Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology
Abstract
1. Introduction
2. Emerging Immunological Markers
2.1. Importance of Immune Processes in Cornea Transplantation
2.2. In Vivo Confocal Microscopy Evaluation of Immune Cells in the Corneal Graft
2.3. Cytokines and Regulatory T cells
2.4. Major Histocompatibility Complex and Antigen-Presenting Cells
3. Endothelial Cell Density and Morphological Indicators as Graft Response Predictors
4. Vascular Dynamics and Graft Survival
4.1. Prior to Transplantation
| Antiangiogenic | Proangiogenic |
|---|---|
| IFN-γ [83] | VEGF-A, C, D [22,85,89,90,91,98,99,100] |
| sVEGFR-1,2,3 [92,93,94,95,96,97,98,99,100] | bFGF [22,85,86,87,88,89] |
| PEDF [94,95,96,97] | VLA-1 [102] |
| Endostatin [94,95,96,97] | PDGF [105,106] |
| ANG2 [107,108] |
4.2. After Transplantation
5. Physical Properties of the Cornea as Biomarkers of Graft Survival
6. Markers of Wound Healing
| Markers of Wound Healing | Clinical Significance | References |
|---|---|---|
| IL-1α, IL-1β | Paracrine regulation of myofibroblasts apoptosis | [143,145,147] |
| TNF-α | Triggers stromal keratocytes responses, including IL-1-mediated synthesis of Fas ligand | [144,145] |
| EGF | Reflects level of intrastromal inflammation. Responds to key inflammatory mediators including IL-1 and TNF. Observed as early as 2 months before rejection. Levels decreased as immunosuppressant treatment progresses. | [145] |
| PDGF | Sub-basal and endothelial immune cell density increases associated with graft rejection. Reflects levels of stromal inflammation by responding to inflammatory mediators. | [145,151] |
| aFGF, bFGF | Binds to VEGF-A; VEGF-C; VEGF-C and D, respectively. Can act as anti-angiogenic factors in the corneal epithelial cells. | [151] |
| uPA | Corneal epithelial cells migration and proliferation | [159] |
7. Future Directions
Author Contributions
Conflicts of Interest
References
- Perez, V.L.; Foulsham, W.; Peterson, K.; Dana, R. Pathophysiology of Corneal Graft Rejection. In Foundations of Corneal Disease; Colby, K., Dana, R., Eds.; Springer: Cham, Germany, 2020; pp. 87–96. [Google Scholar]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M.; et al. High-risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468–2478. [Google Scholar] [CrossRef]
- The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch. Ophthalmol. 1992, 110, 1392–1403. [Google Scholar] [CrossRef]
- Streilein, J.W.; Arancibia-Caracamo, C.; Osawa, H. The role of minor histocompatibility alloantigens in penetrating keratoplasty. Dev. Ophthalmol. 2003, 36, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.C.; Doxiadis, I.I.; Colen, T.P.; Beekhuis, W.H. Long-term outcome in high-risk corneal transplantation and the influence of HLA-A and HLA-B matching. Cornea 2003, 22, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Volker-Dieben, H.J.; Claas, F.H.; Schreuder, G.M.; Schipper, R.F.; Pels, E.; Persijn, G.G.; Smits, J.; D’Amaro, J. Beneficial effect of HLA-DR matching on the survival of corneal allografts. Transplantation 2000, 70, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Zhang, Q.; Liu, Y.; Dana, M.R. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 639–646. [Google Scholar]
- Huq, S.; Liu, Y.; Benichou, G.; Dana, M.R. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J. Immunol. 2004, 173, 4464–4469. [Google Scholar] [CrossRef]
- Kocaba, V.; Colica, C.; Rabilloud, M.; Burillon, C. Predicting Corneal Graft Rejection by Confocal Microscopy. Cornea 2015, 34 (Suppl. 10), S61–S64. [Google Scholar] [CrossRef]
- Chirapapaisan, C.; Abbouda, A.; Jamali, A.; Muller, R.T.; Cavalcanti, B.M.; Colon, C.; Witkin, D.; Sahin, A.; Dana, R.; Cruzat, A.; et al. In Vivo Confocal Microscopy Demonstrates Increased Immune Cell Densities in Corneal Graft Rejection Correlating with Signs and Symptoms. Am. J. Ophthalmol. 2019, 203, 26–36. [Google Scholar] [CrossRef]
- Niederer, R.L.; Sherwin, T.; McGhee, C.N. In vivo confocal microscopy of subepithelial infiltrates in human corneal transplant rejection. Cornea 2007, 26, 501–504. [Google Scholar] [CrossRef]
- Beauregard, C.; Huq, S.O.; Barabino, S.; Zhang, Q.; Kazlauskas, A.; Dana, M.R. Keratocyte apoptosis and failure of corneal allografts. Transplantation 2006, 81, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Falke, K.; Stahnke, T.; Guthoff, R.; Witt, M.; Wree, A.; Stachs, O. Morphological analysis of quiescent and activated keratocytes: A review of ex vivo and in vivo findings. Curr. Eye Res. 2014, 39, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.; Elewahel, S.M.; Kaufman, H.E. Confocal microscopy of corneal wound healing after deep lamellar keratoplasty in rabbits. Arch. Ophthalmol. 2010, 128, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Nishida T, S.S.; Morishige, N. Cornea and sclera: Anatomy and physiology. In Cornea, 4th ed.; Mannis MJ, H.E., Ed.; Elsevier Mosby: London, UK, 2017; Volume 1, pp. 1–22. [Google Scholar]
