Specialized Management of Oral Anticoagulation Therapy Improves Outcome in Patients with Chronic Renal Insufficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Study Procedures
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Usual Medical Care Patients with Regard to Renal Failure Status
3.2. Quality of Oral Anticoagulation Therapy
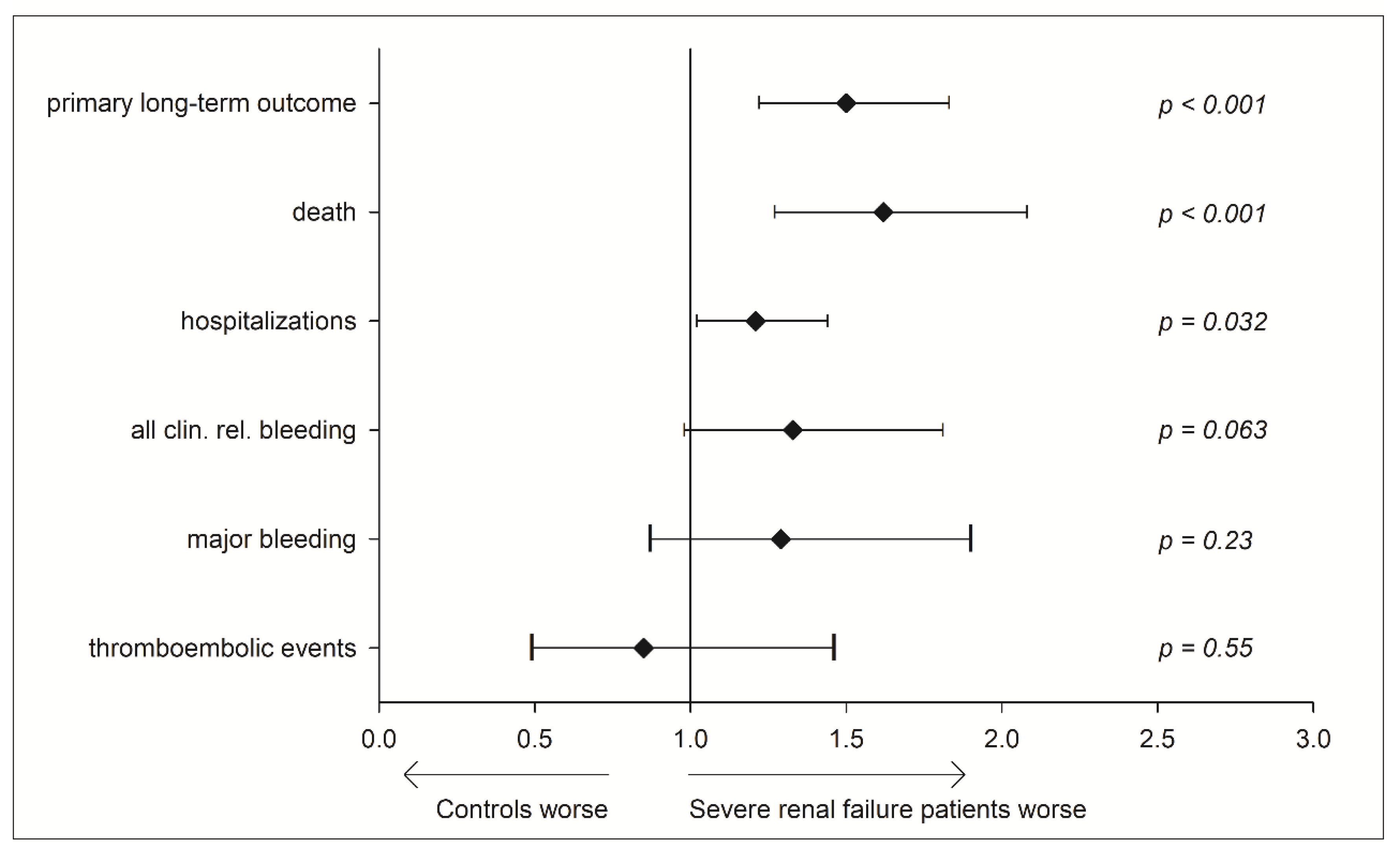
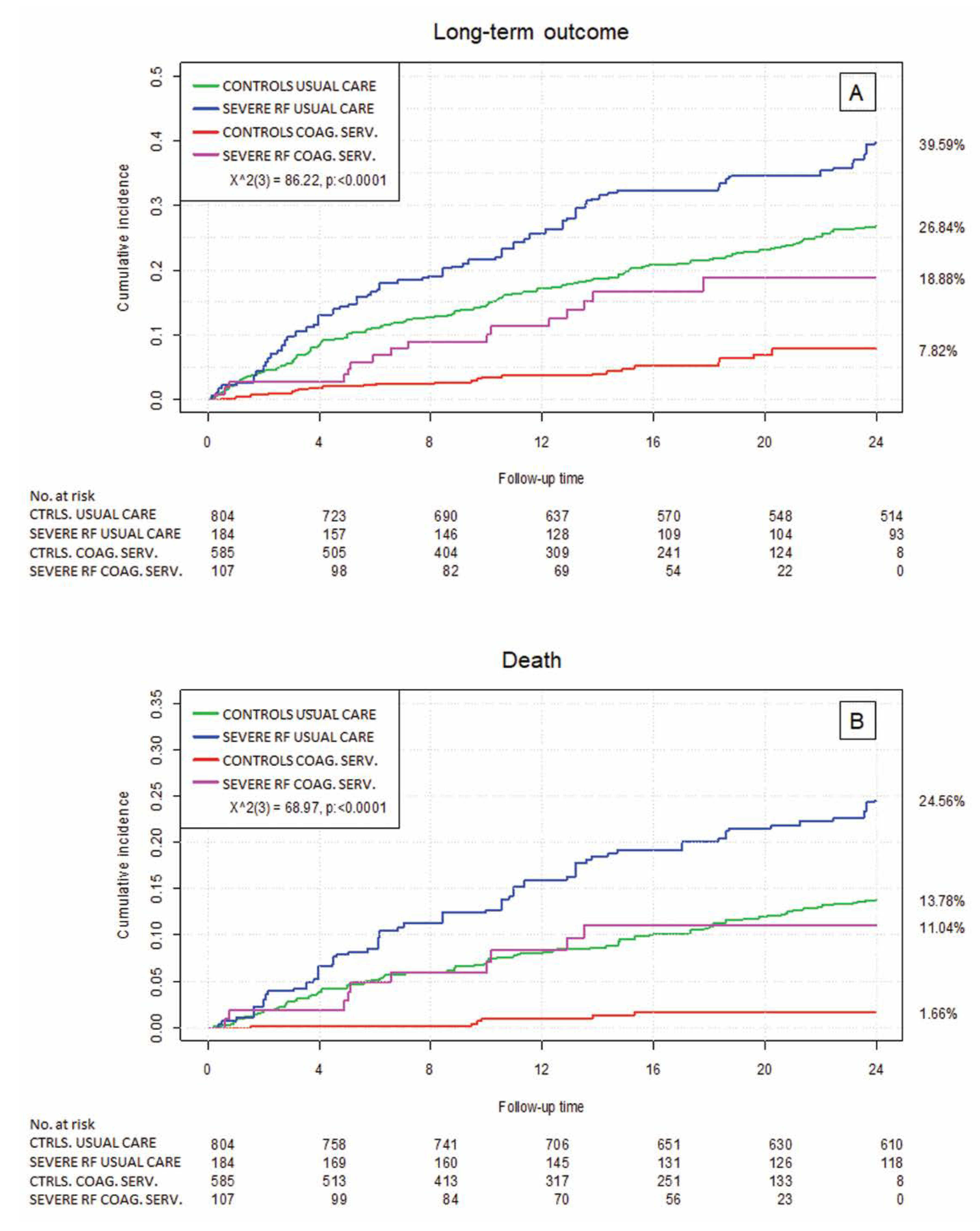
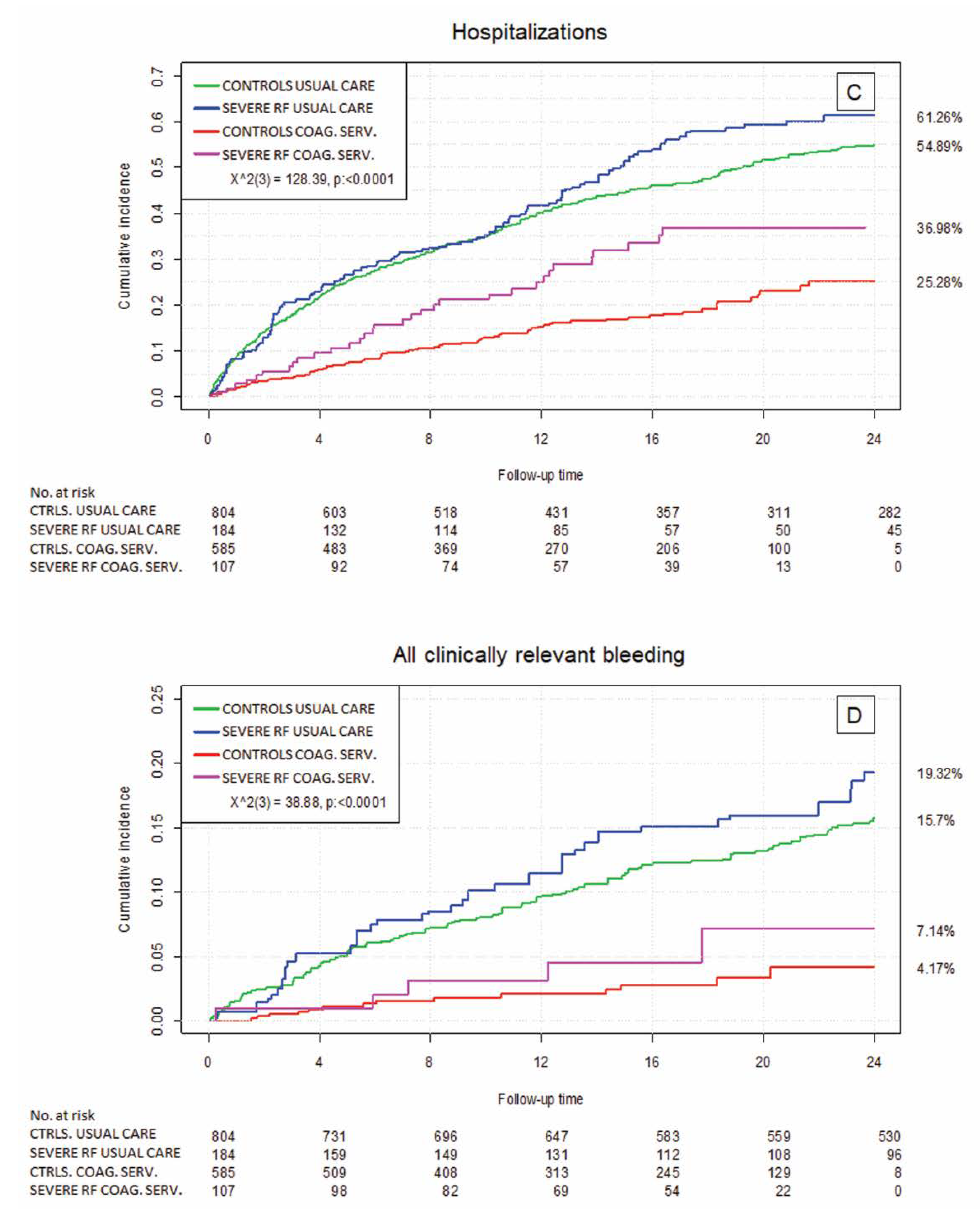
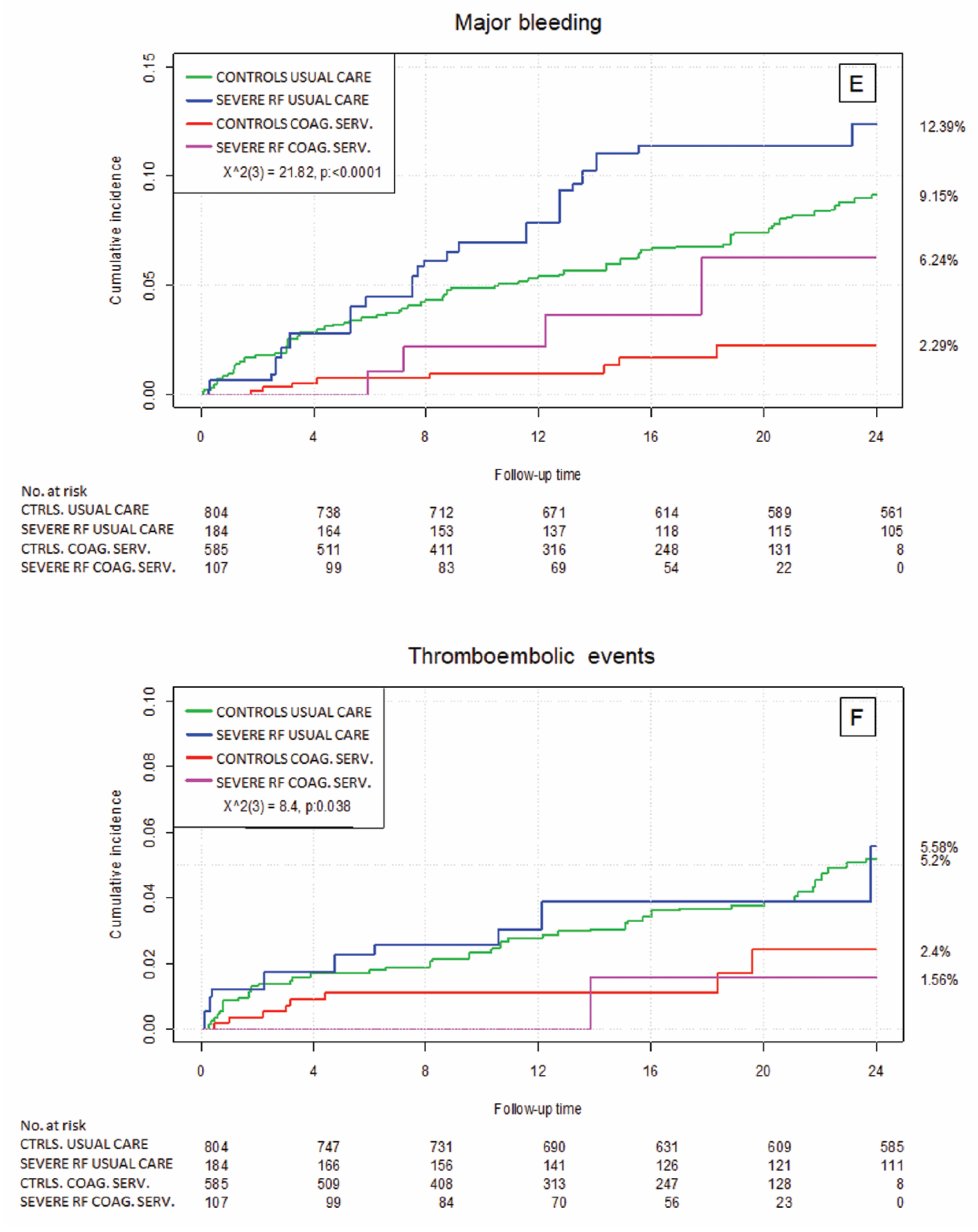
3.3. Clinical Outcome by Renal Failure Status
3.4. Relevance of Health Care Setting—Usual Medical Care vs. the Specialized Coagulation Service
3.5. Usual Medical Care vs. the Specialized Coagulation Service—Adjusted Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Husted, S.; Wallentin, L.; Andreotti, F.; Arnesen, H.; Bachmann, F.; Baigent, C.; Huber, K.; Jespersen, J.; Kristensen, S.D.; et al. Vitamin K antagonists in heart disease: Current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis--Task Force on Anticoagulants in Heart Disease. Thromb. Haemost. 2013, 110, 1087–1107. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.W.; Fahrbach, K.; Hauch, O.; Wygant, G.; Estok, R.; Cella, C.; Nalysnyk, L. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: A systematic review and metaanalysis. Chest 2004, 126, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Antonucci, E.; Testa, S.; Tosetto, A.; Ageno, W.; Palareti, G. Bleeding risk in very old patients on vitamin K antagonist treatment: Results of a prospective collaborative study on elderly patients followed by Italian Centres for Anticoagulation. Circulation 2011, 124, 824–829. