., Albendín-García, L., & Gómez-Urquiza, J. L. (2020). Smartphones and Apps to Control Glycosylated Hemoglobin (HbA1c) Level in Diabetes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(3), 693. https://doi.org/10.3390/jcm9030693