- Inomata, T.; Hua, J.; Nakao, T.; Shiang, T.; Chiang, H.; Amouzegar, A.; Dana, R. Corneal Tissue From Dry Eye Donors Leads to Enhanced Graft Rejection. Cornea 2018, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Funaki, T.; Sadrai, Z.; Hattori, T.; Chauhan, S.K.; Dana, R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1244–1250. [Google Scholar] [CrossRef]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1755–1762. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Dohlman, T.H.; Dana, R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J. Clin. Cell Immunol 2014, 5. [Google Scholar] [CrossRef]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Zhu, S.; Dekaris, I.; Duncker, G.; Dana, M.R. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha after corneal transplantation. J. Interferon Cytokine Res. 1999, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Nishida, T. Commanding roles of keratocytes in health and disease. Cornea 2010, 29 (Suppl. 1), S3–S6. [Google Scholar] [CrossRef]
- King, W.J.; Comer, R.M.; Hudde, T.; Larkin, D.F.; George, A.J. Cytokine and chemokine expression kinetics after corneal transplantation. Transplantation 2000, 70, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Heizmann, U.; Bohringer, D.; Kern, Y.; Reinhard, T. Predicting the risk for corneal graft rejection by aqueous humor analysis. Mol. Vis. 2011, 17, 1016–1023. [Google Scholar] [PubMed]
- Johnson, L.A.; Jackson, D.G. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int. Immunol 2010, 22, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Hamrah, P.; Zhang, Q.; Liu, Y.; Huq, S.; Dana, M.R. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol. Vis. 2005, 11, 632–640. [Google Scholar]
- Jin, Y.; Shen, L.; Chong, E.M.; Hamrah, P.; Zhang, Q.; Chen, L.; Dana, M.R. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol. Vis. 2007, 13, 626–634. [Google Scholar]
- Yamaguchi, T.; Higa, K.; Suzuki, T.; Nakayama, N.; Yagi-Yaguchi, Y.; Dogru, M.; Satake, Y.; Shimazaki, J. Elevated Cytokine Levels in the Aqueous Humor of Eyes with Bullous Keratopathy and Low Endothelial Cell Density. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5954–5962. [Google Scholar] [CrossRef]
- Yoon, C.H.; Choi, S.H.; Lee, H.J.; Kang, H.J.; Kim, M.K. Predictive biomarkers for graft rejection in pig-to-non-human primate corneal xenotransplantation. Xenotransplantation 2019, 26, e12515. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Qian, T.; Dai, Y.; Mashaghi, A.; Xu, J.; Hong, J. IFN-gamma Regulates the Expression of MICA in Human Corneal Epithelium Through miRNA4448 and NFkappaB. Front. Immunol. 2018, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Yamada, J.; Streilein, J.W.; Dana, M.R. ICAM-1 deficiency suppresses host allosensitization and rejection of MHC-disparate corneal transplants. Transplantation 2000, 69, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Grimaldo, S.; Yuen, D.; Ecoiffier, T.; Chen, L. Very late antigen-1 mediates corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4808–4812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Dana, M.R. Expression of cell adhesion molecules on limbal and neovascular endothelium in corneal inflammatory neovascularization. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1427–1434. [Google Scholar]
- Goldberg, M.F.; Ferguson, T.A.; Pepose, J.S. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmol. Ogy 1994, 101, 161–168. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Ophthalmol. 2003, 121, 1132–1140. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 581–589. [Google Scholar] [CrossRef]
- Inomata, T.; Hua, J.; Di Zazzo, A.; Dana, R. Impaired Function of Peripherally Induced Regulatory T Cells in Hosts at High Risk of Graft Rejection. Sci. Rep. 2016, 6, 39924. [Google Scholar] [CrossRef]
- Bestard, O.; Cruzado, J.M.; Rama, I.; Torras, J.; Goma, M.; Seron, D.; Moreso, F.; Gil-Vernet, S.; Grinyo, J.M. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J. Am. Soc. Nephrol. J. Asn 2008, 19, 2020–2026. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and Tolerance in Corneal Transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef]
- Azimzade, Y.; Hong, J.; Mashaghi, A. Immunophysical analysis of corneal neovascularization: Mechanistic insights and implications for pharmacotherapy. Sci. Rep. 2017, 7, 12220. [Google Scholar] [CrossRef]
- Hill, J.C. Systemic cyclosporine in high-risk keratoplasty: Long-term results. Eye 1995, 9 Pt 4, 422–428. [Google Scholar] [CrossRef]