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Weitz, J.I. New anticoagulants. Circulation 2010, 121, 1523–1532. [Google Scholar] [CrossRef]
- Chokesuwattanaskul, R.; Thongprayoon, C.; Tanawuttiwat, T.; Kaewput, W.; Pachariyanon, P.; Cheungpasitporn, W. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: Meta-analysis. PACE Pacing Clin. Electrophysiol. 2018, 41, 627–634. [Google Scholar] [CrossRef]
- Heine, G.H.; Brandenburg, V.; Schirmer, S.H. Oral Anticoagulation in Chronic Kidney Disease and Atrial Fibrillation. Dtsch. Aerztebl. Int. 2018, 115, 287–294. [Google Scholar]
- Chan, K.E.; Giugliano, R.P.; Patel, M.R.; Abramson, S.; Jardine, M.; Zhao, S.; Perkovic, V.; Maddux, F.W.; Piccini, J.P. Nonvitamin K Anticoagulant Agents in Patients With Advanced Chronic Kidney Disease or on Dialysis With AF. J. Am. Coll. Cardiol. 2016, 67, 2888–2899. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.H.; Kamper, A.-L.; Hommel, K.; Køber, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012, 367, 625–635. [Google Scholar] [CrossRef]
- Wizemann, V.; Tong, L.; Satayathum, S.; Disney, A.; Akiba, T.; Fissell, R.B.; Kerr, P.G.; Young, E.W.; Robinson, B.M. Atrial fibrillation in hemodialysis patients: Clinical features and associations with anticoagulant therapy. Kidney Int. 2010, 77, 1098–1106. [Google Scholar] [CrossRef]
