Leptin as a Key Player in Insulin Resistance of Liver Cirrhosis? A Cross-Sectional Study in Liver Transplant Candidates
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Study Design
2.2. Anthropometric Measurements and Laboratory Analysis
2.3. Estimate of Insulin Resistance
2.4. Data Analysis and Statistics
3. Results
3.1. Study Population
3.2. Adiponectin and Leptin
3.3. Insulin Resistance
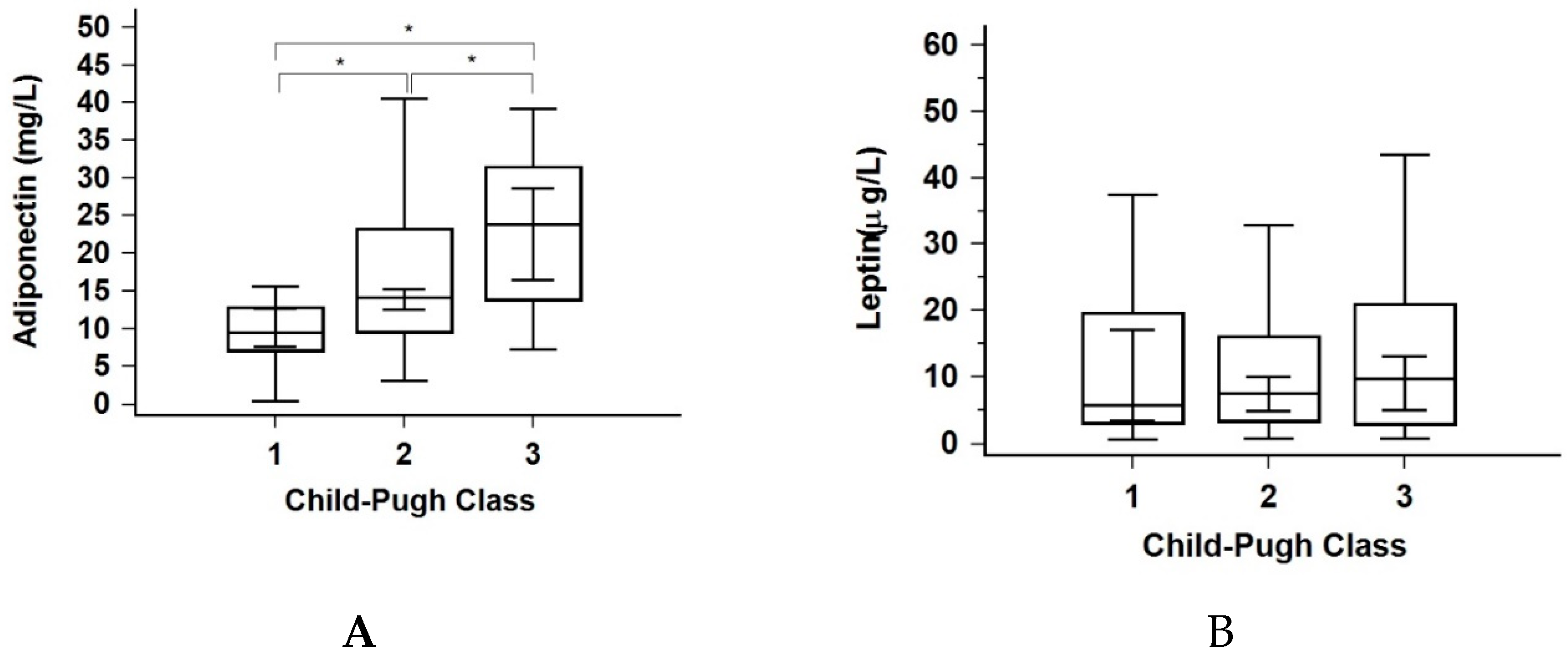
3.4. Liver Disease Stage
4. Discussion
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CHL | cholesterol |
| GGT | gamma-glutamyl transferase |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HOMA-2 | homeostasis model assessment 2 model |
| FPG | fasting plasma glucose |
| HDL | high density lipoprotein cholesterol |
| IRI | insulin resistance index |
| LDL | low density lipoprotein cholesterol |
| MELD | model for end-stage liver disease |
| OR | odds ratio |
| PT-INR | prothrombin time-international normalized ratio |
| TGC | Triglycerides |
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin action and Insulin resistance. Physiol Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Fartoux, L.; Poujol-Robert, A.; Guéchot, J.; Wendum, D.; Poupon, R.; Serfaty, L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut 2005, 54, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Cammà, C.; Bruno, S.; Di Marco, V.; Di Bona, D.; Rumi, M.; Vinci, M.; Rebucci, C.; Cividini, A.; Pizzolanti, G.; Minola, E.; et al. Insulin resistance is associated with steatosis in nondiabetic patients with genotype 1 chronic hepatitis C. Hepatology 2006, 43, 64–71. [Google Scholar] [CrossRef]
- Moucari, R.; Asselah, T.; Cazals-Hatem, D.; Voitot, H.; Boyer, N.; Ripault, M.P.; Sobesky, R.; Martinot-Peignoux, M.; Maylin, S.; Nicolas-Chanoine, M.H.; et al. Insulin resistance in chronic hepatitis C: Association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 2008, 134, 416–423. [Google Scholar] [CrossRef]
- Petta, S.; Cammà, C.; Di Marco, V.; Alessi, N.; Cabibi, D.; Caldarella, R.; Licata, A.; Massenti, M.F.; Tarantino, G.; Marchesini, G.; et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am. J. Gastroenterol. 2008, 103, 1136–1144. [Google Scholar] [CrossRef]
- Megyesi, C.; Samols, E.; Marks, V. Glucose tolerance and diabetes in chronic liver disease. Lancet 1967, 2, 1051–1056. [Google Scholar] [CrossRef]
- Petrides, A.S.; DeFronzo, R.A. Glucose and insulin metabolism in cirrhosis. J. Hepatol. 1989, 8, 107–114. [Google Scholar] [CrossRef]
- Petrides, A.S.; Stanley, T.; Matthews, D.E.; Vogt, C.; Bush, A.J.; Lambeth, H. Insulin resistance in cirrhosis: Prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology 1998, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.J.; Willmann, O.; Rieger, A.; Fenk, A.; Selberg, O.; Lautz, H.U.; Bürger, M.; Balks, H.J.; von zur Mühlen, A.; Schmidt, F.W. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology 1992, 102, 2033–2041. [Google Scholar] [CrossRef]