- Poon, A.C.; Forbes, J.E.; Dart, J.K.; Subramaniam, S.; Bunce, C.; Madison, P.; Ficker, L.A.; Tuft, S.J.; Gartry, D.S.; Buckley, R.J. Systemic cyclosporin A in high risk penetrating keratoplasties: A case-control study. Br. J. Ophthalmol. 2001, 85, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Rumelt, S.; Bersudsky, V.; Blum-Hareuveni, T.; Rehany, U. Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J. Ophthalmol. 2002, 86, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, E.; Papaliodis, G.N.; Lobo, A.M.; Sobrin, L. Side-effects of anti-inflammatory therapy in uveitis. Semin. Ophthalmol. 2014, 29, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, I.; Stapleton, F.; Papas, E.; Sweeney, D.F.; Coroneo, M. In vivo confocal microscopy of the human cornea. Br. J. Ophthalmol. 2003, 87, 225–236. [Google Scholar] [CrossRef]
- Ing, J.J.; Ing, H.H.; Nelson, L.R.; Hodge, D.O.; Bourne, W.M. Ten-year postoperative results of penetrating keratoplasty. Ophthalmol. Ogy 1998, 105, 1855–1865. [Google Scholar] [CrossRef]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Adv Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Chauhan, S.K.; Saban, D.R.; Lee, H.K.; Dana, R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009, 182, 148–153. [Google Scholar] [CrossRef]
- McBride, B.W.; McGill, J.I.; Smith, J.L. MHC class I and class II antigen expression in normal human corneas and in corneas from cases of herpetic keratitis. Immunology 1988, 65, 583–587. [Google Scholar] [PubMed]
- Whitsett, C.F.; Stulting, R.D. The distribution of HLA antigens on human corneal tissue. Investig. Ophthalmol. Vis. Sci. 1984, 25, 519–524. [Google Scholar]
- Chong, E.M.; Dana, M.R. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008, 28, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N.S.; Badian, R.A.; Liu, X.; Feldreich, T.R.; Arnlov, J.; Utheim, T.P.; Dahlin, L.B.; Rolandsson, O. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci. Rep. 2018, 8, 14248. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Jin, Y.; Chen, Y.; Inomata, T.; Lee, H.; Chauhan, S.K.; Petasis, N.A.; Serhan, C.N.; Dana, R. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5944–5951. [Google Scholar] [CrossRef]
- Hua, J.; Stevenson, W.; Dohlman, T.H.; Inomata, T.; Tahvildari, M.; Calcagno, N.; Pirmadjid, N.; Sadrai, Z.; Chauhan, S.K.; Dana, R. Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1457–1467. [Google Scholar] [CrossRef]
- Hatou, S.; Yoshida, S.; Higa, K.; Miyashita, H.; Inagaki, E.; Okano, H.; Tsubota, K.; Shimmura, S. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem. Cells Dev. 2013, 22, 828–839. [Google Scholar] [CrossRef]
- Tuft, S.J.; Coster, D.J. The corneal endothelium. Eye 1990, 4 Pt 3, 389–424. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tanaka, H.; Toda, M.; Sotozono, C.; Hamuro, J.; Kinoshita, S.; Ueno, M.; Tanaka, M. A physical biomarker of the quality of cultured corneal endothelial cells and of the long-term prognosis of corneal restoration in patients. Nat. Biomed. Eng. 2019. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. Jama Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Sayegh RR, B.B.; Lass, J.H. Specular Microscopy. In Cornea, 4th ed.; Mannis MJ, H.E., Ed.; Elsevier Mosby: London, UK, 2017; Volume 1, pp. 160–179. [Google Scholar]
- Nishimura, J.K.; Hodge, D.O.; Bourne, W.M. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmol. Ogy 1999, 106, 1962–1965. [Google Scholar] [CrossRef]
- Lass, J.H.; Sugar, A.; Benetz, B.A.; Beck, R.W.; Dontchev, M.; Gal, R.L.; Kollman, C.; Gross, R.; Heck, E.; Holland, E.J.; et al. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch. Ophthalmol. 2010, 128, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Benetz, B.A.; Lass, J.H.; Gal, R.L.; Sugar, A.; Menegay, H.; Dontchev, M.; Kollman, C.; Beck, R.W.; Mannis, M.J.; Holland, E.J.; et al. Endothelial morphometric measures to predict endothelial graft failure after penetrating keratoplasty. Jama Ophthalmol. 2013, 131, 601–608. [Google Scholar] [CrossRef]
- Rock, T.; Landenberger, J.; Bramkamp, M.; Bartz-Schmidt, K.U.; Rock, D. The Evolution of Corneal Transplantation. Ann. Transpl. 2017, 22, 749–754. [Google Scholar] [CrossRef]
- Patel, S.V. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp. Eye Res. 2012, 95, 40–47. [Google Scholar] [CrossRef]
- Ishii, N.; Yamaguchi, T.; Yazu, H.; Satake, Y.; Yoshida, A.; Shimazaki, J. Factors associated with graft survival and endothelial cell density after Descemet’s stripping automated endothelial keratoplasty. Sci. Rep. 2016, 6, 25276. [Google Scholar] [CrossRef]
- Oellerich, S.; Ham, L.; Frank, L.E.; Gorges, S.; Bourgonje, V.J.A.; Baydoun, L.; van Dijk, K.; Melles, G.R.J. Parameters associated with endothelial cell density variability after Descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Ivarsdottir, E.V.; Benonisdottir, S.; Thorleifsson, G.; Sulem, P.; Oddsson, A.; Styrkarsdottir, U.; Kristmundsdottir, S.; Arnadottir, G.A.; Thorgeirsson, G.; Jonsdottir, I.; et al. Sequence variation at ANAPC1 accounts for 24% of the variability in corneal endothelial cell density. Nat. Commun. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Joyce, N.C.; Zhu, C.C. Human corneal endothelial cell proliferation: Potential for use in regenerative medicine. Cornea 2004, 23, S8–S19. [Google Scholar] [CrossRef]