- Chan, K.E.; Lazarus, J.M.; Thadhani, R.; Hakim, R.M. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J. Am. Soc. Nephrol. 2009, 20, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-M.; Disthabanchong, S. Are there ways to attenuate arterial calcification and improve cardiovascular outcomes in chronic kidney disease? World J. Cardiol. 2014, 6, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Turgut, F.; Covic, A.; Goldsmith, D. Statin treatment for dyslipidemia in chronic kidney disease and renal transplantation: A review of the evidence. J. Nephrol. 2009, 22, 598–609. [Google Scholar] [PubMed]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.-W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Prochaska, J.H.; Coldewey, M.; Göbel, S.; Keller, K.; Hendelmeier, M.; Konstantinides, S.; Münzel, T.; Wild, P.S. Evaluation of oral anticoagulation therapy: Rationale and design of the thrombEVAL study programme. Eur. J. Prev. Cardiol. 2014, 22, 622–628. [Google Scholar] [CrossRef]
- Prochaska, J.H.; Göbel, S.; Keller, K.; Coldewey, M.; Ullmann, A.; Lamparter, H.; Jünger, C.; Al-Bayati, Z.; Baer, C.; Walter, U.; et al. Quality of oral anticoagulation with phenprocoumon in regular medical care and its potential for improvement in a telemedicine-based coagulation service--results from the prospective, multi-center, observational cohort study thrombEVAL. BMC Med. 2015, 13, 14. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Piccini, J.P.; Stevens, S.R.; Chang, Y.; Singer, D.E.; Lokhnygina, Y.; Go, A.S.; Patel, M.R.; Mahaffey, K.W.; Halperin, J.L.; Breithardt, G.; et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: Validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K ant. Circulation 2013, 127, 224–232. [Google Scholar] [CrossRef]