- Blei, A.T.; Robbins, D.C.; Drobny, E.; Baumann, G.; Rubenstein, A.H. Insulin resistance and insulin receptors in hepatic cirrhosis. Gastroenterology 1982, 83, 1191–1199. [Google Scholar] [CrossRef]
- Grancini, V.; Trombetta, M.; Lunati, M.E.; Zimbalatti, D.; Boselli, M.L.; Gatti, S.; Donato, M.F.; Resi, V.; D’Ambrosio, R.; Aghemo, A.; et al. Contribution of β-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: Role of severity of liver disease. J. Hepatol. 2015, 63, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Erice, E.; Llop, E.; Berzigotti, A.; Abraldes, J.G.; Conget, I.; Seijo, S.; Reverter, E.; Albillos, A.; Bosch, J.; García-Pagán, J.C. Insulin resistance in patients with cirrhosis and portal hypertension. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1458–G1465. [Google Scholar] [CrossRef] [PubMed]
- De Minicis, S.; Agostinelli, L.; Rychlicki, C.; Sorice, G.P.; Saccomanno, S.; Candelaresi, C.; Giaccari, A.; Trozzi, L.; Pierantonelli, I.; Mingarelli, E.; et al. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS ONE 2014, 9, e97136. [Google Scholar] [CrossRef]
- Hung, C.-H.; Wang, J.-H.; Hu, T.-H.; Chen, C.-H.; Chang, K.-C.; Yen, Y.-H.; Kuo, Y.-H.; Tsai, M.C.; Lu, S.N.; Lee, C.M. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J. Gastroenterol. 2010, 16, 2265–2271. [Google Scholar] [CrossRef]
- Nkontchou, G.; Bastard, J.-P.; Ziol, M.; Aout, M.; Cosson, E.; Ganne-Carrie, N.; Grando-Lemaire, V.; Roulot, D.; Capeau, J.; Trinchet, J.C.; et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J. Hepatol. 2010, 53, 827–833. [Google Scholar] [CrossRef]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Testa, R.; Franceschini, R.; Giannini, E.; Cataldi, A.; Botta, F.; Fasoli, A.; Tenerelli, P.; Rolandi, E.; Barreca, T. Serum leptin levels in patients with viral chronic hepatitis or liver cirrhosis. J. Hepatol. 2000, 33, 33–37. [Google Scholar] [CrossRef]
- Bouloumié, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999. [Google Scholar] [CrossRef]
- Ikejima, K.; Honda, H.; Yoshikawa, M.; Hirose, M.; Kitamura, T.; Takei, Y.; Sato, N. Leptin augments inflammatory and profibrogenic responses in the murine liver induced by hepatotoxic chemicals. Hepatology 2001, 34, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Titus, M.A.; Ding, X.; Floyd, J.; Srinivasan, S.; Sitaraman, S.V.; Anania, F.A. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: Mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004, 18, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Haberl, E.M.; Rein-Fischboeck, L.; Aslanidis, C. Adipokines in liver cirrhosis. Int. J. Mol. Sci. 2017, 18, 1392. [Google Scholar] [CrossRef]
- Balmer, M.L.; Joneli, J.; Schoepfer, A.; Stickel, F.; Thormann, W.; Dufour, J.F. Significance of serum adiponectin levels in patients with chronic liver disease. Clin. Sci. 2010, 119, 431–436. [Google Scholar] [CrossRef]
- Tietge, U.J.F.; Böker, K.H.W.; Manns, M.P.; Bahr, M.J. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E82–E89. [Google Scholar] [CrossRef]