- Shen, L.; Sun, P.; Zhang, C.; Yang, L.; Du, L.; Wu, X. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Sci. Rep. 2017, 7, 13400. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, E.; Hatou, S.; Higa, K.; Yoshida, S.; Shibata, S.; Okano, H.; Tsubota, K.; Shimmura, S. Skin-Derived Precursors as a Source of Progenitors for Corneal Endothelial Regeneration. Stem. Cells Transl. Med. 2017, 6, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shima, N.; Kimoto, M.; Ebihara, N.; Murakami, A.; Yamagami, S. Markers for distinguishing cultured human corneal endothelial cells from corneal stromal myofibroblasts. Curr. Eye Res. 2015, 40, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Dorfmueller, S.; Tan, H.C.; Ngoh, Z.X.; Toh, K.Y.; Peh, G.; Ang, H.P.; Seah, X.Y.; Chin, A.; Choo, A.; Mehta, J.S.; et al. Isolation of a recombinant antibody specific for a surface marker of the corneal endothelium by phage display. Sci. Rep. 2016, 6, 21661. [Google Scholar] [CrossRef] [PubMed]
- Bartakova, A.; Alvarez-Delfin, K.; Weisman, A.D.; Salero, E.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kunzevitzky, N.J.; Goldberg, J.L. Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2749–2762. [Google Scholar] [CrossRef]
- Hamuro, J.; Toda, M.; Asada, K.; Hiraga, A.; Schlotzer-Schrehardt, U.; Montoya, M.; Sotozono, C.; Ueno, M.; Kinoshita, S. Cell Homogeneity Indispensable for Regenerative Medicine by Cultured Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4749–4761. [Google Scholar] [CrossRef]
- Cheong, Y.K.; Ngoh, Z.X.; Peh, G.S.; Ang, H.P.; Seah, X.Y.; Chng, Z.; Colman, A.; Mehta, J.S.; Sun, W. Identification of cell surface markers glypican-4 and CD200 that differentiate human corneal endothelium from stromal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4538–4547. [Google Scholar] [CrossRef]
- Yoshihara, M.; Ohmiya, H.; Hara, S.; Kawasaki, S.; Consortium, F.; Hayashizaki, Y.; Itoh, M.; Kawaji, H.; Tsujikawa, M.; Nishida, K. Discovery of molecular markers to discriminate corneal endothelial cells in the human body. PLoS ONE 2015, 10, e0117581. [Google Scholar] [CrossRef]
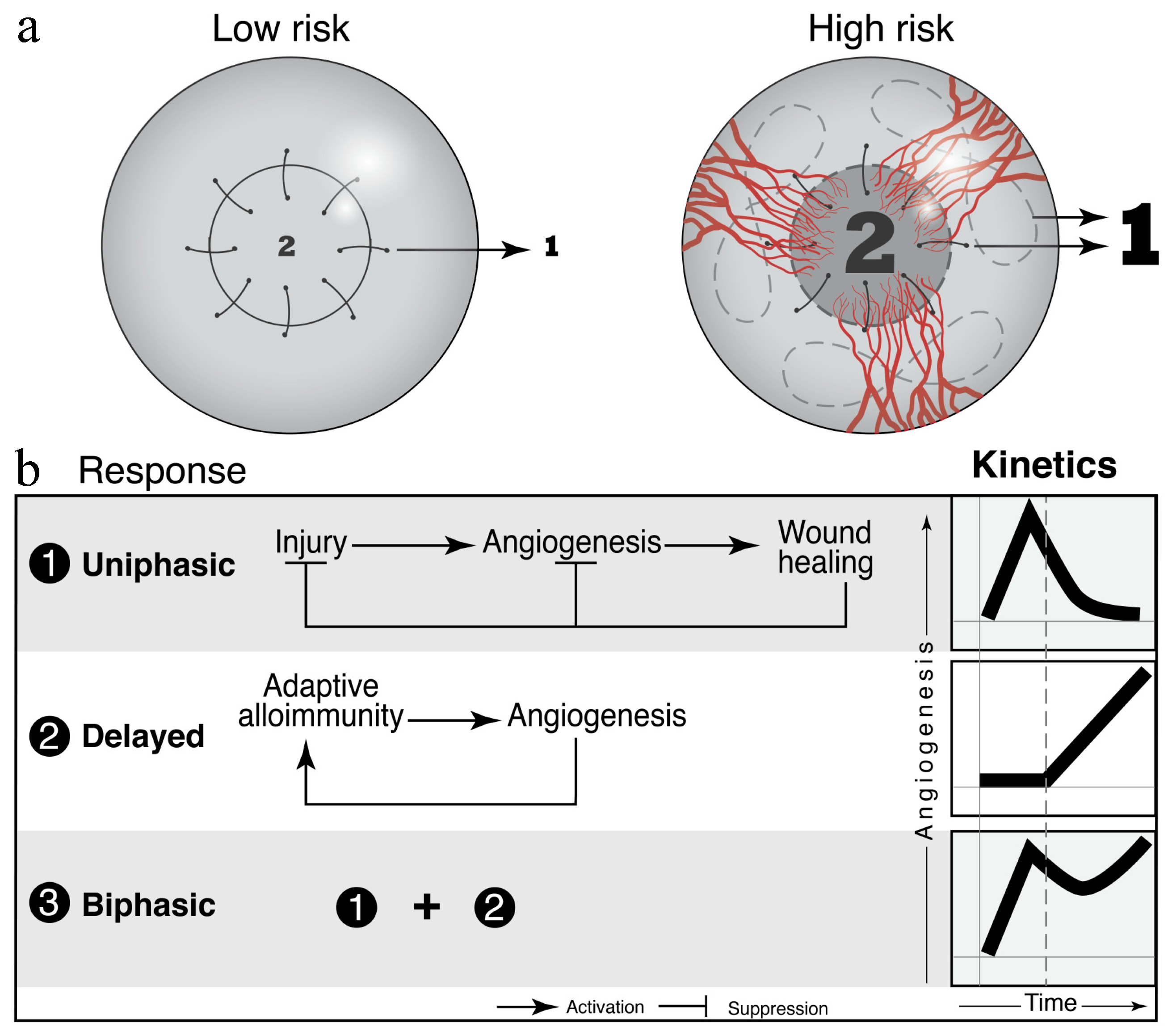
- Inomata, T.; Mashaghi, A.; Di Zazzo, A.; Lee, S.M.; Chiang, H.; Dana, R. Kinetics of Angiogenic Responses in Corneal Transplantation. Cornea 2017, 36, 491–496. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Kheirkhah, A.; Abud, T.B.; Goyal, S.; Dana, R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017, 62, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Salem, H.; Elkhanany, A.E.; Hussein, H.; Abd El-Baky, N. Clinical correlates of common corneal neovascular diseases: A literature review. Int. J. Ophthalmol. 2015, 8, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care (New Rochelle) 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Mizia-Malarz, A.; Sobol, G.; Wos, H. Proangiogenic factors: Vascular-endothelial growth factor (VEGF) and basic fibroblast growth factor--the characteristics and function. Przegl Lek 2008, 65, 353–357. [Google Scholar] [PubMed]
- Niu, G.; Chen, X. Why integrin as a primary target for imaging and therapy. Theranostics 2011, 1, 30–47. [Google Scholar] [CrossRef]
- Voiculescu, O.B.; Voinea, L.M.; Alexandrescu, C. Corneal neovascularization and biological therapy. J. Med. Life 2015, 8, 444–448. [Google Scholar]
- Cauchi, P.A.; Ang, G.S.; Azuara-Blanco, A.; Burr, J.M. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am. J. Ophthalmol. 2008, 146, 251–259. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Anshu, A.; Price, M.O.; Price, F.W., Jr. Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmol. Ogy 2012, 119, 536–540. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Hornig, C.; Weich, H.A. Soluble VEGF receptors. Angiogenesis 1999, 3, 33–39. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef]
- Han, K.Y.; Chang, J.H.; Dugas-Ford, J.; Alexander, J.S.; Azar, D.T. Involvement of lysosomal degradation in VEGF-C-induced down-regulation of VEGFR-3. Febs Lett. 2014, 588, 4357–4363. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Makarem, J.A.; Shamseddine, A.I. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol. Dis. 2007, 38, 258–268. [Google Scholar] [CrossRef]
- Zhong, W.; Montana, M.; Santosa, S.M.; Isjwara, I.D.; Huang, Y.H.; Han, K.Y.; O’Neil, C.; Wang, A.; Cortina, M.S.; de la Cruz, J.; et al. Angiogenesis and lymphangiogenesis in corneal transplantation-A review. Surv. Ophthalmol. 2018, 63, 453–479. [Google Scholar] [CrossRef]
- Chen, L.; Huq, S.; Gardner, H.; de Fougerolles, A.R.; Barabino, S.; Dana, M.R. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch. Ophthalmol. 2007, 125, 783–788. [Google Scholar] [CrossRef][Green Version]