- Schmitt, L.; Speckman, J.; Ansell, J. Quality assessment of anticoagulation dose management: Comparative evaluation of measures of time-in-therapeutic range. J. Thromb. Thrombolysis 2003, 15, 213–216. [Google Scholar] [CrossRef]
- Prochaska, J.H.; Göbel, S.; Keller, K.; Coldewey, M.; Ullmann, A.; Lamparter, H.; Schulz, A.; Schinzel, H.; Bickel, C.; Lauterbach, M.; et al. e-Health-based management of patients receiving oral anticoagulation therapy: Results from the observational thromb EVAL study. J. Thromb. Haemost. 2017, 15, 1375–1385. [Google Scholar] [CrossRef]
- Kleinow, M.E.; Garwood, C.L.; Clemente, J.L.; Whittaker, P. Effect of chronic kidney disease on warfarin management in a pharmacist-managed anticoagulation clinic. J. Manag. Care Pharm. 2011, 17, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bonde, A.; Lip, G.; Kamper, A.-L.; Staerk, L.; Torp-Pedersen, C.; Gislason, G.; Olesen, J. Renal Function, Time in Therapeutic Range and Outcomes in Warfarin-Treated Atrial Fibrillation Patients: A Retrospective Analysis of Nationwide Registries. Thromb. Haemost. 2017, 117, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.; Delate, T.; Witt, D.M.; Shaw, P.B.; McCool, K.H.; Clark, N.P. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb. Res. 2018, 163, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Roldán, V.; Marín, F.; Manzano-Fernandez, S.; Fernández, H.; Gallego, P.; Valdés, M.; Vicente, V.; Lip, G.Y.H. Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb. Haemost. 2013, 109, 956–960. [Google Scholar] [CrossRef]
- Malhotra, K.; Ishfaq, M.F.; Goyal, N.; Katsanos, A.H.; Parissis, J.; Alexandrov, A.W.; Alexandrov, A.V.; Tsivgoulis, G. Oral anticoagulation in patients with chronic kidney disease: A systematic review and meta-analysis. Neurology 2019, 92, e2421–e2431. [Google Scholar] [CrossRef]

| Controls (n = 1183) | Severe Renal Failure (n = 333) | p | |||
|---|---|---|---|---|---|
| Age | 73.0 | (64.0/78.9) | 75.0 | (68.0/81.0) | <0.001 |
| Male sex | 63.2% | (748/1183) | 65.8% | (219/333) | 0.40 |
| CHA2DS2-VASc | 3.95 | (1.78 SD) | 4.72 | (1.59 SD) | <0.001 |
| HAS-BLED | 2.57 | (1.18 SD) | 3.93 | (1.04 SD) | <0.001 |
| Charlson index | 5.54 | (2.23 SD) | 6.97 | (2.26 SD) | <0.001 |
| Care level present | 5.5% | (64/1171) | 9.8% | (32/328) | 0.007 |
| Traditional CV risk factors | |||||
| Diabetes | 26.8% | (317/1183) | 45.6% | (152/333) | <0.001 |
| Dyslipidemia | 53.0% | (626/1181) | 62.2% | (207/333) | 0.003 |
| FH of MI/Stroke | 40.5% | (479/1182) | 41.4% | (138/333) | 0.80 |
| Hypertension, any grade | 76.7% | (907/1182) | 86.2% | (287/333) | <0.001 |
| Obesity | 29.7% | (459/1183) | 32.2% | (107/332) | 0.28 |
| Smoker, current | 8.3% | (98/1183) | 5.7% | (19/333) | 0.13 |
| Comorbidities | |||||
| Atrial Fibrillation | 71.5% | (842/1177) | 80.9% | (266/329) | <0.001 |