- Bolukbas, F.F.; Bolukbas, C.; Horoz, M.; Gumus, M.; Erdogan, M.; Zeyrek, F.; Yayla, A.; Ovunc, O. Child-pugh classification dependent alterations in serum leptin levels among cirrhotic patients: A case controlled study. BMC Gastroenterol. 2004, 4, 23. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Sheu, W.H.H. Increased serum leptin concentrations correlate with soluble tumour necrosis factor receptor levels in patients with cirrhosis. Clin. Endocrinol. (Oxf.) 2002, 57, 805–811. [Google Scholar] [CrossRef]
- Ataseven, H.; Bahcecioglu, I.H.; Kuzu, N.; Yalniz, M.; Celebi, S.; Erensoy, A.; Ustundag, B. The levels of ghrelin, leptin, TNF-α, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators Inflamm. 2006. [Google Scholar] [CrossRef]
- Sadik, N.A.H.; Ahmed, A.; Ahmed, S. The significance of serum levels of adiponectin, leptin, and hyaluronic acid in hepatocellular carcinoma of cirrhotic and noncirrhotic patients. Hum. Exp. Toxicol. 2012, 31, 311–321. [Google Scholar] [CrossRef]
- Kaser, S.; Moschen, A.; Kaser, A.; Ludwiczek, O.; Ebenbichler, C.F.; Vogel, W.; Jaschke, W.; Patsch, J.R.; Tilg, H. Circulating adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis. J. Intern. Med. 2005, 258, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Yamada, C.; Moriyama, K.; Takahashi, E. Optimal cut-off point for homeostasis model assessment of insulin resistance to discriminate metabolic syndrome in non-diabetic Japanese subjects. J. Diabetes Investig. 2012, 3, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Kaser, S.; Föger, B.; Waldenberger, P.; Nachbaur, K.; Propst, A.; Jaschke, W.R.; Vogel, W.; Patsch, J.R. Transjugular intrahepatic portosystemic shunt (TIPS) augments hyperinsulinemia in patients with cirrhosis. J. Hepatol. 2000. [Google Scholar] [CrossRef]
- Angulo, P.; Alba, L.M.; Petrovic, L.M.; Adams, L.A.; Lindor, K.D.; Jensen, M.D. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J. Hepatol. 2004, 41, 943–949. [Google Scholar] [CrossRef]
- Havel, P.J. Update on adipocyte hormones: Regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 2004. [Google Scholar] [CrossRef]
- Friedman, J. The long road to leptin. J. Clin. Investig. 2016, 126, 4727–4734. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996. [Google Scholar] [CrossRef]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003. [Google Scholar] [CrossRef]
- Marra, F. Leptin and liver fibrosis: A matter of fat. Gastroenterology 2002. [Google Scholar] [CrossRef] [PubMed]
- Borer, K.T. Counterregulation of insulin by leptin as key component of autonomic regulation of body weight. World J. Diabetes 2014. [Google Scholar] [CrossRef]
- Kieffer, T.J.; Habener, J.F. The adipoinsular axis: Effects of leptin on pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1–E14. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Kolaczynski, J.W.; Polansky, M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J. Clin. Investig. 1997. [Google Scholar] [CrossRef]