- Yamagami, S.; Dana, M.R. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1293–1298. [Google Scholar]
- Patel, S.P.; Dana, R. Corneal lymphangiogenesis: Implications in immunity. Semin. Ophthalmol. 2009, 24, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004, 15, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; Gerhardt, H.; Kalen, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001, 153, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Giacomini, C.; Bignami, F.; Moi, D.; Ranghetti, A.; Doglioni, C.; Naldini, L.; Rama, P.; Mazzieri, R. Angiopoietin 2 expression in the cornea and its control of corneal neovascularisation. Br. J. Ophthalmol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef]
- Diamond, M.A.; Chan, S.W.S.; Zhou, X.; Glinka, Y.; Girard, E.; Yucel, Y.; Gupta, N. Lymphatic vessels identified in failed corneal transplants with neovascularisation. Br. J. Ophthalmol. 2019, 103, 421–427. [Google Scholar] [CrossRef]
- Baluk, P.; McDonald, D.M. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann. N. Y. Acad. Sci. 2008, 1131, 1–12. [Google Scholar] [CrossRef]
- Kong, L.L.; Yang, N.Z.; Shi, L.H.; Zhao, G.H.; Zhou, W.; Ding, Q.; Wang, M.H.; Zhang, Y.S. The optimum marker for the detection of lymphatic vessels. Mol. Clin. Oncol. 2017, 7, 515–520. [Google Scholar] [CrossRef]
- Schroedl, F.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; Lutty, G.A.; Maruyama, K.; Chen, L.; Lutjen-Drecoll, E.; et al. Consensus statement on the immunohistochemical detection of ocular lymphatic vessels. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6440–6442. [Google Scholar] [CrossRef]
- Randleman, J.B.; Woodward, M.; Lynn, M.J.; Stulting, R.D. Risk assessment for ectasia after corneal refractive surgery. Ophthalmol. Ogy 2008, 115, 37–50. [Google Scholar] [CrossRef]
- Stewart, W.C.; Jenkins, J.N.; Stewart, J.A. Corneal thickness after refractive surgery. Ophthalmol. Ogy 2005, 112, 1637. [Google Scholar] [CrossRef]
- Jonas, J.B.; Holbach, L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Verdier, D.D.; Sugar, A.; Baratz, K.; Beck, R.; Dontchev, M.; Dunn, S.; Gal, R.L.; Holland, E.J.; Kollman, C.; Lass, J.H.; et al. Corneal thickness as a predictor of corneal transplant outcome. Cornea 2013, 32, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the Cornea Donor Study Research, G.; Sugar, A.; Gal, R.L.; Kollman, C.; Raghinaru, D.; Dontchev, M.; Croasdale, C.R.; Feder, R.S.; Holland, E.J.; Lass, J.H.; et al. Factors associated with corneal graft survival in the cornea donor study. Jama Ophthalmol. 2015, 133, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Su, D.H.; Wong, T.Y.; Wong, W.L.; Saw, S.M.; Tan, D.T.; Shen, S.Y.; Loon, S.C.; Foster, P.J.; Aung, T.; Singapore Malay Eye Study, G. Diabetes, hyperglycemia, and central corneal thickness: The Singapore Malay Eye Study. Ophthalmol. Ogy 2008, 115, 964–968. [Google Scholar] [CrossRef]
- Wang, D.; Huang, W.; Li, Y.; Zheng, Y.; Foster, P.J.; Congdon, N.; He, M. Intraocular pressure, central corneal thickness, and glaucoma in chinese adults: The liwan eye study. Am. J. Ophthalmol. 2011, 152, 454–462. [Google Scholar] [CrossRef]
- Maurice, D.M. The location of the fluid pump in the cornea. J. Physiol 1972, 221, 43–54. [Google Scholar] [CrossRef]
- Levenson, J.E. Corneal edema: Cause and treatment. Surv. Ophthalmol. 1975, 20, 190–204. [Google Scholar] [CrossRef]
- Kopplin, L.J.; Przepyszny, K.; Schmotzer, B.; Rudo, K.; Babineau, D.C.; Patel, S.V.; Verdier, D.D.; Jurkunas, U.; Iyengar, S.K.; Lass, J.H.; et al. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch. Ophthalmol. 2012, 130, 433–439. [Google Scholar] [CrossRef]
- Lu, Y.; Dimasi, D.P.; Hysi, P.G.; Hewitt, A.W.; Burdon, K.P.; Toh, T.; Ruddle, J.B.; Li, Y.J.; Mitchell, P.; Healey, P.R.; et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010, 6, e1000947. [Google Scholar] [CrossRef]
- Vitart, V.; Bencic, G.; Hayward, C.; Skunca Herman, J.; Huffman, J.; Campbell, S.; Bucan, K.; Navarro, P.; Gunjaca, G.; Marin, J.; et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum. Mol. Genet. 2010, 19, 4304–4311. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Aung, T.; Khor, C.C.; Cornes, B.K.; Tay, W.T.; Sim, X.; Lavanya, R.; Wu, R.; Zheng, Y.; Hibberd, M.L.; et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum. Mol. Genet. 2011, 20, 649–658. [Google Scholar] [CrossRef]
- Nicholls, S.; Pong-Wong, R.; Mitchard, L.; Harley, R.; Archibald, A.; Dick, A.; Bailey, M. Genome-Wide Analysis in Swine Associates Corneal Graft Rejection with Donor-Recipient Mismatches in Three Novel Histocompatibility Regions and One Locus Homologous to the Mouse H-3 Locus. PLoS ONE 2016, 11, e0152155. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Roberts, C.J.; Mahmoud, A.M.; Colin, J.; Maurice-Tison, S.; Kerautret, J. Screening of forme fruste keratoconus with the ocular response analyzer. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, D.P.; Burdon, K.P.; Craig, J.E. The genetics of central corneal thickness. Br. J. Ophthalmol. 2010, 94, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, B. Tenuity of cornea with Ehlers-Danlos syndrome. Acta Ophthalmol. (Copenh) 1969, 47, 704–708. [Google Scholar] [CrossRef]