| Coronary Artery Disease | 40.6% | (459/1131) | 49.4% | (157/318) | 0.006 |
| Myocardial Infarction | 20.3% | (238/1175) | 27.4% | (90/329) | 0.007 |
| Heart Failure, any grade | 37.3% | (433/1161) | 62.4% | (204/327) | <0.001 |
| History of Bleeding | 30.3% | (342/1129) | 38.3% | (118/308) | 0.009 |
| History of DVT a | 17.2% | (202/1175) | 17.5% | (58/332) | 0.93 |
| History of PE a | 11.2% | (132/1181) | 9.6% | (32/332) | 0.48 |
| History of stroke/TIA | 16.1% | (190/1179) | 20.2% | (67/331) | 0.082 |
| Peripheral Arterial Disease | 22.3% | (257/1151) | 25.8% | (84/326) | 0.21 |
| Chronic Lung Disease | 18.6% | (218/1170) | 34.1% | (113/331) | <0.001 |
| Sleep Apnea | 9.3% | (105/1126) | 10.1% | (32/317) | 0.67 |
| Autoimmune Disease | 8.2% | (95/1165) | 11.1% | (37/332) | 0.10 |
| Liver Disease | 4.2% | (49/1177) | 10.6% | (35/329) | <0.001 |
| Mental Illness | 10.4% | (122/1177) | 12.0% | (40/332) | 0.37 |
| Neoplasm | 15.4% | (181/1173) | 20.6% | (68/330) | 0.029 |
| Controls (n = 1183) | Severe Renal Failure (n = 364) | p | |||
|---|---|---|---|---|---|
| Atrial Fibrillation | 64.6% | (764/1183) | 72.1% | (240/333) | 0.011 |
| Venous Thromboembolism | 13.6% | (161/1183) | 7.2% | (24/333) | 0.001 |
| Peripheral Bypass | 10.7% | (126/1183) | 6.0% | (20/333) | 0.011 |
| Mechanical Heart Valve | 9.1% | (108/1183) | 10.2% | (34/333) | 0.53 |
| Embolism | 7.9% | (93/1183) | 4.5% | (15/333) | 0.04 |
| Thrombosis | 5.7% | (68/1182) | 2.7% | (9/333) | 0.024 |