- Herrera, J.L. Complications of cirrhosis. Pract. Manag. Liver Diseases 2008. [Google Scholar] [CrossRef]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Pares, A. EASL clinical practice guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Cammisotto, P.; Bendayan, M. A review on gastric leptin: The exocrine secretion of a gastric hormone. Anat. Cell Biol. 2012, 45, 1–16. [Google Scholar] [CrossRef]
- Beier, J.I.; McClain, C.J. Mechanisms and cell signaling in alcoholic liver disease. Biol. Chem. 2010, 391, 1249–1264. [Google Scholar] [CrossRef]
- Meikle, P.J.; Mundra, P.A.; Wong, G.; Rahman, K.; Huynh, K.; Barlow, C.K.; Duly, A.M.P.; Haber, P.S.; Whitfield, J.B.; Seth, D. Circulating lipids are associated with alcoholic liver cirrhosis and represent potential biomarkers for risk assessment. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Andreelli, F.; Foretz, M.; Knauf, C.; Cani, P.D.; Perrin, C.; Iglesias, M.A.; Pillot, B.; Bado, A.; Tronche, F.; Mithieux, G.; et al. Liver AMPK alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not by insulin. Endocrinology 2006, 147, 2432–2441. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, B.; Lo, K.A.; Patterson, H.C.; Sun, Y.; Lodish, H.F. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc. Natl. Acad. Sci. USA 2012, 109, 14568–14573. [Google Scholar] [CrossRef]
- Ragheb, R.; Medhat, A.M. Mechanisms of Fatty Acid-Induced Insulin Resistance in Muscle and Liver. J. Diabetes Metab. 2011, 2, 127. [Google Scholar] [CrossRef]
| Variable | N | % |
|---|---|---|
| Gender (Male:Female) | 91:35:00 | 72:28:00 |
| Etiology | ||
| • Alcohol | 69 | 54.8 |
| • Viral | 28 | 22.2 |
| • Autoimmune | 12 | 9.5 |
| • Metabolic | 1 | 0.8 |
| • Cryptogenic | 12 | 9.5 |
| • Other | 4 | 3.2 |
| CP class | ||
| • A | 14 | 11.1 |
| • B | 43 | 34.1 |
| • C | 69 | 54.8 |
| Insulin resistance | 104 | 82.5 |
| Mean | SD | |
| Age | 57 | 9 |
| Variable | Total (n = 126) | Insulin Resistant (n = 104) | Insulin Sensitive (n = 22) | ||
|---|---|---|---|---|---|
| Median ± IQR | Range | Median ± IQR | Median ± IQR | p * | |
| Age (years) | 58 ± 12 | 32–77 | 58 ± 12 | 56 ± 13 | 0.203 |
| MELD score | 15.43 ± 7 | 7–38 | 14.95 ± 7 | 16.44 ± 8 | 0.386 |
| Leptin (ug/L) | 7.55 ± 15.34 | 0.66–58.10 | 9.58 ± 16.18 | 3.00 ± 2.45 | <0.001 |
| Adiponectin (mg/L) | 16.26 ± 18.04 | 3.01–137.17 | 16.95 ± 18.08 | 14.15 ± 15.58 | 0.951 |
| BMI (kg/m2) | 26.06 ± 5.81 | 15.94–44.19 | 26.86 ± 5.28 | 22.86 ± 5.01 | <0.001 |
| FPG (mmol/L) | 5.3 ± 1.0 | 3.3–6.9 | 5.4 ± 0.9 | 4.63 ± 1.0 | <0.001 |
| HbA1c (%) | 4.80 ± 0.6 | 3.4–6.0 | 4.8 ± 0.8 | 4.7 ± 0.5 | 0.163 |
| Insulin (pmol/L) | 113.0 ± 118.1 | 18.0–731.5 | 155.0 ± 115.6 | 68.1 ± 20.0 | <0.001 |
| C-peptide (nmol/L) | 0.97 ± 0.66 | 0.39–5.32 | 1.05 ± 0.67 | 0.57 ± 0.44 | <0.001 |
| IRI | 2.5 ± 2.3 | 0.40–15.90 | 3.3 ± 2.3 | 1.4 ± 0.45 | <0.001 |
| Triglycerides (mmol/L) | 0.75 ± 0.59 | 0.28–3.88 | 0.72 ± 0.53 | 1.00 ± 1.14 | 0.035 |
| Cholesterol (mmol/L) | 3.2 ± 2.0 | 0.3–12.7 | 3.1 ± 2.0 | 3.35 ± 2.03 | 0.787 |