- Pesudovs, K. Orbscan mapping in Ehlers-Danlos syndrome. J. Cataract Refract. Surg. 2004, 30, 1795–1798. [Google Scholar] [CrossRef]
- Blumcke, S.; Niedorf, H.R.; Thiel, H.J.; Langness, U. Histochemical and fine structural studies on the cornea with osteogenesis imperfecta congenita. Virchows Arch. B Cell Pathol. 1972, 11, 124–132. [Google Scholar] [CrossRef]
- Surquin, M.; Le Moine, A.; Flamand, V.; Nagy, N.; Rombaut, K.; Demoor, F.X.; Stordeur, P.; Salmon, I.; Guery, J.C.; Goldman, M.; et al. Skin graft rejection elicited by beta 2-microglobulin as a minor transplantation antigen involves multiple effector pathways: Role of Fas-Fas ligand interactions and Th2-dependent graft eosinophil infiltrates. J. Immunol. 2002, 169, 500–506. [Google Scholar] [CrossRef]
- Bohringer, D.; Spierings, E.; Enczmann, J.; Bohringer, S.; Sundmacher, R.; Goulmy, E.; Reinhard, T. Matching of the minor histocompatibility antigen HLA-A1/H-Y may improve prognosis in corneal transplantation. Transplantation 2006, 82, 1037–1041. [Google Scholar] [CrossRef]
- Qazi, Y.; Hamrah, P. Gene therapy in corneal transplantation. Semin. Ophthalmol. 2013, 28, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Lee, J.; Ferrara, N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer 2010, 10, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 2014, 219, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Sumioka, T.; Saika, S. The role of extracellular matrix in corneal wound healing. Cornea 2013;32(suppl): s43-s45-retracted. Cornea 2014, 33, 100. [Google Scholar] [CrossRef]
- Chung, E.S.; Chauhan, S.K.; Jin, Y.; Nakao, S.; Hafezi-Moghadam, A.; van Rooijen, N.; Zhang, Q.; Chen, L.; Dana, R. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am. J. Pathol. 2009, 175, 1984–1992. [Google Scholar] [CrossRef]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998, 30, 1019–1030. [Google Scholar] [CrossRef]
- Boosani, C.S.; Sudhakar, Y.A. Proteolytically Derived Endogenous Angioinhibitors Originating from the Extracellular Matrix. Pharmaceuticals 2011, 4, 1551–1577. [Google Scholar] [CrossRef]
- Jackson, J.R.; Seed, M.P.; Kircher, C.H.; Willoughby, D.A.; Winkler, J.D. The codependence of angiogenesis and chronic inflammation. Faseb J. 1997, 11, 457–465. [Google Scholar] [CrossRef]
- Maycock, N.J.; Marshall, J. Genomics of corneal wound healing: A review of the literature. Acta Ophthalmol. 2014, 92, e170–e184. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Tuominen, I.S.; Tervo, T.M.; Teppo, A.M.; Valle, T.U.; Gronhagen-Riska, C.; Vesaluoma, M.H. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp. Eye Res. 2001, 72, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, K.; Senn, B.C.; Reiser, P.; Pech, M.; Flammer, J.; Meyer, P. Connective tissue growth factor in retrocorneal membranes and corneal scars. Ophthalmol. Ogica 2000, 214, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Flanders, K.C.; Bertolette, D.; Lyakh, L.A.; Wurthner, J.U.; Parks, W.T.; Letterio, J.J.; Ruscetti, F.W.; Roberts, A.B. Levels of phospho-Smad2/3 are sensors of the interplay between effects of TGF-beta and retinoic acid on monocytic and granulocytic differentiation of HL-60 cells. Blood 2003, 101, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Jumblatt, M.M.; Willer, S.S. Corneal endothelial repair. Regulation of prostaglandin E2 synthesis. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1294–1301. [Google Scholar]
- van Setten, G.B.; Tervo, T.; Viinikka, L.; Perheentupa, J.; Tarkkanen, A. Epidermal growth factor in human tear fluid: A minireview. Int. Ophthalmol. 1991, 15, 359–362. [Google Scholar] [CrossRef]
- Lim, M.; Goldstein, M.H.; Tuli, S.; Schultz, G.S. Growth factor, cytokine and protease interactions during corneal wound healing. Ocul. Surf. 2003, 1, 53–65. [Google Scholar] [CrossRef]
- Vesaluoma, M.; Teppo, A.M.; Gronhagen-Riska, C.; Tervo, T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: A potential modulator of corneal wound healing following photorefractive keratectomy. Curr. Eye Res. 1997, 16, 825–831. [Google Scholar] [CrossRef]
- Morita, S.; Shirakata, Y.; Shiraishi, A.; Kadota, Y.; Hashimoto, K.; Higashiyama, S.; Ohashi, Y. Human corneal epithelial cell proliferation by epiregulin and its cross-induction by other EGF family members. Mol. Vis. 2007, 13, 2119–2128. [Google Scholar]