| Other | 4.5% | (53/1183) | 6.0% | (20/333) | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauterbach, M.; Uhrich, E.; Eggebrecht, L.; Göbel, S.; Panova-Noeva, M.; Nagler, M.; ten Cate, V.; Bickel, C.; Espinola-Klein, C.; Münzel, T.; et al. Specialized Management of Oral Anticoagulation Therapy Improves Outcome in Patients with Chronic Renal Insufficiency. J. Clin. Med. 2020, 9, 645. https://doi.org/10.3390/jcm9030645
Lauterbach M, Uhrich E, Eggebrecht L, Göbel S, Panova-Noeva M, Nagler M, ten Cate V, Bickel C, Espinola-Klein C, Münzel T, et al. Specialized Management of Oral Anticoagulation Therapy Improves Outcome in Patients with Chronic Renal Insufficiency. Journal of Clinical Medicine. 2020; 9(3):645. https://doi.org/10.3390/jcm9030645
Chicago/Turabian StyleLauterbach, Michael, Eduard Uhrich, Lisa Eggebrecht, Sebastian Göbel, Marina Panova-Noeva, Markus Nagler, Vincent ten Cate, Christoph Bickel, Christine Espinola-Klein, Thomas Münzel, and et al. 2020. "Specialized Management of Oral Anticoagulation Therapy Improves Outcome in Patients with Chronic Renal Insufficiency" Journal of Clinical Medicine 9, no. 3: 645. https://doi.org/10.3390/jcm9030645
APA StyleLauterbach, M., Uhrich, E., Eggebrecht, L., Göbel, S., Panova-Noeva, M., Nagler, M., ten Cate, V., Bickel, C., Espinola-Klein, C., Münzel, T., S. Wild, P., & H. Prochaska, J. (2020). Specialized Management of Oral Anticoagulation Therapy Improves Outcome in Patients with Chronic Renal Insufficiency. Journal of Clinical Medicine, 9(3), 645. https://doi.org/10.3390/jcm9030645