| HDL-C (mmol/L) | 0.91 ± 0.6 | 0.16–2.38 | 0.94 ± 0.63 | 0.8 ± 0.6 | 0.106 |
| LDL-C (mmol/L) | 2.00 ± 1.6 | 0.20–10.8 | 1.9 ± 1.7 | 2.1 ± 1.8 | 0.626 |
| AST (IU/L) | 63 ± 47 | 22–903 | 64 ± 41 | 56 ± 89 | 0.619 |
| ALT (IU/L) | 35 ± 36 | 10–241 | 36 ± 31 | 27 ± 69 | 0.458 |
| AP (IU/L) | 53 ± 65 | 13–1088 | 117 ± 78 | 108 ± 111 | 0.437 |
| GGT (IU/L) | 117 ± 82 | 42–567 | 54 ± 80 | 53 ± 72 | 0.529 |
| Bilirubin (µmol/L) | 53 ± 65 | 5–647 | 49 ± 63 | 74 ± 131 | 0.188 |
| PV-INR | 1.5 ± 1.3 | 1.0–3.0 | 1.5 ± 1.2 | 1.5 ± 1.3 | 0.672 |
| Variable | Odds Ratio | 95% Conf. Interval | p * |
|---|---|---|---|
| CP score | 0.954 | 0.777–1.171 | 0.651 |
| Age | 1.018 | 0.956–1.084 | 0.581 |
| Gender | 2.284 | 0.647–8.069 | 0.199 |
| Leptin | 1.247 | 1.076–1.447 | 0.003 |
| Adiponectin | 1.001 | 0.969–1.041 | 0.802 |
| HbA1c | 1.779 | 0.690–4.893 | 0.233 |
| Triglycerides | 0.357 | 0.139–0.917 | 0.032 |
| Cholesterol | 1.079 | 0.832–1.399 | 0.569 |
| HDL-C | 2.757 | 0.874–8.701 | 0.084 |
| LDL-C | 0.979 | 0.708–1.355 | 0.900 |
| Variable | CP class A (n = 14) | CP class B (n = 43) | CP class C (n = 69) | p * |
|---|---|---|---|---|
| Age (years) | 60.18 ± 11 | 56.56 ± 12 | 58.38 ± 12 | 0.321 |
| MELD score | 9.06 ± 2 | 13.77 ± 4 | 18.64 ± 8 | <0.001 |
| BMI (kg/m2) | 26.13 ± 6.43 | 25.93 ± 7.26 | 26.24 ± 5.66 | 0.653 |
| FPG (mmol/L) | 5.3 ± 0.8 | 5.1 ± 1.2 | 5.3 ± 1.0 | 0.686 |
| HbA1c (%) | 5.2 ± 0.5 | 4.9 ± 0.7 | 4.6 ± 0.4 | <0.001 |
| Insulin (pmol/L) | 127.85 ± 163.1 | 114.6 ± 117.9 | 110.8 ± 108.1 | 0.963 |
| C-peptide (nmol/L) | 0.78 ± 0.45 | 0.98 ± 0.77 | 1.03 ± 0.64 | 0.01 |
| IRI | 2.75 ± 3.35 | 2.5 ± 2.5 | 2.5 ± 2.2 | 0.959 |
| TGC (mmol/L) | 1.17 ± 0.56 | 0.94 ± 0.6 | 0.65 ± 0.32 | <0.001 |
| CHL (mmol/L) | 4.5 ± 2.0 | 3.7 ± 2.5 | 2.7 ± 1.5 | <0.001 |
| HDL-C (mmol/L) | 1.28 ± 0.86 | 0.97 ± 0.48 | 0.75 ± 0.7 | 0.002 |
| LDL-C (mmol/L) | 3.1 ± 1.4 | 2.4 ± 2.1 | 1.6 ± 1.3 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Košuta, I.; Mrzljak, A.; Kolarić, B.; Vučić Lovrenčić, M. Leptin as a Key Player in Insulin Resistance of Liver Cirrhosis? A Cross-Sectional Study in Liver Transplant Candidates. J. Clin. Med. 2020, 9, 560. https://doi.org/10.3390/jcm9020560
Košuta I, Mrzljak A, Kolarić B, Vučić Lovrenčić M. Leptin as a Key Player in Insulin Resistance of Liver Cirrhosis? A Cross-Sectional Study in Liver Transplant Candidates. Journal of Clinical Medicine. 2020; 9(2):560. https://doi.org/10.3390/jcm9020560
Chicago/Turabian StyleKošuta, Iva, Anna Mrzljak, Branko Kolarić, and Marijana Vučić Lovrenčić. 2020. "Leptin as a Key Player in Insulin Resistance of Liver Cirrhosis? A Cross-Sectional Study in Liver Transplant Candidates" Journal of Clinical Medicine 9, no. 2: 560. https://doi.org/10.3390/jcm9020560
APA StyleKošuta, I., Mrzljak, A., Kolarić, B., & Vučić Lovrenčić, M. (2020). Leptin as a Key Player in Insulin Resistance of Liver Cirrhosis? A Cross-Sectional Study in Liver Transplant Candidates. Journal of Clinical Medicine, 9(2), 560. https://doi.org/10.3390/jcm9020560