- Zhou, M.; Li, X.M.; Lavker, R.M. Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells. A comparison of limbal and corneal epithelial basal cells. J. Biol. Chem. 2006, 281, 19600–19609. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Tseng, S.C. Differential regulation of cytokine and receptor transcript expression in human corneal and limbal fibroblasts by epidermal growth factor, transforming growth factor-alpha, platelet-derived growth factor B, and interleukin-1 beta. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2068–2080. [Google Scholar]
- Kakazu, A.; Sharma, G.; Bazan, H.E. Association of protein tyrosine phosphatases (PTPs)-1B with c-Met receptor and modulation of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Saghizadeh, M.; Kramerov, A.A.; Yu, F.S.; Castro, M.G.; Ljubimov, A.V. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem. Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yano, W.; Kondo, S.; Hattori, Y.; Yamada, N.; Yanai, R.; Nishida, T. Up-regulation of urokinase-type plasminogen activator in corneal epithelial cells induced by wounding. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3332–3338. [Google Scholar] [CrossRef]
- Paladini, S.V.; Pinto, G.H.; Bueno, R.H.; Calloni, R.; Recamonde-Mendoza, M. Identification of Candidate Biomarkers for Transplant Rejection from Transcriptome Data: A Systematic Review. Mol. Diagn. Ther. 2019, 23, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Mark, E.; Goldsman, D.; Gurbaxani, B.; Keskinocak, P.; Sokol, J. Using machine learning and an ensemble of methods to predict kidney transplant survival. PLoS ONE 2019, 14, e0209068. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Hirata, Y.; Tomioka, R.; Kashima, H.; Yamanishi, K.; Hayashi, N.; Egawa, S.; Aihara, K. Predicting disease progression from short biomarker series using expert advice algorithm. Sci. Rep. 2015, 5, 8953. [Google Scholar] [CrossRef]
- Senanayake, S.; White, N.; Graves, N.; Healy, H.; Baboolal, K.; Kularatna, S. Machine learning in predicting graft failure following kidney transplantation: A systematic review of published predictive models. Int. J. Med. Inf. 2019, 130, 103957. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.; Park, J.; Lee, K.P.; Lee, S.; Lee, D.M.; Kim, K.H.; Kim, H.K.; Cho, D.W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Woman is First to Receive Cornea Made from ‘Reprogrammed’ Stem Cells. Available online: https://www.nature.com/articles/d41586-019-02597-2 (accessed on 6 December 2019).
| Biomarkers | Clinical Significance | References |
|---|---|---|
| ABO Blood Group | Minor histocompatibility complex antigen mismatch implicated in allograft rejection. | [3,4] |
| HLA-DR | Major histocompatibility complex antigen mismatch implicated in allograft rejection for high-risk bed. | [5,6,7,8] |
| Activated Keratocytes | Reflect level of intrastromal inflammation. Respond to key inflammatory mediators including IL-1 and TNF-α. Observed as early as 2 months before rejection. Levels decrease as immunosuppressant treatment progresses. | [9,10,11,12,13,14,15] |
| Immune Cell Density | Sub-basal and endothelial immune cell density increase associated with graft rejection. Reflects levels of stromal inflammation by responding to inflammatory mediators. | [10,16] |
| Angio-/Lymphangiogenic Markers VEGFR-1, 2, 3 | Binds to VEGF-A; VEGF-C; VEGF-D, respectively. Can act as anti-angiogenic factors in the corneal epithelial cells. | [17,18,19,20,21,22] |
| VEGF-A, C, D | Directly promotes corneal angio/lymphangiogenesis in the absence of above anti-angiogenic receptor. | |
| Inflammatory Markers IL-1, IL-6, IL-8, IL-17A, TNF-α | Proinflammatory cytokines upregulated post-transplantation. | [13,23,24,25,26,27] |
| MIP-1α, MIP-1β, MIP-2, RANTES, CCL2, CCL20, CCL21 | Proinflammatory chemokines upregulated post-transplantation. Promote corneal acquisition of MHC class II cells and APC. | [21,24,28,29,30,31] |
| IL-2, IL-4, IL-5, IFN-γ | Protective factors (IL-2 and IL-5) and hazardous factors (IL-4 and IFN-γ) within the AqH. Candidate markers for prognosticating post-operative immune responses. | [27] |
| C3a | Complement pathway product. High levels in the AqH associated with graft rejection. | [32] |
| MHC class I-related chain A (MICA) | Expression induced by IFN-γ in corneal epithelial and endothelial cells. Connection to stimulation of CD8+ cells and subsequent promotion of immune response. | [33] |
| ICAM-1, VLA-1 | Adhesion molecules targeted by immune cells. Expression upregulated in inflammatory states and promote acquisition of MHC class II cells and APC in the cornea. | [34,35,36,37] |
| Antigen Presenting Cells and Surface Proteins CD11c+(Dendritic cells) | Upregulation within 24 h of inflammation. Showed increased expression of MHC class II molecules in inflammatory states. | [38] |
| CD11c−/CD11b+ (Monocyte/Macrophage) | Migrates throughout the stroma (normally confined to posterior stroma) during inflammatory states. | [38] |
| CD80, CD86, CD40 | Co-stimulatory molecules expressed on APCs, of which their expression is increased due to increased proinflammatory cytokines post-transplantation. | [7,38,39] |
| CCR7 | Promotes CCL21-dependent APC migration to the cornea through afferent lymphatics. | [30] |
| T Cells and Surface Proteins Foxp3 (Treg) | Releases IL-10 and TGF. Correlated with reduced allograft rejection. | [16,40,41] |
| CD8+/IFN-γ+ | High levels in the AqH associated with prognostication of allograft rejection. | [32] |
| Biomarkers | Clinical Significance | References |
|---|---|---|
| Endothelial cell density (ECD, cell counts/mm2) | Lower ECD preoperatively and 2 months postoperatively was significantly correlated with the development of late endothelial failure after PKP. The lower ECD at 6 months postoperatively showed strong correlation with graft failure from endothelial decompensation. | [63,65] [66,67] |
| Lower graft ECD was identified as a significant predisposing factor for lower postoperative ECD, but not for graft failure after DSAEK. Lower graft ECD was found as a significant risk factor for higher postoperative ECD loss by multinominal regression analysis after DMEK. | [70] [71] | |
| Endothelial cell morphology Polymegethism (coefficient of variation of cell area, %) | Clinically valuable marker of the state of the endothelium | [15] |
| Pleomorphism (hexagonality, %) Spring constant K * | Valuable morphometric parameter of the state of the endothelium Lower hexagonality at 6 months after PKP showed a suggestive trend of higher graft failure. Positive correlation with CD166+/CD24–/CD105–/CD44– effector cell fraction for injection of cultured HCECs with a ROCK inhibitor. Preoperative K showed best classification accuracy with ECD at postoperative 6 months compared with other parameters, including effector cell fraction, preoperative ECD, and preoperative hexagonality. | [15] [67] [61] |
| Genes ANAPC1 | A cell cycle-regulated E3 ubiquitin ligase which controls progression through mitosis and the G1 phase of the cell cycle. An intergenic variant (rs78658973[A]) close to ANAPC1 was found to have a strong association with decreased ECD. | [72] |
| Associated Factors | Clinical Significance | References |
|---|---|---|
| Graft Failure | CCT was associated with graft failure independent of the prediction made through ECD. The possibility of an unknown mechanism connecting CCT to graft failure has been posited. | [116,117] |
| Diabetes and Hyperglycemia | Associated with corneal endothelial dysfunction and resultant stromal hydration of the cornea. Osmotic fluid shifts and collagen cross-linkage are likely etiologies. | [118,119] |
| Endothelial Decompensation, Corneal Edema | Diseases involving endothelial dysfunction, such as Fuchs’ endothelial corneal dystrophy, progress into corneal edema. Resultant increase in CCT is a reliable method to measure disease progression. | [120,121,122] |
| ΔIOP > 25 mmHg (post-operation) | CCT was predictive of IOP increase 1 month postoperatively. Preoperative glaucoma was associated with early graft failure. CCT may represent the underlying physiologic link that connects glaucoma and graft failure. | [116,117] |
| GenesZNF469 | Possible regulator of collagen synthesis and/or organization. Implicated in the development of Brittle Cornea syndrome, which exhibits markedly reduced CCT. | [123] |
| COL5A1 | Encodes for the alpha-1 chain of type V collagen. Associated with a variation of Ehlers–Danlos syndrome, which also exhibits reduced CCT. | [124,125] |
| COL8A2 | Encodes for the alpha-2 chain of type VIII collagen. Associated with posterior polymorphous corneal dystrophy and Fuchs’ endothelial corneal dystrophy, characterized by changes in the endothelial layer and Descemet’s membrane. | [124,125] |
| ZFP106 | Contains an mHag loci which encodes for H-3a epitopes. These loci were previously shown to mediate corneal graft allograft rejection. | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Zazzo, A.; Lee, S.-M.; Sung, J.; Niutta, M.; Coassin, M.; Mashaghi, A.; Inomata, T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. J. Clin. Med. 2020, 9, 586. https://doi.org/10.3390/jcm9020586
Di Zazzo A, Lee S-M, Sung J, Niutta M, Coassin M, Mashaghi A, Inomata T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. Journal of Clinical Medicine. 2020; 9(2):586. https://doi.org/10.3390/jcm9020586
Chicago/Turabian StyleDi Zazzo, Antonio, Sang-Mok Lee, Jaemyoung Sung, Matteo Niutta, Marco Coassin, Alireza Mashaghi, and Takenori Inomata. 2020. "Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology" Journal of Clinical Medicine 9, no. 2: 586. https://doi.org/10.3390/jcm9020586
APA StyleDi Zazzo, A., Lee, S.-M., Sung, J., Niutta, M., Coassin, M., Mashaghi, A., & Inomata, T. (2020). Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. Journal of Clinical Medicine, 9(2), 586. https://doi.org/10.3390/jcm9020586







